Different culture conditions applied to in vitro shoot multiplication of two Eucalyptus benthamii explant sources
2020-05-19NataliaPimentelEspositoPolesiLeandroSilvadeOliveiraFranciscoJosBenediniBaccarinCristinaVieiradeAlmeidaMarcliodeAlmeida
Natalia Pimentel Esposito-Polesi · Leandro Silva de Oliveira ·Francisco José Benedini Baccarin · Cristina Vieira de Almeida ·Marcílio de Almeida
Abstract Eucalyptus adult material requires more successive subcultures in the in vitro multiplication phase for increased vigor and cellular activity. This study evaluated the endophytic manifestation and shoot multiplication of one 13-year-old Eucalyptus benthamii clone under different culture conditions and used canopy branches (CB) and trunk base material as explant sources. The culture media were wood plant medium (WPM), Murashige and Skoog medium(MS)and JADS(Correia and co-authors medium).Based on the results of the initial multiplication experiment, further tests examined sucrose concentrations and pH. Morphophysiology, dry mass production, endophytic manifestation and histochemical were determined. Explant sources responded differently to MS and JADS media, but the WPM medium promoted homogeneous development.The responses were similar for both explant sources when sucrose concentrations varied. Shoots died in the absence of sucrose,showed high oxidation at 60 g L-1 and optimal development at 30 g L-1. Endophytes were more evident for shoots from the CB origin. Explant sources responded distinctively to treatment due to physiological and intrinsic genetic factors. Therefore, explant sources, different culture media, sucrose concentration and pH may determine micropropagation success and influence the presence and/or intensity of endophytic manifestation.
Keywords Plant tissue culture · Explant sources · Culture media · Sucrose · pH
Introduction
Eucalyptus spp. are widely cultivated in different tropical and subtropical regions of the world to address the growing global demand for biomass, and represent a renewable resource (Rockwood et al. 2008). Plantations of this genus in environmentally constrained areas with drought or low temperatures are restricted in their expansion. However,Eucalyptus benthamii Maiden & Cambage is a potential species for planting in colder regions due to its frost tolerance (Brondani et al. 2012; Baccarin et al. 2015) but its cultivation has been limited by propagation difficulties.The seeds, when available are expensive, and asexual propagation has restrictions on adventitious rooting of cuttings and grafting incompatibilities (Brondani et al.2012).
Micropropagation is an alternative propagation method.However,there are several factors that determine its success.Genetic characteristics, culture medium, sugar concentrations and pH may significantly affect in vitro response.Among these factors, the culture medium has a primary influence on in vitro multiplication (George and Debergh 2008).The media most used in Eucalyptus micropropagation are Murashige and Skoog(MS;Murashige and Skoog 1962),wood plant medium(WPM;Lloyd and McCown 1980)and the JADS medium(Correia et al.1995).
Sugar may improve in vitro propagation and influence various metabolic processes with direct effects on growth and tissue differentiation (KubeŠet al. 2014). The pH may change nutrient and plant growth regulator availabilities(Pasqual et al. 2002). However, studies of Eucalyptus micropropagation focus on analyzing growth regulators,and there is insufficient information regarding the effect of other factors on in vitro culture of this genus.
The explant source may also influence micropropagation success, especially during the multiplication and adventitious rooting phases.Explant selection criteria involves the physiological state of the plant and the cutting season(Borges et al. 2011; Hartmann et al. 2011). Additionally,Eucalyptus micropropagation has used adult material as explant sources, but the difficulty of shoot rooting has required more subcultures (serial propagation) (Brondani et al. 2012; Wendling et al. 2014) and 10-12 successive subcultures has been recommended (Dutra et al. 2009;Brondani et al. 2012). This subculturing process is termed reinvigoration/rejuvenation and aims to improve the level of adventitious rooting of cuttings(Sulichantini et al.2014;Wendling et al. 2014).
The successive subculture in micropropagation can induce latent endophyte growth in the medium due to excessive time or micro-environmental variations(Thomas and Kumari 2010; Esposito-Polesi et al. 2015). This phenomenon is conventionally known as endophytic manifestation (Esposito-Polesi et al. 2015). It is not a common parameter in the evaluation of shoot multiplication but in our research it was considered as an auxiliary tool to verify the effects of the subculture numbers and changes in cultivation.
Endophytic manifestation is the appearance of small colonies around the explant or the increase in turbidity of the culture medium (Almeida et al. 2009). However, this sudden bloom does not always affect the plantlets and discarding the material immediately is unnecessary. An endophytic display under control may not be harmful(Almeida et al. 2009; Abreu-Tarazi et al. 2010; Esposito-Polesi 2011; Esposito-Polesi et al. 2015).
Therefore, the aim of this study was to evaluate the growing conditions and the endophytic manifestation in shoot multiplication from two explant sources of one E.benthamii clone material.
Materials and methods
Plant material was collected from a 13-year-old E. benthamii tree. A clonal mini-garden of E. benthamii ministumps was established by vegetative rescue from a mature tree.This was carried out by collecting plant material from the pruning of epicormic shoots from the lowest canopy branches (CB) and cuttings from the girdling at the trunk base (TB) as described by Baccarin et al. (2015).
Eucalyptus benthamii mini-stumps from both CB and TB sources were maintained under greenhouse conditions of >80% relative humidity and temperatures between 26 and 30 °C.
In vitro explantestablishment and shoot multiplication
Before collection, a 2.4 g L-1fungicide solution was applied to the clonal mini-garden. Nodal segments with a pair of axillary buds (1.5 cm explants) from the middle portion of the shoot were collected. The explants were immersed in deionized water and transported to the tissue culture laboratory.
Initially, the explants were washed in running water with 0.05% Tween 20 (v/v) for 25 min, then washed with autoclaved deionized water and immersed for 15 min in fungicidal solution (2.4 g L-1, 50% captan), and rinsed again with sterile deionized water. The explants were immersed 5 min in a sodium hypochlorite solution (1.5%active chlorine, v/v, NaOCl) supplemented with Tween 20(0.05%,v/v).Subsequently,they were put in a laminar flow chamber and triple rinsed with autoclaved deionized water.
At the end of this aseptic treatment, the explants were established in vitro using MS medium with 0.5 mg L-1BAP (6-benzylaminopurine), sucrose (30.0 g L-1) and agar (4.5 g L-1).
After 40 days, the first subculture had developed shoots with one to three buds and without microbial contamination. The shoots were transferred to glass test tubes containing 5 mL of WPM medium supplemented with 0.5 mg L-1BAP,0.05 mg L-1NAA(a-naphthaleneacetic acid), 30.0 g L-1sucrose and 4.5 g L-1agar. After 20 days, the shoots (approximately 10 axillary buds) were subcultured in fresh medium of the same composition.
Shoot multiplication experiments
The first experiment was carried out after 10 successive subcultures (20 days in each subculture). Thirty shoots more than 5 mm in length from each explant source were subcultured in either WPM,MS,or JADS to evaluate their development.The shoot clusters were cultured in glass test tubes containing 5 ml of each medium supplemented with 0.5 mg L-1BAP, 0.05 mg L-1NAA, 30 g L-1sucrose and 4.5 g L-1agar.
The second experiment was based on the results of the first.Shoot clusters of each explant source were cultured in WPM medium supplemented with 0.5 mg L-1BAP,0.05 mg L-1NAA and 4.5 g L-1agar. In addition, three concentrations of sucrose (zero, as a control, 30 and 60 g L-1) and three pH values (4.8, 5.8 and 6.8) were evaluated.
For each experiment, there were two successive subcultures each lasting 20 days, renewing the medium but keeping all of the conditions of the previous subculture.
Evaluated parameters
The shoot multiplication experiments were evaluated for morphophysiological characteristics (visual analysis of the appearance, development and shoot multiplication), shoot oxidation, dry mass and endophytic manifestation. The morphophysiological characteristics were assessed after 20 days and after 40 days. By visual analysis, a score of 0-4 was assigned, with (0) shoot death, (1) shoot multiplication much lower than expected, (2) shoot multiplication lower than expected, (3) shoot multiplication as expected, (4) excellent shoot multiplication.
Shoot oxidation and endophytic manifestation were determined after 20 days and after 40 days. The classification was (0) absent or (1) present. In our work, ‘‘microbial contamination’’ refers to the growth of epiphytic microorganisms shortly after in vitro establishment. This may be due to an inefficient disinfection protocol(incorrect use of disinfectants, i.e., inadequate exposure time and/or concentration of these antimicrobial agents) or to a failure in the subculturing process. The term has already been attributed to the sudden growth in the medium after lengthy cultivation of latent endophytic microorganisms in response to stress or physiological imbalances in the host plant (Esposito-Polesi 2011).
Dry mass was assessed at the end of each experiment(after 40 days) by weighing the samples (eight in the first experiment and three in the second). The samples were weighed fresh,individually wrapped in Kraft paper and dried in an oven at 60 °C for 72 h to obtain a constant weight.
In vitro conditions
All culture media was prepared with deionized water, pH adjusted to 5.8 with HCl (1M) and NaOH (1M) and autoclaved 20 min at 121 °C (≈1.0 kgf cm-2). The explants were cultured under controlled conditions (25 °C ± 2 °C,a photoperiod of 16 h, and a light intensity of 42 μmol m-2s-1).
Experimental design and statistical analysis
The experiments were a completely randomized, factorial arrangement. The first experiment tested two explant sources, two subcultures and three media (WPM, MS,JADS) with 24 replications. For the second experiment, a factorial arrangement tested two sources, two subcultures,three sucrose concentrations (0 sucrose, as a control, 30 and 60 g L-1) and three pH values (4.8, 5.8 and 6.8) with six replications.
The results were subjected to ANOVA), p <0.05 and p <0.01), and Tukey’s test (p >0.05) for comparison of means using ASSISTAT program version 7.6 beta (Silva and Azevedo 2009).
Histochemical analysis
This was carried out at the end of the second experiment.Samples from the centre of leaf blades were removed from each shoot cluster and fixed in a paraformaldehyde(4%;v/v) and glutaraldehyde (1% in phosphate buffer, pH 7.0)solution (Karnovsky 1965), and subjected to a series of three vacuum infiltrations (≈620 kgf cm-2) for 15 min each. The samples were then dehydrated in an ethyl-alcohol series for 10 min each before embedding in a hydroxyethyl methacrylate resin (Leica®, Heidelberg,Germany).
Sample blocks were sectioned transversely(5 μm thick)with a rotary microtome. The sections were stained with periodic acid-Schiff stain (PAS) and naphthol blue-black(Almeida et al. 2012) and mounted on slides with a synthetic resin. Polysaccharides in the cell walls, cytoplasm and amyloplasts (non-pigmented organelles) were identified by their pink color while phenolic compounds were orange from the periodic acid-Schiff stain. Proteins were stained blue by the naphthol blue-black stain.
The histological slides were analyzed and photomicrographed with a light microscope. Evaluation criteria were absence (-) or presence (+) of substances. Presence was quantitatively classified as slightly present(+),moderately present (++) or intensely present (+++) according to the intensity of the reactions (Esposito-Polesi et al. 2013).
Results
Culture media
Morphophysiological characteristics were equal for shoots from both explant sources when grown in WPM, but different in MS and JADS media. Shoots from the CB(canopy branches) origin showed no differences according to culture media. But TB (trunk base) shoots increased slightly in MS and JADS media compared to WPM(Table 1). In the CB shoots there was hyperhydricity (excessive hydration) in addition to multiplication.
There were differences in shoot development between the two subcultures with the best score in the first subculture for both explant sources, and a reduction in the appearance and multiplication in the second subculture(Table 1).
In the first subculture, there was no oxidation of trunkbased shoots on any culture medium. However, canopy -branches shoots showed 100% oxidation in the JADS medium but not in the WPM and MS media.In the second subculturing, there was oxidation of trunk base shoots in the MS and JADS media (58.3% and 29.2%, respectively)but absent in the WPM medium. Canopy branches shoots did not show oxidation in the WPM and MS media; there was oxidation reduction in the JADS medium (16.2%).
The largest gains in dry mass were for the CB shoots in WPM (215.7 mg) and MS (229.9 mg) media. With the JADS medium, shoots from the two explant sources showed equal mass (205.1 mg for trunk base shoots and 200.9 mg for canopy branches shoots). There was no change in the dry mass of trunk base shoots regardless of the medium. These shoots had lower gains of dry mass compared to the canopy branches shoots (Table 2).
Sucrose concentration and pH
Canopy branches shoots showed improved morphophysiological characteristics when grown in a medium supplemented with 30 g L-1sucrose (Table 3, Fig. 1).Nevertheless, CB shoots showed a gradual decrease in vitality in the 60 g L-1concentration and controls.Trunk base shoot responses were better when subculturedin 30 and 60 g L-1sucrose concentrations (Table 3,Fig. 2). The morphophysiological characteristics of canopy branches shoots did not change with pH (Table 3,Fig. 1). However, there was multiple reduction of trunkbased shoots subcultured at pH 6.8 (Table 3, Fig. 2).
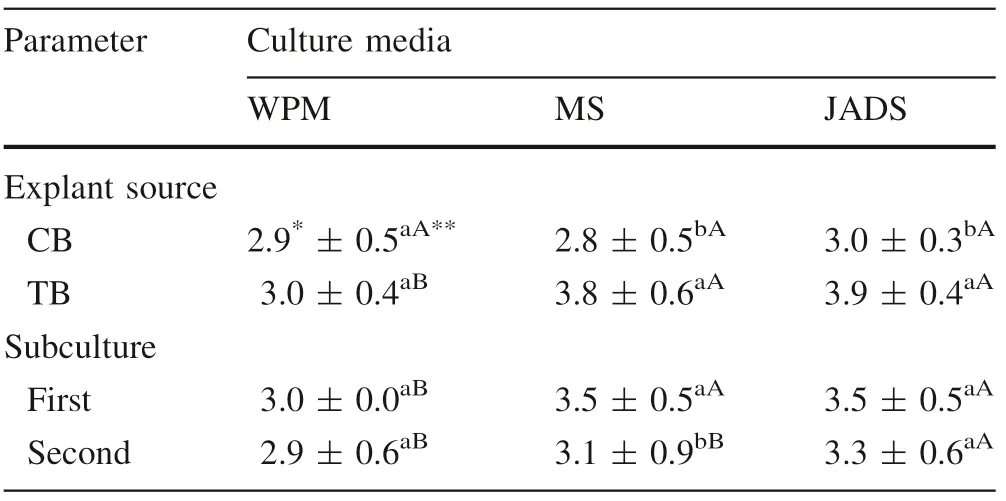
Table 1 Means and standard deviation of morphophysiological characteristics from CB and TB cultured in the first (after 20 days)and second (after 40 days) subcultures in three culture media
Shoot morphophysiological characteristics were better in the first subculture compared to the second (Table 4,Figs. 1, 2). In the sucrose treatments, the medium without sucrose resulted in the death of most shoots. In addition,the morphophysiological characteristics from both explant sources were higher in the first subculture regardless of pH.In contrast, the second subculture had greater reduction in shoot vitality at pH 6.8 and a higher drop rate compared to sucrose treatments. This shows a greater sensitivity of the shoots to pH variation over time compared to variation in sucrose concentrations.
The interaction between pH and sucrose concentrations resulted in higher multiplication rates on a medium with 30 g L-1and pH 4.8 (Table 5). For treatments without sucrose or with 60 g L-1sucrose,the response was similar regardless of pH. However, at a sucrose concentration of 30 g L-1, response varied according to pH. Therefore,morphophysiological characteristics of the shoots decreased with increased pH.
Shoot oxidation was not significant;the only differences were observed when comparing the subcultures. For the first subculture, oxidation occurred in 15.7% of the treatments. In the second, only 1.9% of the cultures showed oxidation.
The dry mass of shoots from both explant sources varied according to sucrose concentration and pH (Table 6). The lowest weight was on medium without sucrose regardless of pH. The highest shoot dry mass was on medium supplemented with 60 g L-1sucrose for all pH values.
Histochemical analysis
Distribution patterns in the cells varied for each component evaluated. In general, polysaccharides (pink arrows) were more restricted to the walls of the few vascular cells,whereas proteins (blue arrows) and phenolic compounds(yellow arrows) were distributed in the cytoplasm of mesophyll cells (Fig. 3).
There were no reaction to phenolic compounds and polysaccharides by shoots on medium without sucrose(Table 7) regardless of pH and explant source. However,proteins ranged from moderately present(CB shoots at pH 4.8 and 5.8 and TB shoots at pH 4.8)to extensively present for CB shoots at pH 6.8 and TB shoots at pH 5.8 and 6.8).Furthermore,the TB shoots in the medium without sucrose at pH 5.8 showed mesophyll cells hypertrophy without clear differentiation of palisade and spongy parenchyma tissues (Fig. 3).
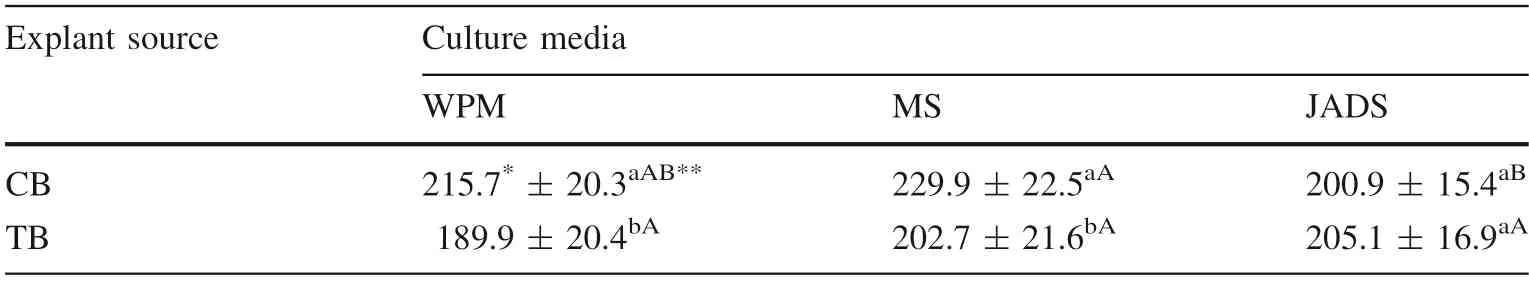
Table 2 Mean values and standard deviation of dry mass(mg) of shoots from the first(lowest) canopy branches (CB)and trunk base of the tree (TB)in three culture media

Table 3 Mean values and standard deviation of morphophysiological characteristics of E. benthamii shoots from the first (lowest) canopy branches (CB) and trunk base of the tree (TB) cultured in three sucrose concentrations and three pH values
In a medium with 30 g L-1of sucrose, explant sources had different responses (Table 7). In CB shoots the reaction for polysaccharides and proteins at pH 4.8 and 5.8 was slightly present, however, for phenolic compounds the reaction was moderately present. At pH 6.8, there was an increase in all components. For TB shoots in pH 4.8 and 5.8 medium, proteins and phenolic compounds showed moderate accumulation, whereas polysaccharides were lower.At pH 6.8 there was decreased intensity of phenolic compounds, proteins and polysaccharides remained the same.
Finally, CB shoots cultured in a 60 g L-1sucrose concentration(Table 7)showed an increase in polysaccharides with increasing pH. There was no variation in protein content at different pH levels. However, phenolic compounds increased from pH 4.8 to pH 5.8 and decreased above pH 5.8. In the TB shoots, the accumulation of polysaccharides was higher at pH 4.8 and pH 5.8(strongly present)with a drastic reduction at pH 6.8(barely present).Proteins had little intensity at pH 4.8 and remained moderately present at pH 5.8 and 6.8. Phenolic compounds decreased with increasing pH values (intensely present at pH 4.8, moderately present at pH 5.8 and insignificant at pH 6.8).
Endophytic manifestation
MS,WPM and JADS media in the first experiment did not display endophytic manifestations regardless of explant source. However, the second experiment (sucrose concentrations and pH values) induced endophytic manifestation with different intensities for the two explant sources along the subcultures (Fig. 4).
Comparing explant sources,CB shoots produced a more intense endophytic manifestation display in all treatments(Fig. 4A).Considering the two subculture stages,there was decrease in endophytic growth in the CB shoots from the first to the second subculture, while for the TB shoots, the degree of endophytic manifestation remained the same(Fig. 4B).
There was no significant difference in the control treatment (without sucrose) in terms of endophytic symptoms. However, with 30 and 60 g L-1of sucrose, regardless of explant source, there was a reduction in endophytic manifestation between the first and the second subculture(Fig. 4C).
The pH combined with different sucrose concentrations influenced the endophytic manifestation for both explant sources (Fig. 4D). At pH 4.8, the effect was more intense in the absence of sucrose but decreased at pH 5.8 with increasing sucrose concentration. With pH 6.8 the endophytic manifestation was similar in all treatments for both sucrose concentrations.
Discussion
The culture medium is an important factor for E.benthamii in vitro multiplication.However,depending on the explant source, canopy branch or trunk base, (CB or TB), the results were different. As reported by Souza et al. (2006),shoots grown on different types and compositions of media not only varied according to species but between genotypes of the same species and explants from the same genotype.
For multiplying CB shoots, the optimum medium was WPM, widely used in promoting callus formation and organogenesis of woody species (Glocke et al. 2006).According to the literature, among the most commonly used culture media for the micropropagation of Eucalyptus spp., WPM has been the most suitable for the multiplication of adventitious buds of E. benthamii compared with MS or JADS (Correia et al. 1995; Glocke et al. 2006;Brondani et al. 2012).
In contrast, for trunk base shoots the best medium was JADS as it promoted better growth and multiplication,expressed by morphophysiological characteristics, and had a low rate of oxidation and higher dry mass. The positive result of JADS could be that it was developed for Eucalyptus grandis W.Hill ex Maiden.This allows the medium to have a composition closer to that required for in vitro micropropagation of the genus (Lima and Gonc¸alves 1998).
The MS medium was not appropriate for the multiplication of TB shoots (high oxidation) and CB shoots (excessive multiplication with a reduction of leaf growth and hyperhydricity which may explain high dry mass). The possible reason for this is the high concentration of salts in the culture medium(Debergh et al.1981).The MS medium has been reported as a possible agent due to its high salt concentration (Radmann et al. 2009) which corroborates the results observed in the CB shoots.
The explant source can also induce hyperhydricity.Tsay et al. (2006) working with Scrophulariayo shimurae found significant differences in hyperhydricity to two explant sources. With greater numbers of hyperhydric shoots from apical meristems than in nodal segments, under the same growing conditions.This phenomenon was observed in this work (high intensity in CB shoots and absence in TB).
Shoot oxidation is closely related to genotype and explant sources(Bassan et al.2006).Oxidation is a processintimately influenced by mechanical and chemical damage in the shoot cultures. This influenced the choice of the optimum medium. WPM did not cause any oxidation in both explant sources. The MS medium showed intermediate oxidation and the JADS medium was responsible for the highest oxidation,and according to Borges et al.(2011)also released phenolic compounds in the in vitro multiplication of E. urophylla × E. globulus.

Table 4 Mean values and standard deviation of morphophysiological characteristics of shoots from the both explant sources cultured in the first (after 20 days) and second (after 40 days) subcultures in three sucrose concentrations and three pH values
Therefore the WPM medium was considered optimum for the development and propagation of E. benthamii.Regardless of the specific responses of each explant source,the WPM was more suited to the second experiment because it exhibited homogeneity. This allowed less influence when altering other parameters of the in vitro culture such as sucrose concentrations and pH.
In the second experiment, the CB shoots were superior to the TB shoots for all treatments.One possible reason for this may be related to the intrinsic abilities of each tissue or organ. When different explant sources are chosen, the responses to in vitro development may be different under the same conditions and stimuli (George and Debergh 2008; Hartmann et al. 2011).
Sucrose concentrations influenced morphophysiological characteristics and shoot development from both explant sources. However, the ideal concentration for shoot development was 30 g L-1. This was also observed in histochemical analysis in which both the high or zero concentration of sucrose affected the accumulation of compounds.
The high concentration of sucrose restricts the photosynthetic efficiency of plants by reducing levels of chlorophyll and important enzymes for photosynthesis(Hazarika 2006). The reddening of shoot leaves from both explant sources in medium with 60 g L-1of sucrose wasalso observed and is usually associated with excess production of anthocyanins which is closely related to the sucrose concentration in the system (Dai et al. 2014).

Table 5 Mean values and standard deviation of morphophysiological characteristics of E.benthamii shoots from both explant sources showing the interaction between pH and sucrose concentrations

Table 6 Mean values and standard deviation of dry mass(mg) of E. benthamii shoots from the both explant sources cultured in three sucrose concentrations combined with three pH values
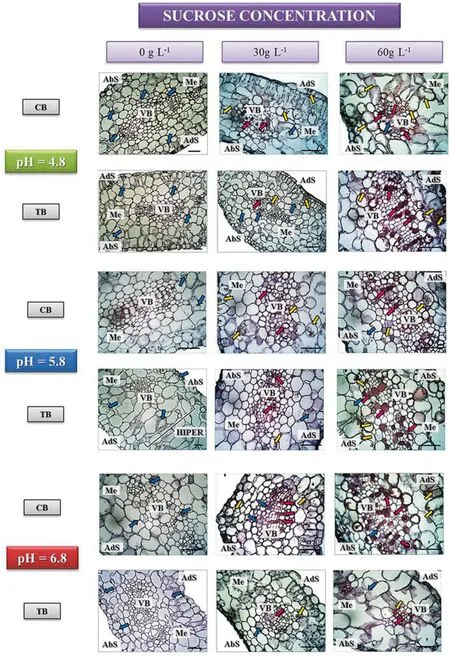
Fig. 3 Histochemical results of shoot leaves from the CB and TB sources. Polysaccharide accumulation (rose arrows),proteins (blue arrows) and phenolic compounds (yellow arrows). Polysaccharides and phenolic compounds increased in intensity with the addition of sucrose and protein decreased.AbS abaxial surface,AdS adaxial surface,VB vascular bundle,Me mesophyll,HIPPER mesophyll cells hypertrophy. Bar: 50 μm
On the other hand, the absence of sucrose inhibits development because the amount of CO2present in the vial is insufficient for the plant tofully exert its autotrophism(Jo et al. 2009). There also was a marked increase in protein accumulation, signaling stress or programmed cell death.
The pH level did not result in significant changes in the morphophysiological characteristics of both CB and TB shoots. However, studies have demonstrated that the change in pH could affect the absorption of other components such as salts, vitamins and growth regulators, and impair the micropropagated species development (KubeŠ et al.2014).Furthermore,pH may interfere in biochemical reactions by activating or denaturing enzymes (Parveen and Shahzad 2014).Although there is some consensus that a pH of 5.8 is ideal, optimum values may vary with the stage of morphogenesis, as in the establishment of culture,in shoot proliferation or in adventitious rooting induction(Parveen and Shahzad 2014).
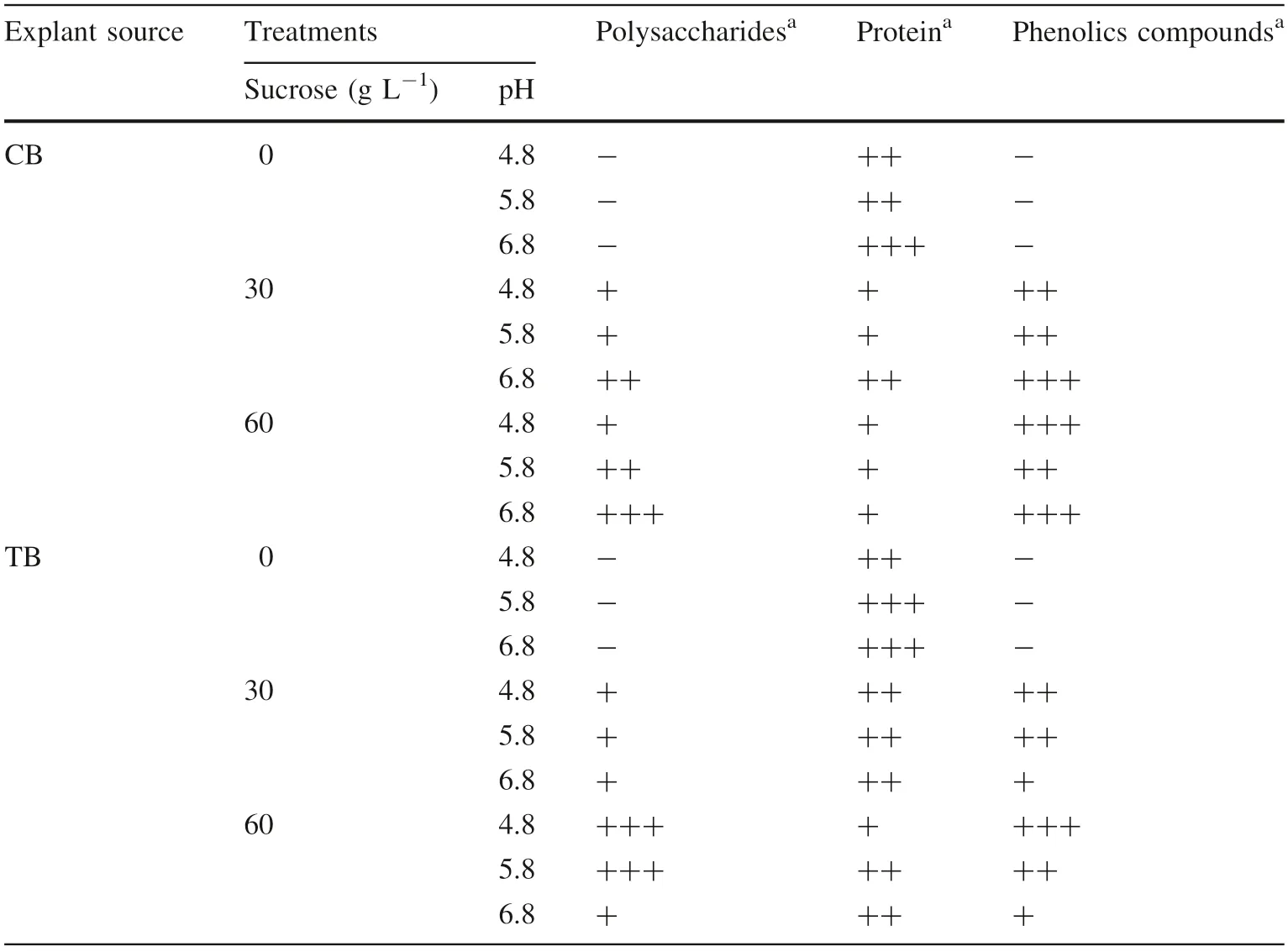
Table 7 Histochemical results for polysaccharides, proteins and phenolic compounds of shoot leaves from the lowest canopy branches(CB)and trunk base (TB). Both in vitro multiplication phase and subcultured at different sucrose concentrations and pH
In all treatments, sucrose concentrations and pH values,morphophysiological characteristics differed from the two explant sources over time,with improved multiplication for the first subculture. This may demonstrate the cumulative effect of different treatments (negatively influenced)among the subcultures.
The variation in dry mass gain with pH from 4.8 to 6.8(Table 6), was 7.5 mg (0 g L-1sucrose), 22.4 mg(30 g L-1sucrose)and 29.2(60 g L-1sucrose).However,dry mass increment with sucrose increase from 30 to 60 g L-1, (Table 6), was more significant, 161.8 mg (4.8 pH), 125.1 mg (5.8 pH) and 147 mg (6.8 pH). This result shows a weak pH role in shoot development while sucrose levels had a significant influence.
There was no significant difference between material origin(CB or TB)for dry mass gain.Therefore,while there are intrinsic differences between explant sources, the mass gain capacity was similar.However,dry mass is not solely responsible for the choice of a particular protocol over another. For this reason, others aspects were evaluated for the selection of the best shoot multiplication protocol for E.benthamii culture medium WPM supplemented with 30 g L-1sucrose and pH adjusted to 5.8. Additionally, it was possible to select the best explant source (canopy branches, CB).
Histochemistry clarifies how the various treatments affected shoot metabolism to some degree. In general, the presence of polysaccharides gradually increased as sucrose increased regardless of the explant source.The majority of polysaccharides was starch.Studies show that an excess of sucrose for in vitro culture may result in significant starch production (Capellades et al. 1991). In the case of E.benthamii,high levels of polysaccharides may be attributed not only to starch but to shorter chain soluble polysaccharides. This is an important feature which makes this species frost resistant (Floriani et al. 2013).
Polysaccharide synthesis in treatments with the addition of 30 g L-1sucrose was less evident and restricted to the vascular bundle cells. In the absence of sucrose, there was no accumulation of polysaccharides due to shoot death.This suggests a strong heterotrophism of micropropagated plants. Additionally, during the process of senescence,starch and simple sugars are consumed due to the high energetic demand of the cell death process (Elmore 2007;Graner et al. 2015).
Individual shoot assessment demonstrated different responses for each explant source with pH fluctuation. For CB shoots at pH 4.8, there was no increase in polysaccharides when the sucrose concentration increased from 30 to 60 g L-1. However, for pH 5.8 and 6.8 polysaccharide accumulation was higher with increasing sucrose. In contrast, for the TB shoots, there was an increase in polysaccharides with increasing sucrose at pH 4.8 and 5.8 and no difference at pH 6.8. The results show that pH did not cause significant macroscopic alterations but promoted a differential accumulation of polysaccharides according to explant origin.
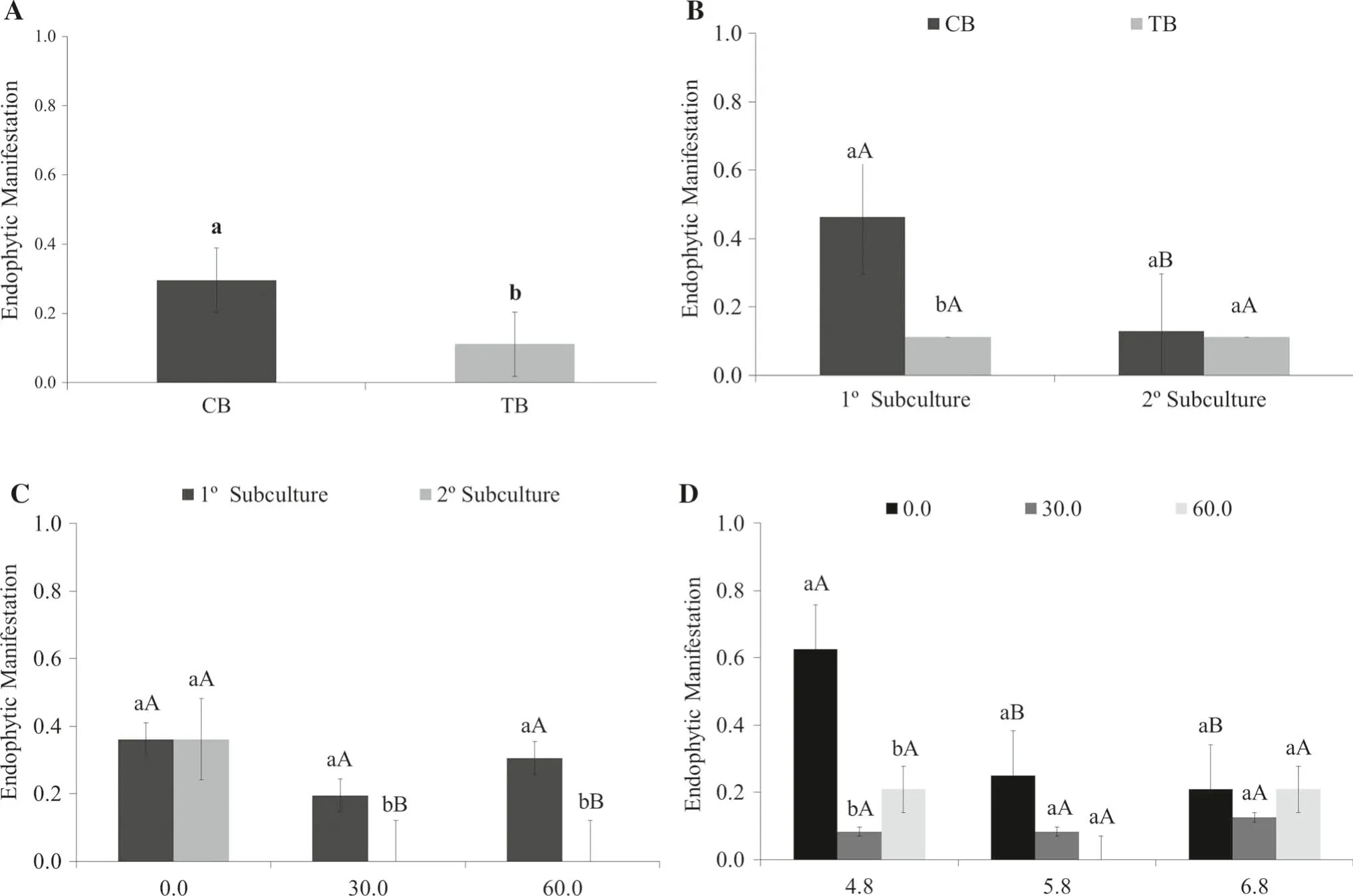
Fig. 4 Endophytic manifestation mean values from CB and TB.A Each explant source; B different subcultures; C three sucrose concentrations (g L-1); and, D three pH values. (Lowercase letters equal to two different explant sources or subcultures in sucrose concentration and pH, and to equal capital letters for different concentrations of sucrose added to the culture medium, or pH values within a same explant source ofor subculture do not differ at 5%probability by Tukey’s test)
Phenolic compounds and polysaccharides increased with increasing sucrose levels for both explant sources. This shows intense metabolic activity of alternative pathways for the disposal of excess sucrose. Some phenolic compounds are widely used as indicators of stress and high metabolic rates(Almeida et al.2012;Esposito-Polesi et al.2013; Graner et al. 2015). Thus the absence of sucrose in the medium may indicate reduced metabolic activity, and the high concentrations presence can generate high metabolic activity. Proteins decreased when sucrose concentrations increased. Treatments without sucrose showed higher protein concentrations and decreased when sucrose increased. This may indicate stress or even cell death which occurred in the treatment without sucrose. During cell death, numerous enzymes and protein complexes that degrade organelles are activated and paralyze the cell cycle(Palavan-Unsal and Arisan 2011; Graner et al. 2015).
Endophytic manifestation was another parameter evaluated. The CB shoots displayed more endophytes compared with the TB shoots. CB shoots were also superior in development and multiplication.Therefore,we believe that endophytic manifestation was beneficial and possibly favored the multiplication of CB shoots.
Some studies report that endophytes are not an imminent danger to the in vitro culture and therefore the attribution of ‘‘contamination’’ for all microbial manifestation is arbitrary. All plant species have endophytic bacterial microbiota as they are found in plants considered axenic(Almeida et al. 2009; Abreu-Tarazi et al. 2010; Thomas and Kumari 2010).
Our results show a tendency for endophytic manifestation when the environment conditions (sucrose concentration and pH) are disturbed. The sudden manifestation in a medium may be explained by environmental fluctuations during in vitro culture (Thomas and Kumari 2010). However, this manifestation may be modulated, particularly to intrinsic plant factors such as genotype, variety, age,growth stage and physiological status(Hardoim et al.2008;Ardanov et al. 2012; Gagne-Bourgue et al. 2013).
Another possible explanation for endophytic manifestation is how the organisms have adapted to the plant.If the endophytes evolutionarily relate to the host plant or not.The endophytes are restricted to a specific group of hosts,to a single species,to a single individual or even to certain organs of the same individual (Cook et al. 2012; Moricca et al. 2012). Thus when choosing an explant, one endophytic microbiota may be more prone to manifest,depending on the degree of intimacy every organism has with its host or its parts. Therefore, when the endophyte is an ‘‘obligate endophyte’’, it will be closer evolutionarily and less prone to survive in the absence of its host (in this case probably, the manifestation will not occur). On the other hand, if it when the endophyte is a ‘‘facultative endophyte’’it will be less dependent on the host(Hardoim et al. 2008), with greater chance of growing in the culture medium if conditions are abruptly altered. In other research, the endophytic community in TB shoots was mainly composed of obligate endophytes due to its constancy over successive subcultures (Esposito-Polesi et al.2015). In turn, in CB shoots this population varied significantly. This could indicate that the residing endophytic communities in CB shoots were more casual or opportunistic endophytes. This finding can be confirmed by the results in this study. It suggests that the relationship established between the endophytic bacterial community and the host explant could favor endophytic manifestation.However, the degree of manifestation was higher in CB shoots due to less specialized endophytic bacterial populations.
Conclusions
The specific characteristics of each explant source allowed for the measurement of responses to environmental conditions (culture media, sucrose concentration, and pH).Canopy branches shoots were superior under the different treatments and also displayed more endophytes compared with trunk base shoots. This indicates that the sudden growth of endophytes was not detrimental to the development of canopy branches shoots. The endophytic manifestation, even at low intensity, may signal the need to change environmental media conditions so as not to hinder the development of E. benthamii shoots. We believe that endophytic manifestation may be controlled before it becomes harmful to the species micropropagation to avoid unnecessary discards.
杂志排行
Journal of Forestry Research的其它文章
- Do increasing respiratory costs explain the decline with age of forest growth rate?
- At what carbon price forest cutting should stop
- Mapping the risk of winter storm damage using GIS-based fuzzy logic
- Effects of seed moisture content, stratification and sowing date on the germination of Corylus avellana seeds
- Comparison of seed morphology of two ginkgo cultivars
- De novo assembly of the seed transcriptome and search for potential EST-SSR markers for an endangered, economically important tree species: Elaeagnus mollis Diels
