The population structure and sex ratios of Bursaphelenchus xylophilus under α-pinene stress
2020-05-19JingCuiYongXiaLiWeiZhangXuanWangLongPanYuQianFengXingYaoZhang
Jing Cui · Yong-Xia Li,2 · Wei Zhang · Xuan Wang · Long Pan ·Yu-Qian Feng · Xing-Yao Zhang,2
Abstract Pine wood nematode (PWN), Bursaphelenchus xylophilus, is a serious pathogen of pines throughout the world. Previous work indicated that different concentrations of α-pinene could affect nematode reproduction,however the mechanism of that influence is not clear. In order to examine the reproductive strategies of PWN in response to the stress of the volatile material α-pinene, we investigated different aspects of population changes of B.xylophilus under two concentrations of α-pinene. The results show that a high concentration (214.5 mg ml-1)promoted population growth while a low concentration(56.33 mg ml-1) decreased the population. Population structure was analyzed and it was found that a high concentration of α-pinene decreased the percentage of adults but increased the percentages of larvae and eggs.Furthermore,from the results of an evaluation of sex ratios(female/male), it was determined that a high concentration could elevate sex ratios but a low concentration decreased ratios sharply. These results suggest that the PWN could regulate its population by changing sex ratios under stress of α-pinene.This study has provided a theoretical basis for the prevention and control of pine wilt disease caused by the pine wood nematode.
Keywords Pine wood nematode · α-Pinene · Population structure · Sex ratios
Introduction
Pine wilt disease (PWD), an intricate disease system caused by the pine wood nematode (PWN), Bursaphelenchus xylophilus Steiner & Buhrer (Nickle), (Nematoda:Aphelenchoididae), is native to North America (Nickle et al. 1981) as the pathogen, Monochamus spp. as the vector and Pinus spp. as the host, and their associated microbes.PWD is a serious pine disease worldwide and the pathogen responsible for the disease is an object of quarantine (Yang 2003; Zhang and Luo 2003).
The PWN develops through four moults(i.e.,four larval stages-eggs,L2,L3 and L4)and reproduces within woody tissue while food is available. When conditions are adverse, (i.e., food becomes limiting), B. xylophilus enters a specialized dauer larva third stage or alternative development stage (DL 3). When stimulated by the presence of the Monochamus vector beetle,the DL 3 moults to become the fourth stage dispersal larva (DL 4) in preparation to board the vector for invading healthy trees(Zhang and Luo 2003;Kikuchi et al.2011).After nematode invasion,pines produce volatile terpenes as a defense;α-and β-pinenes are the main terpenes (Mamiya 2008; Niu et al. 2012; Santos et al. 2012).
In the conflict between the pine wood nematode and pines, the population of the PWN is a key factor responsible for pine wilt disease (Mamiya 1974). Under ideal conditions, the PWN needs only 3 days to breed a generation (Liao 2007). Population structure and sex ratio (female/male) are additional factors affecting reproduction rates. The sex ratio determines mating efficiency and reproduction rate,as the higher the sex ratio,the higher the reproduction ability(Rutherford et al.1992).When the sex ratio is 3:1, mating efficiency is higher and populations increases at a faster rate (Liu 2014).
Reproduction rate and sex ratio are affected by chemical stress (Huang 2014). Pines produce volatile terpenes such as α-pinene and β-pinene which reduce biological stress(Niu et al. 2012). The ratio of α-pinene and β-pinene in healthy pines is generally 1:0.1 (Zhao et al. 2007; Futai 2013). However, when PWN invades the pine and the tree develops disease symptoms,the ratio changes to 1:0.8(Niu et al. 2012). Kuroda et al. (1988) inoculated seedlings of Pinus thunbergii Parl. with PWN and observed that monoterpene and sesquiterpene contents increased up tofour-fold compared to non-inoculated pines. Vaporization of monoterpenes and sesquiterpenes occurred through the parenchyma cell walls and permeated into the tracheids which ultimately led to tube cavitation. The PWN populations increased which suggested an increased amount of terpene in the xylem, leading to increased reproduction of PWN (Umebayashi et al. 2011). Niu et al. (2012) found that low concentrations of α-pinene and β-pinene inhibited PWN breeding, whereas high concentrations promoted breeding. The effect of α-pinene was superior to β-pinene(Niu et al. 2012). However, the mechanism of terpene influence on the reproduction rate, sex ratios, and population structure of PWN is not clear.This study carried out a bioassay to measure the influence of high and low concentrations of α-pinene in order to analyze the reproductive strategies of PWN with the aim of providing an effective control of pine wilt disease (PWD).
Materials and methods
Strain and cultivation of PWN
The strain Nxy61 of PWN used in this study was isolated from wood chips of infested Pinus massoniana Lamb.from Ningbo,Zhejiang province in October 2012,and conserved in the culture collection of the Chinese Academy of Forestry,Beijing.PWN was cultured on cornmeal agar(CMA)with Botrytis cinerea Pers.,a grey mould fungus,for longterm preservation. To obtain nematodes from CMA using the Baermann funnel technique (Southey 1986), the nematodes were centrifuged at 5000 rpm for 5 min,washed three times with distilled water, and grown on potato dextrose agar (PDA) with B. cinerea for 7 days at 25 °C in the dark.They were then extracted from the PDA after centrifugation at 5000 rpm for 5 min, washed three times with distilled water and collected in 15 ml centrifuge tubes. Aqueous suspensions were prepared for the additional experiments.
Picking unmated PWN
A 20 μl liquid suspension containing PWN was collected from 15 ml tubes(a suspension of mixed-aged nematodes)using a pipette and dripped on to glass slides.According to descriptions by Zhang and Luo (2003), Gu and Wang(2011) and Liu et al. (2014) on PWN morphological features such as lips, scalpella, tails and genital organs, male(L4)and female(L4)were observed.L4 are unmated male and female pine wood nematodes, picked and examined under an optical microscope.Thirty ♀and ten ♂nematodes were picked as mating systems and transferred to 50 μl distilled water in 1.5 ml centrifuge tubes. These 50 μl suspensions were transferred onto potato dextrose agar with B. cinerea using a pipette.
Reproduction of PWN
Sixteen time intervals(3,4,4.5,5,5.5,6,6.5,7,8,10,11,12, 14, 15, 17 and 19 days) were selected. The mating systems(30 ♀and 10 ♂nematodes)were cultured on PDA plates with B. cinerea at 25 °C in the dark. Five replicates were maintained for each cultivation period. The nematodes were washed three times with distilled water, centrifuged at 5000 rpm for 5 min, collected in 15 ml centrifuge tubes, and the numbers of nematodes counted.
Population, structure and sex ratios of PWN
To differentiate between male and female nematodes, a 50 μl liquid suspension was placed on a glass slide each time. Nematodes with female external genitals were considered female, and ones with copulatory spicules as male(Gu and Wang 2011). The nematodes were divided into adults, larvae (L2, L3 and L4) and eggs based on morphology, length and genital characteristics (Mamiya 1975;Ishibashi et al. 1978). The total number of adults, larvae and eggs were regarded as one population and population structure was the ratio of adults, larvae and eggs in the populations. Sex ratios were calculated depending on the number of females and males in the population. These values were determined 10 times in each centrifuge tube.
Influences of α-pinene on quantity,structure and sex ratios of PWN
Two different concentrations of α-pinene (Acros OrganicsLtd,Japan,purity 97%,dissolved in 0.5%Triton X-100)were prepared showing inhibiting or facilitating effects on PWN reproduction. Based on results of previous studies and on our research, the lower concentration of α-pinene(56.3 mg/ml, AL) could inhibit reproduction, the higher one (214.5 mg/ml, AH) could facilitate reproduction. The controls were 0.5%Triton X-100(Niu et al.2012;Fig.S1).So we explored the reproduction characters of PWN affected by these two concentrations of α-pinene using a cotton ball bioassay (Kong et al. 2007). The definite methods were as follows:Holes approximately 5 mm were drilled at the center of PDA plates and cotton balls were placed over the holes with two concentrations of α-pinene and controls. Nematode suspensions containing 30 ♀and 10 ♂were dropped on to the fungal plates,and incubated at 25 ± 1 °C in the dark. The reproduction of PWN was examined after 4, 6, 7, 9, 10, 11, and 12 days. For each treatment, there were five replicates.
Statistical analysis
Data were processed and analyzed by Excel 2010 and independent sample group t-test using SPSS 19.0 (Vandepitte et al. 2014).
Results
Changes of population structures and sex ratios in PWN
After culturing on PDA (potato dextrose agar), the populations of PWN at each cultivation time were counted. As PWN needs 3 days to complete a generation, data collection started from the 3rd day. The most rapid growth occurred within seven, especially between days 5 and 7.Mating and breeding started from the 3rd to the 5th day,and the peak population was reached by the 10th day, and then started to decline from day 11. The rate of reproduction tended to stabilize and populations on day 14 were the same as on day 15. A similar trend was also observed on days 17 and 19(Fig. 1).Sex ratios(female/male)began to decline after culturing on PDA, but increased between day 4 and day 5.5.The highest sex ratio was 2.2 on day 5.5 and then continued to decline. The lowest sex ratio of 0.4 was reached on day 10. From day 11 onward, a sharp rise in sex ratio occurred and ranged between 1.1 and 1.3,with a sharp decline at the 19th day(Fig. 1),suggesting that sex ratios tended stabilize. According to the changes of population structures and sex ratios, typical observation times were selected: days 4, 6, 7, 9, 10, 11, and 12.
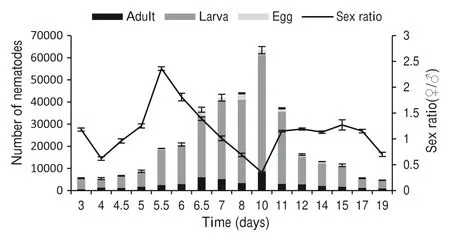
Fig. 1 The changes of population structures(adults,larvae and eggs)and sex ratios of PWN
To verify the accuracy of the empirical data,the changes of populations was analyzed. We built logistic model as follow: N = K/1 + B*e-rt= 66,178/1 + 368*e-0.866tFig. S2(A). N is the population of PWN, t the observation time,population maximum is K = 66,178,the intrinsic rate of increase is r.This figure showed that with the increase in incubation time,populations of PWN tended to increase as well. Populations maximized by day 10. Fig. S2(B) represents the curvilinear equation, N = eb0+b1/twhere N is the population of PWN, b0 the constant value 5.585, b1 the constant value 53.595, and t is the observation time. This figure demonstrates that, with food decreasing, the populations began to decline from day 11 and gradually leveled off. The results verify the accuracy of the experimental results and the reliability of observation periods selected.
The influence of α-pinene on population changes
In the entire reproductive process, populations under low α-pinene concentration was always lower than under the higher concentration (P <0.01). At every cultivation period, the control populations were slightly smaller than populations under the high concentration. Populations under the low concentration were about half of those under the higher concentration. The fastest population growth occurred within 7 days after culturing (Fig. 2). The largest population number occurred on day 10, and began to decline with a noticeable decline on day 12. According to the breeding characteristics of PWN,3 days are required to complete a generation on PDA with B. cinerea. As food remained abundant in the plates up to 7 days, the fastest growth of PWN populations occurred within 7 days. With a decrease in available food with time as well as the effects of α-pinene, the numbers of PWN within a population declined and stabilized in later days (Fig. 2).

Fig. 2 The change in populations of PWN under two α-pinene concentrations. *Statistically different from controls (P <0.05),**statistically different from controls (P <0.01). (CK controls, AL low concentration, AH high concentration)
The changes of PWN population structure under αpinene stress
During the reproductive period (Fig. 3), the quantity of adults was higher under the high concentration of α-pinene from day 4 to day 7.Whereas,under the low concentration,the quantity of adults was higher starting from day 9.There was a significant difference between treatments(P <0.01).The lowest proportion of adults occurred on day 6 under the low concentration α-pinene, and then increased to become higher than. In the whole phase, the proportion of adults declined a little under the high concentration of αpinene. The proportions stabilized between the 11th and 12th days under both high and low concentrations. The quantity of larvae was lower under the AH than under the AL, from day 4 to day 6 (P <0.01) (Fig. 3). To total number of one population, the highest proportion of larva was on day 7 under the high dosage, and then higher than under the low dosage from the 7th to the 12th days(Fig. 3).The proportion of larva up to day 10 showed a similar trend under the high and low concentrations. The proportion of eggs began to decline under the low and high concentrations after culturing on PDA.The proportion of eggs under the low concentration was higher than under the AH from day 4 to day 9 and day 11 (P <0.01) (Fig. 3).
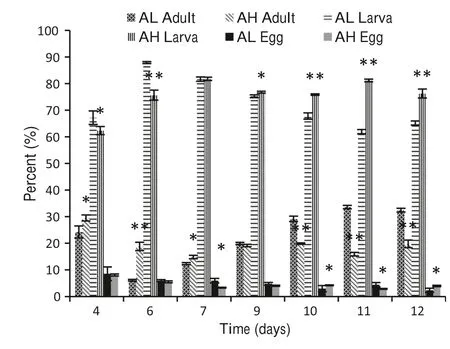
Fig. 3 Population structure ratio of PWN under two concentrations of α-pinene. *Statistically different from AL (P <0.05), **statistically different from AL (P <0.01)
The changes of sex ratios of PWN under α-pinene stress
Sex ratios under high and low concentrations of α-pinene began to rise from day 4 after culturing, peaked on day 6,and declined gradually(Fig. 4).Over the whole cultivation period,sex ratios under the high dosage were always higher than under the low dosage (P <0.01) (Fig. 4). Sex ratios under the high α-pinene concentration were higher than the controls until the 10th day. However, sex ratios under the low dosage were always lower than the controls.Sex ratios under the low concentration were similar to controls on day 9 and day 10. With the high concentration, the ratios remained same as controls on the 11th and 12th days(Fig. 4).
Discussion
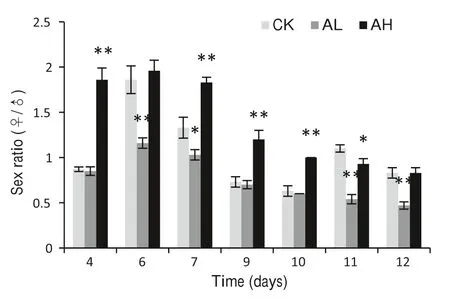
Fig. 4 The sex ratio of PWN under the two concentrations of αpinene. *Statistically different from controls (P <0.05), **statistically different from controls (P <0.01)
A main secondary metabolite of Pinus spp. is α-pinene, a defensive component for the colonization of the PWN(pine wood nematode). However, our study shows that the PWN adjusted breeding efficiency according to the concentration of α-pinene. The results indicate that sex ratio was adjusted to affect population structure, i.e., the ratios of adults,larvae and eggs affected the populations under αpinene stress. PWN produced more female nematodes and adjusted population structure (the ratios of larva and eggs were kept stable) under the higher α-pinene concentration(214.5 mg/ml). On the other hand, PWN produced more male nematodes and adjusted population structure (the ratios of larvae and eggs declined) under the lower αpinene concentration (56.33 mg/ml) to sustain population development. Niu et al. (2012) also demonstrated that low concentrations of α-pinene reduced the propagation of PWN on culture plates, whereas the highest concentration increased propagation.
In this study, variations in populations of PWN show significant differences under high and low concentrations of α-pinene compared to the controls. Populations under the high concentration were higher than controls, while populations under the low concentration were lower. This suggests that the differences in α-pinene concentrations could promote or inhibit PWN population development.The largest populations observed on day 10 under high and low dosages due tofood sufficiency within 1 week after culturing,might have benefited breeding.According to the key breeding period of PWN, accumulation of the second generation of nematodes peaked on the 10th day. The maximum amount of fungal food was consumed by PWN within 7 days after culturing, resulting in a shortage of food.Moreover,α-pinene may have reduced the volume of volatiles and decreased the effect on nematode reproduction. Populations started to decline from the 11th day.According to the logistic growth model, the saturation point of the populations peaked on day 10 after culturing.During this whole growth period, when the population densities were largest,growth rates were zero,and nutrition deplete started to decline the populations.As a result,PWN breeding was kept low and tended to stabilize in the later periods. The populations then began to decline and finally died off. This finding is similar to a study on PWN population dynamics on shoot segments of Pinus thunbergii Parl. colonized by Ceratocystis spp. (Fukushige 1991).
Several studies have demonstrated that chemical substances such as ascarosides, (a group of glycolipids containing the sugar ascarylose), can affect the development and breeding of Caenorhabditis elegans Maupas, a freeliving non-parasitic nematode, and B. xylophilus by receptor proteins GPCRs, G protein-coupled receptors(Kim et al. 2009; Kikuchi et al. 2011), and some plant extracts can affect the hatching rate of eggs of Meloidogyne incognita Kofoid&White(Fabiyi et al.2012).The females produce fewer number of eggs in adverse environments,but adjust the size of eggs, and then keep hatching eggs to promote populations under different environmental conditions(Olofsson et al.2009).Our results have demonstrated that to the total number of one population,the proportion of adults first declined, then raised under both concentrations of α-pinene. The proportion of larvae changed little, but was higher under the high concentration of α-pinene. The proportion of larvae and eggs declined under the low concentration of α-pinene. It is inferred that the higher concentration might have acted on the specific receptor proteins or neurons of nematodes like GPCRs, and then increased the development of PWN, shortened the growth cycle and quickened the reproductive stages. While low concentrations of α-pinene did not activate the receptors,the populations clearly declined in later periods and had little growth potential. Population structure played an important role for populations and their development. As the juvenile stages reduce, the number of populations will also be reduced (Sun 1987).
Sex ratio is an important factor affecting population structure,reproduction efficiency and the differentiation of sexual dimorphism(Prohl 2002).The sex ratio of different species or the same species changes at different times or under different environmental conditions. When populations start to decline, the quantity of females is lower than males. However, the opposite occurs when populations start to rise (Wang et al. 2004, 2010). Populations will produce surplus males in adverse environmental conditions; when environmental conditions are suitable for a population’s development, there will be more females(Blackmore and Charnov 1989; Hulin and Guillon 2007).For example, Heterodera rostochiensis Wollenweber, the potato cyst nematode, produces much more female offspring when nutrient contents are higher in host root cells,and many more male offspring when nutrients are scarce(Trudgill 1967).Our study has shown that sex ratios under high and low concentrations of α-pinene first rose and then declined after culturing on PDA, and the highest sex ratio was on the 6th day. The sex ratio of PWN was 1.0 on day 10, and remained stable until the 12th day under the high concentration of α-pinene, but the ratios were always higher than with the low concentration and the controls.These results suggest that a high concentration of α-pinene and adequate nutrition promotes female growth and development to increase the population of PWN. The pine wood nematode adjusted sex ratios and population structure to control populations based on the concentration of αpinene. Therefore, the exact impact of naturally occurring monoterpenes in the PWN system is worthy of further exploration.
AcknowledgementsThis project was financially supported by Fundamental Research Funds of Research Institute of Forest New Technology,CAF(CAFYBB2018SY037)and National Key Research and Development Program (2016YFC1200604).
杂志排行
Journal of Forestry Research的其它文章
- Do increasing respiratory costs explain the decline with age of forest growth rate?
- At what carbon price forest cutting should stop
- Mapping the risk of winter storm damage using GIS-based fuzzy logic
- Effects of seed moisture content, stratification and sowing date on the germination of Corylus avellana seeds
- Comparison of seed morphology of two ginkgo cultivars
- De novo assembly of the seed transcriptome and search for potential EST-SSR markers for an endangered, economically important tree species: Elaeagnus mollis Diels
