An assessment of population structure and regeneration status of Magnolia punduana Hk.f.&Th.(Magnoliaceae)in fragmented forests of northeast India
2020-05-19VihenoIraluNgakhainiiTrunePaoKrishnaUpadhaya
Viheno Iralu · Ngakhainii Trune Pao · Krishna Upadhaya
Abstract The population structure and regeneration status of Magnolia punduana Hk. f. & Th., an endemic tree species of northeast India were investigated in fragmented forests in the Jarain Hills and in adjoining areas of Meghalaya state. The population structure was discontinuous in all the fragments with the absence of individuals in higher diameter classes. The number of individuals increased with the size of the fragment patches(p <0.004).The density of mature trees(≥5 cm dbh)was high (24-30 individuals ha-1) in the largest fragment(>105 ha). The number of seedlings and saplings were also higher in the larger fragments. Human-related disturbances had a negative impact on the species population(p <0.002). Variation in the population density in different forest patches has been attributed tofragment size,site characteristics and ongoing human disturbances. The restricted distribution of the species coupled with exploitation and habitat destruction underlines the need for its conservation.
Keywords Conservation · Disturbance · Fragmentation ·Magnolia punduana · Restricted distribution
Introduction
The world’s biodiversity is depleting rapidly owing to anthropogenic activities such as urbanization, agricultural expansion and overexploitation. These activities are leading to the fragmentation of large forests into smaller patches. Fragmentation is a dynamic process in which the habitat is progressively reduced to smaller patches(Franklin 2001;McGarigal 2002) and is recognized as one of the major threats to biological diversity. In the tropics,fragmentation is considered as one of the major threats to natural tree populations (Heywood et al. 1994; Trejo and Dirzo 2000). It alters the ecology of forest remnants by reducing species richness, declining their population and disrupting their recruitment dynamics and spatial structure(Tang et al. 2011).
Regeneration processes in forests can be interpreted by the frequency distribution of individuals in different size classes in a population (Veblen et al. 1980; Fensham and Bowman 1992). Studies have focused on identifying ecological factors that affect population dynamics and have established that populations and species more often become extinct for ecological and demographic reasons than for a lack of genetic variation(Jennersten 1988;Aizen and Feinsinger 1994). The distribution of a species is determined mainly by its adaptability to the habitat and by barriers to dispersal,as well as by external events(Cox and Moore 1993). Habitat fragmentation may affect species differently according to their life history traits. According tofarwig and Berens (2012), fragmentation affects plants with specific dispersal modes.Some species that depend on biotic pollinators and dispersers have become rare as a result of forest loss and fragmentation (Bustamante and Castor 1998). Even common species that are wind pollinated may be affected by habitat fragmentation, especially in areas with extensive deforestation. Species that are endemic, often with localised distribution, are more susceptible to threat due tofragmentation.
Northeast India,being part of the Indo-Burma biological diversity hotspot, is rich in endemic species (Mittermeier et al.2004).However,such species are under various threat categories due to a number of human activities. These species are,therefore key for biodiversity conservation and need immediate attention(Upadhaya et al.2013).There are very few studies that have examined the population and regeneration ecology of endemic and threatened plants of the region (Khan et al. 2003; Duchok et al. 2005; Choudhury et al. 2007; Upadhaya et al. 2009; Choudhury and Khan 2010; Saikia and Khan 2013). One of several effective measures suggested for the conservation of endemic and threatened species is the close monitoring of existing populations(Upadhaya et al.2013).Magnolia punduana is an endemic tree species of Meghalaya state in northeast India. Habitat destruction and exploitation for timber are the main threats to the species (Haridasan and Rao 1985),and at present,it occurs only in a few localized areas(Iralu and Upadhaya 2015).Therefore,this study was undertaken in the Jaintia Hills of Meghalaya state to: (1) assess the current natural population status of M. punduana along a fragment size gradient; (2) identify the factors responsible for its depletion; and, (3) suggest strategies for its conservation.
Materials and methods
Study sites
The study was conducted in Jarain and adjoining areas of Meghalaya state over an area of approximately 20 km2with elevations of 900-1500 m, a.s.l. The forest is in fragmented patches with sizes ranging from 4 ha to>105 ha (Table 1), and represents the remnants of a subtropical broad- leaved humid forest (Champion and Seth 1968). The climate of the area is monsoonal with distinct wet and dry seasons.The wet season extends from May to October, and the dry season from November to March. The average annual rainfall is ca. 3500 mm. Mean monthly temperatures vary from a maximum of 26 °C in April to a minimum of 5 °C in January.
Study species
Magnolia punduana Hk. f. & Th. (synonym: Michelia punduana)of the family Magnoliaceae is a mid-sized tree attaining heights of 25 m, often forming the canopy layer in a stand. It grows in subtropical broad-leaved forests of the Khasi-Jaintia Hills up to 1500 m a.s.l. and is endemic to Meghalaya state(Balakrishnan 1981;Haridasan and Rao 1985). The species is largely exploited for timber and is listed as ‘‘Rare’’ in the Red Data Book of Indian Plants(Nayar and Sastry 1990) and in the IUCN Red List of Threatened Plants (Walter and Gillett 1998). The species was previously classified as ‘‘Vulnerable’’ by the IUCN(2014), but recently it has been categorised as ‘‘Data Deficient’’ (Wheeler and Rivers 2015). The species is of botanical interest because it is a primitive species with good timber value (Haridasan and Rao 1985; Nayar and Sastry 1990).
Population status
Since the species often occurs in fragmented habitats,each fragment of the forest was initially surveyed for the presence of M. punduana. Of all the fragments surveyed, the species was found in 10 that were classified as small (abbreviated as S1 and S2), medium (M1, M2 and M3), large(L1, L2 and L3), and very large (VL). In each fragment, a representative belt transect 20 m wide × 250 m long(0.5 ha)was laid out.However,owing to the unavailability of replicate fragments of the largest size, two sampling plots (VL1 and VL2) were established. Each transect was further divided into 10 × 10 m quadrats for systematic sampling of M.punduana and other associated species.All mature trees ≥5 cm diameter at breast height (dbh) were counted and measured. For saplings (<5 cm dbh and≥1 m height)and seedlings(<1 m height), 5 × 5 m and 2 × 2 m quadrats were laid out in the centre of the larger quadrats.
For each fragment, the population structure and regeneration status of the species were assessed by classifying the species into:(1)mature individuals assigned to six dbh classes (5-15, 16-25, 26-35, 36-45, 46-55 and>56 cm),and(2)regenerating individuals that included saplings and seedlings. The state of regeneration was based on the following categories: (a) ‘‘good’’ if seedling >sapling>mature; (b) ‘‘fair’’ if seedling >sapling ≤mature;(c) ‘‘poor’’ if no individuals in the seedling stage were found, (although saplings may be less, more or equal to mature trees);(d)‘‘none’’if individuals in the seedling and sapling stages were absent but present as mature trees;and,(e) ‘‘new’’ if no mature individuals were found but only seedlings and/or saplings (Sukumar et al. 1992).

Table 1 Site characteristics and location of the study area
In order to assess the site characteristics,the disturbance index adopted by Tang et al.(2010a,b,2011)and Mir et al.(2015) was followed. Based on the number of human disturbance factors (viz. selective logging for timber, harvesting of wood for fuel, NTFP collections, creating agriculture lands, cattle grazing and building roads), each fragment was assigned one of the following disturbance values: 5 if there was only one disturbance factor; 10 if there were 2; 20 if there were 3; and, 30 if there were 4.The relationships of the species population tofragment size and to disturbance factors were analysed using linear regression.
Results
Stand characteristics and density
In all fragments, M. punduana was associated with: Castanopsis tribuloides (Sm.) DC., Styrax serrulatum Roxb.,Schima wallichii (DC.) Korth, Calophyllum polyanthum Choisy,Quercus semiserrata Roxb.,Lithocarpus dealbatus Hk. & Th. ex Miq.) Rehder., Helicia nilagirica Bedd.,Lithocarpus fenestratus(Roxb.)Rehder,Glycosmis cymosa(Kurz) Narayanaswamy, Cinnamomum zeylanicum Blume,Dysoxylum gobara (Buch.-Ham.) Merr., Myrica esculenta Buch.-Ham.ex D.Don,Syzygium tetragonum(Wt.)Kurz.,Persea odoratissima (Nees) Kosterm., and Eurya japonica Thunb. These forest fragments are under various anthropogenic disturbances due to the extraction for timber (C.polyanthum, P. odoratissima, Q. semiserrata, Schima wallichii),for poles(Dysoxylon gobara,Helicia nilagirica,L. dealbatus, Styrax serrulatum) and for firewood (C.tribuloides, E. japonica, L. dealbatus, L. fenestratus, S.wallichii, Styrax serrulatum). Apart from these species,non-timber forest products (NTFPs) are collected from species such as C. zeylanicum, Myrica esculenta, and S.tetragonum. Other anthropogenic activities in and around the forest fragments includes the construction of roads,small water reservoirs, the mining of sandstone,encroachment on forest lands due to expanding villages,and cattle grazing. Based on human activities, fragments S1 and L1 were the most affected (disturbance index DI = 30), and VL2 was the least disturbed (DI = 5).
Stand density was 1246 and 1126 (Individuals ha-1) in the small- sized fragments (S1 and S2, mean 1186 ± 60),1186, 1360 and 902 in medium- sized fragments (M1, M2 and M3, mean 1149 ± 133), 896, 822 and 1426 in large fragments (L1, L2 and L3, mean 1048 ± 190), and 1400 and 1260 in very large fragments (VL1 and VL2, mean 1330 ± 70)(Table 2).The contribution of M.punduana to stand density was minimal, and accounted for <1% in fragment S1 (2 individuals ha-1), S2 (6 individuals ha-1),M1 (10 individuals ha-1), L1 (4 individuals ha-1) and L3(6 individuals ha-1),whereas,it was<2%in M3 and VL1(14 and 24 individuals ha-1). In sites M2, (30 individuals ha-1), L2 (20 individuals ha-1) and VL2 (30 individuals ha-1), M. punduana was >2% of the stand density(Table 2). There was a positive relation between the number of M. punduana individuals and fragment size(Y = 112.348 + 2.915x, R = 0.804, N = 10, p <0.004,Fig. 1a). Disturbance was greater in smaller patches compared to larger ones. Site characteristics had a marked effect on species numbers as evident by a decline in the number of individuals with increasing disturbance factors(Y = 428.104 - 13.594x, R = - 0.843, N = 10, p <0.002,Fig. 1b).
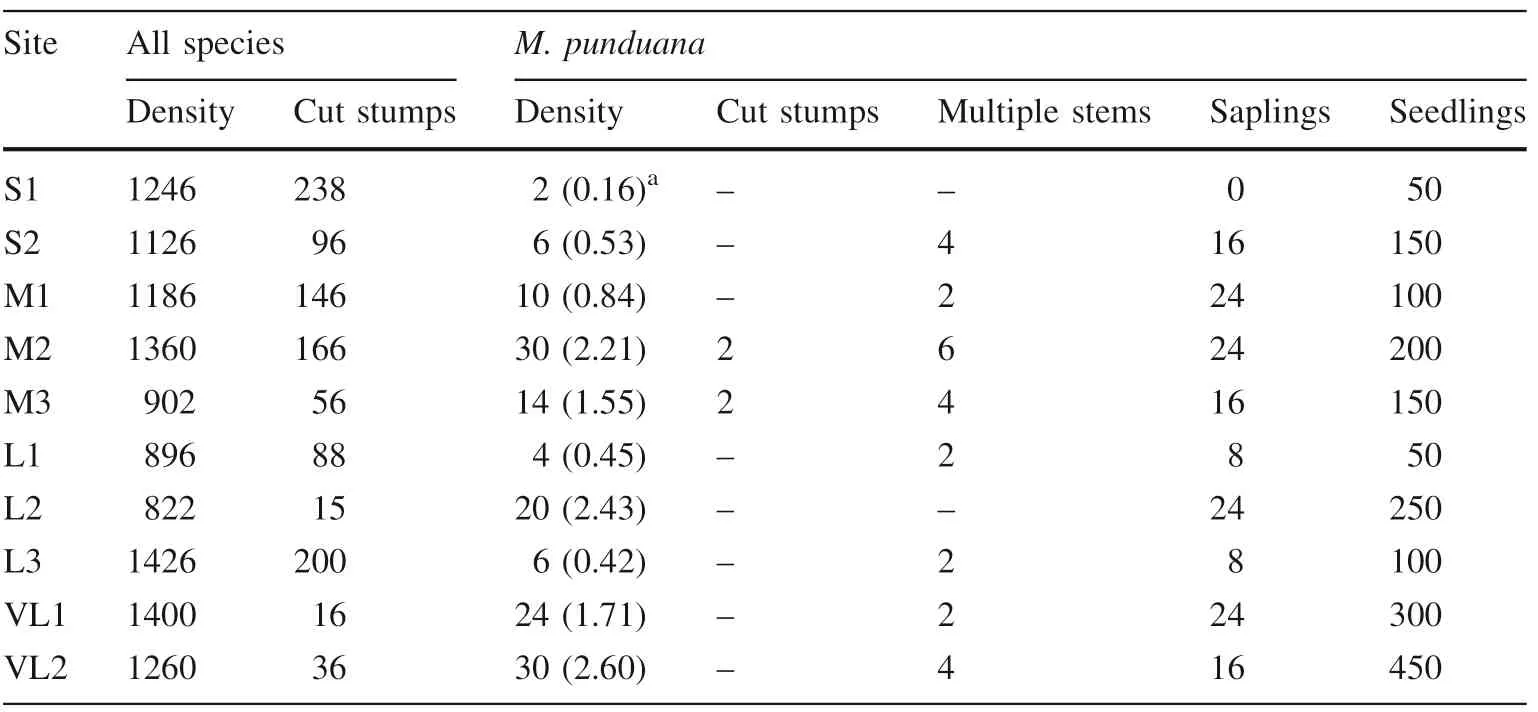
Table 2 Population characteristics of M. punduana along a fragment size gradient(individuals ha-1)

Fig. 1 Relationship between a population density of M. punduana and fragment size, and b disturbance index
Population structure and regeneration status
Overall the population structure of mature trees (≥5 cm dbh) depicted through density diameter distribution a reverse J-shaped distribution (Fig. 2). However, at the fragments level, the population structure exhibited a discontinuous distribution evident by the absence of trees in some dbh classes. Trees in the 5-15 cm dbh class were present in all fragments, followed by the 16-25 cm dbh class(except in S1,S2 and L1).There were only two trees in the 46-55 cm dbh class in VL1 and only four in the>55 cm dbh class in fragment S2 and M1 (Fig. 2).
The number of seedlings and saplings of M. punduana also varied among different forest fragments. The highest seedling density (450 Individuals ha-1) was in fragment VL2 and the lowest (50 Individuals ha-1) in fragments S1 and L1. Sapling density was high in M1, M2, L2 and VL1(24 Individuals ha-1each), followed by S2, M3, VL2 (16 Individuals ha-1), and L1 and L3 (8 Individuals ha-1).There were no saplings in S1. Based on the number of seedlings, saplings and mature trees, fragments S2, M1,M3, L1 and L2 depicted ‘‘good’’ regeneration. Fragments M2,VL1 and VL2 showed‘‘fair’’regeneration and S1 had‘‘poor’’ regeneration (Table 2). The overall population structure of M.punduana,based on seedlings,saplings and mature plants showed good regeneration, as evident by the presence of individuals in different life stages (Table 2).
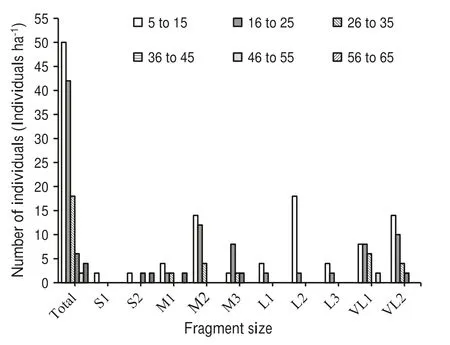
Fig. 2 Distribution of M.punduana trees in different diameter classes
The species regenerated naturally by seeds. In addition to natural regeneration, the species also regenerated by coppicing. The number of cut stumps (two each) was recorded in fragments M2 and M3. The cut stumps were from dbh classes 16-25 and 36-46 cm (Table 2). The species coppiced well with up to 8 shoots emerging from cut stumps.
Discussion
The variation in the density of the species could be due to differences in the methods of past and present land use,local topography, as well as species restricted distribution to moist and open habitat (Kanjilal et al. 1934; Balakrishnan 1981). Moxham and Turner (2011) established the decline in the ecological condition of vegetation as well as regional extinction of many species due toforest fragmentation(Galetti et al.2006).The low species population may be due to extraction for its timber value (Nayar and Sastry 1990) and to habitat loss due tofragmentation. The effects of habitat fragmentation often vary with the effects of local disturbance(Cayuela et al.2006)and could be the reason for the low population of the species.A decrease in population density with an increase in disturbance was observed in this present study.This result is similar to that of Tang et al.(2011)for Michelia coriacea Hung.T.Chang that was represented by a few individuals in the most disturbed areas.
The high population density of mature M. punduana in fragments M2 and VL2 (30 Individuals ha-1) could be attributed to low disturbance events and inaccessibility of the sites by the local community.These fragments are also characterized by favourable environmental conditions due to seasonal streams.Fragments that were subjected to more frequent disturbances due to human activities such as NTFPs extraction, cattle grazing and exploitation of biomass had low species population densities. Although the fragments L1 (21.7 Individuals ha-1) and L3 (28.7 Individuals ha-1)were comparatively larger than the small and medium-sized fragments,population density of the species was low. This could be attributed to the location of the stands as L1 was located near the roadside and L3 close to the village.In addition,L3 was steep and rocky that might have resulted in low species population.
The regeneration of M. punduana appeared to be satisfactory in all the fragments; there was a high density of seedlings and saplings compared to mature trees except in fragment S1(Sukumar et al.1992).Most of these seedlings were observed around parent trees. The higher ratio of seedling to parent densities in this study is similar to that observed for Alphonsea sclerocarpa Thw., an evergreen species of the Eastern Ghats with a restricted distribution(Kadavul and Parthasarathy 2001) and Grewia pandaica J.R. Drummond, a rare and endemic species of the southern Western Ghats (Parthasarathy and Karthikeyan 1997). The low population of seedlings in fragments S1 and L1 and the absence of saplings in fragment S1 can be attributed to low numbers of mature individuals. Such small densities of mature trees, coupled with high human disturbances, may have drastically affected the recruitment of the species.Such a disturbance-linked decline in population size has been observed with Ilex khasiana Purakaystha (Upadhaya et al. 2009), Shorea siamensis Miq. (Gazoul et al. 1998)and A. sclerocarpa (Kadavul and Parthasarathy 2001).Disturbance in the form of extraction drastically reduces the densities of naturally occurring flowering trees.
The regeneration of this species by seeds is often impaired by unfavourable environmental conditions during periods of germination and seedling establishment. M.punduana fruit mature during August which overlaps with the peak rainy season. Since seeds are dormant, there is a high risk of being washed away. Field observation indicates that the fruit are predated by birds and ants.The seeds germinate during the winter season (December-March)which is characterized by dry, cold conditions. Therefore,desiccation of the newly germinated seeds during this period might be a reason for low recruitment (Iralu and Upadhaya 2016).As growth conditions become favourable after a few showers in the following spring(April),there is also the germination and growth of other species.Thus,the low density of species under natural conditions may possibly be due to competition with other associated species.Such observations have been made for Ilex khasiana(Upadhaya et al. 2009) and Aquilaria malaccensis Lam.(Saikia and Khan 2013). M. punduana has a good coppicing ability as evident by the presence of numerous sprouts from cut stumps. This is another important regeneration mechanism by many tree species to maintain a viable population (Luoga et al. 2004) and is often viewed as an important characteristic feature in many species typical of disturbed habitats with high light intensity(Rundel 1991).
Endemic and endangered species often have small populations (Holsinger and Gottlieb 1989) and are restricted to specific habitats. The low population of this locally endemic species in the study area is the cause of its rarity. In this study, seedling and sapling densities were directly correlated to the presence and absence of mature trees. Another important factor for long-term persistence and survival of such a species is its habitat. As a result,detailed knowledge of the species-habitat relationship is vital to understanding the current status of rare plants(Kalliovirta et al. 2006). Therefore, protection of the existing habitat from further degradation is of utmost importance.
The presence of mature individuals of the species is vital to maintaining a viable population in the wild and alsofor future recovery. However, the number of mature trees is very limited(Table 2).Therefore,they should be a priority for conservation so as to ensure the ongoing recruitment of the species through seeds. In-situ conservation is undoubtedly the most effective method for conserving threatened species (Navarro and Guitián 2003; Herbert 2006). In addition, aided natural regeneration (ANR)through artificial propagation from seed or stem cuttings and their introduction in suitable habitats would play a major role for conserving the species (Choudhury et al.2007).Reducing anthropogenic disturbances by sensitizing villagers and providing incentives for conserving existing patches would be important for the conservation of the species. However, the major threat to decline of species was habitat degradation. Therefore, in addition to in situ conservation,ex situ conservation measures should also be implemented. Since the species belongs to ancient family,it has attracted considerable attention of evolutionary biologists and biogeographers(Qiu et al.1995).Therefore,planting the species in botanical gardens and in home gardens for timber is also recommended. The species restricted distribution, low population and its exploitation for timber underline the need for its conservation.
AcknowledgementsWe thank the Buam Raid of Thungbulli village,Headman of Jarain,Umladkur and Amlarem villages and the CEO of Jaintia Hills Autonomous District Council for allowing us to work in the area.
杂志排行
Journal of Forestry Research的其它文章
- Do increasing respiratory costs explain the decline with age of forest growth rate?
- At what carbon price forest cutting should stop
- Mapping the risk of winter storm damage using GIS-based fuzzy logic
- Effects of seed moisture content, stratification and sowing date on the germination of Corylus avellana seeds
- Comparison of seed morphology of two ginkgo cultivars
- De novo assembly of the seed transcriptome and search for potential EST-SSR markers for an endangered, economically important tree species: Elaeagnus mollis Diels
