Simple phenols in tropical woods determined by UHPLC-PDA and their antioxidant capacities: an experimental design for Randall extraction using environmentally friendly solvents
2020-05-19MarieBartakovaEvaDvorackovaLucieChromcovaPetrHrdlicka
Marie Bartakova · Eva Dvorackova · Lucie Chromcova · Petr Hrdlicka
Abstract A comprehensive experimental design was developed to determine suitable conditions (volume, percentage of solvent, extraction temperature) for the ethanol extraction of phenolic compounds from ten tropical woody plants. Wenge, angelim vermelho, zebrano, merbau,tigerwood, angelim pedra, jatoba, angelim amargoso,massaranduba, and doussie woods were used in experiments.The effects of three independent variables and their interactions on the yields of cinnamic and benzoic acid derivates were analyzed using UHPLC-PDA. The most significant parameters were found to be solvent percentage,extraction volume, and extraction temperature. Optimal conditions for the extraction of phenolic compound contents were an 80 °C extraction temperature, a 30 mL extraction volume, and the use of pure water as the extraction solvent. The tested species of woods contained mainly cinnamic acid derivates. Water extracts after hydrolysis contained greater amounts of cinnamic acid derivates than those extracts from non-hydrolyzed material.The total phenolics content was highest in water extracts of tigerwood, wenge and merbau; however, the extract of merbau wood was a stronger radical scavenger in ABTS+·assays: 34.11 ± 0.02 mM Trolox equivalents per g of dry extract. The main compounds possessing antioxidant activity in the extracts of vermelho wood assessed by UHPLC analysis were hydroxycinnamic acids.
Keywords Phenolic compounds · Tropical woods ·Ultrahigh-performance liquid chromatography ·Antioxidant capacity
Abbreviations
AC Antioxidant capacity
ACN Acetonitrile
DW Dry weight
EtOH Ethanol
MeOH Methanol
PDA Photodiode array detector
SPE Solid-phase extraction
TEAC Total equivalent antioxidant capacities
TPC Total polyphenol content
UHPLC Ultrahigh-performance liquid chromatography
Introduction
Wood is a lignocellulosic natural product. Regarding the chemical composition of wood,there is a significant group of phenols and their derivatives. The macromolecular phenolic substance is lignin, which significantly contributes to the construction of the walls of wood cells. The wood also contains tannins,flavonoids,and others phenolic compounds.Simple phenols are usually found to be bound as esters oforganic acids or glycosides (Fengel and Wegener 1984; Wallace and Fry 1994).
Phenolic substances obtained from wood are widely used.Up to now,several studies have shown that woods is a rich source of polyphenolic compounds capable of eliminating radicals, substances effective against cancer and cardiovascular or neurodegenerative diseases (Bursal and Köksal 2011; Nenkova et al. 2011; Aspé and Fernández 2011;Lamounier et al.2012;Kougan et al.2013;Aadil et al. 2014). Low molecular weight phenolic substances such as hydroxycinnamic and hydroxybenzoic acids may be used as allelopathic compounds (Strack 1997) and/or may be used as functional ingredients in food,cosmetics or pharmaceuticals products.
At present, the procedure for the isolation of phytochemicals from plant origin,based on liquid extraction,has come to the fore. Solvent extraction allows for a good transfer of substances and heat from the plant to the solvent, which increases the extraction efficiency. Most have been concerned with the solvent extraction of natural matrices, even in the light of the development of newer extraction methods (supercritical fluid extraction, pressurized extraction etc.)(Šliumpaite et al.2013).A wide range of phytochemicals from simple to complex structures have been screened in various plant materials (Häkkinen 2000;Kougan et al. 2013).
Commonly used solvents for the extraction of polar compounds are ethanol and/or its water mixtures. The use of ethanol under relatively mild conditions has been well documented. A number of methodological experiments have been carried out to optimize the conditions for the solvent extraction of phenols and organic acids from wood and its products (Bursal and Köksal 2011; Gironi and Piemonte 2011; Celhay et al. 2014).
The aim of this study was to optimize experimental conditions for the extraction of phenolic compounds from ten tropical wood using ethanol/water as a solvent. In relation to the yield, the following parameters were optimized: ethanol concentration in the solvent, extraction temperature, extraction volume and the relationship of these parameters. The effect of these parameters on the antioxidant capacity and content of total phenols was also monitored.
Materials and methods
Chemical and equipment
Analyses were conducted in a specialized laboratory at the Mendel University in Brno, Czech Republic, Department of Chemistry and Biochemistry. All chemicals and equipment were listed in Dvorackova et al. (2015).
Woody plants samples
Wood samples (Table 1) were taken from commercial floorboards MAGNUM Parket a.s.and ToTEM s.r.o.(Baar et al.2012,2016).Milled pieces of wood(particle size max 0.5 mm), one mixed sample of each floorboard (Fig. 1),were placed before analysis in PE bottles under laboratory conditions.
Extraction of woody plants
The phenolic compounds extraction from woody plants was conducted using the Randall extraction technique(Dvorackova et al. 2015). First, to determine optimum extraction conditions 1 g of dried,milled and homogenized representative wenge wood sample in 20 mL of various aqueous solutions of ethanol (20%, 40%, 60%, 80% and 96%,v/v)or pure water was extracted at 80 °C for 30 min.In the following steps,different extraction volumes(20,30,and 40 mL) and extraction temperatures (50, 80, and 110 °C)were used.After each extraction,the extracts were evaporated to dryness and reconstituted with water acidified by 6 M HCl (pH ~2). These extracts were put on solid-phase extraction (SPE) cartridges using a modified procedure according to Inbaraj et al. (2010) and the individual phenols were analyzed using UHPLC(the procedure was detailedly descripted in Dvorackova et al. 2015). The final extracts were hydrolyzed. Thus, the contents of all forms of phenolic substances (free, ester and glycosidebound) were obtained. The acquired contents of phenolic substances (each extraction three times) are given in dry weight (mg/g, DW).
Determination of antioxidant capacity
Free radical-scavenging capacity was determined as described by Dvorackova et al.(2015)and Re et al.(1999).A concentration of Trolox in calibration curve was used in the scale 0-50 μmol/L. Exactly 20 min after the initial mixing, the absorbance of the blue color solution was measured at the 734 nm.The quantification was based on a standard Trolox curve. The results were adjusted by dilution and converted to mM of Trolox per 1 g dry weight of the sample equivalents as Trolox (TEAC). The obtained values are given as means ± standard deviations (n = 3).
Determination of total polyphenols
The determination of total phenolic substances was carried out with the modified colorimetric method of Folin-Ciocalteu (Medina 2011). As the reference and calibration standard was used gallic acid in the concentration scale 0-100 μmol/L. Exactly 40 min after the initial mixing,the absorbance of the blue color solution was measured at the 765 nm. The quantification was based on a standard gallic acid curve. The results were adjusted by dilution and converted to mg of gallic acid per 1 g dry weight of the sample equivalents as gallic acid (GAE). The obtained values are given as means ± standard deviations (n = 3).
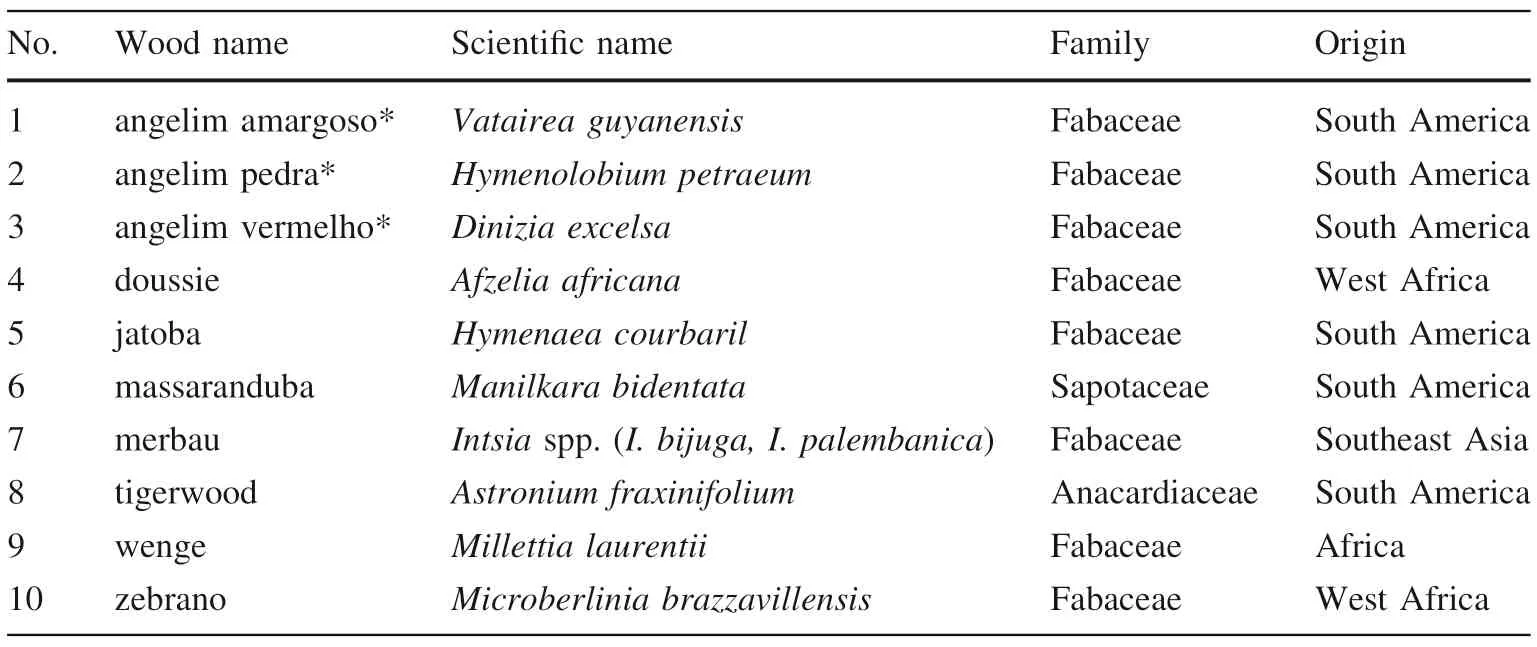
Table 1 Different types of tropical woods
Separation and determination of individual wood phenols
This process was performed by means of Ultra-High Performance Liquid Chromatography with a photodiode array detector. The mobile phase, acetonitrile-water, with 0.8%acetic acid, was applied according to the solvent program.Content of acetic acid (%); content of acetonitrile was the rest to 100%: 0.0 min 90%; 1.0 min 92%; 1.1-4.0 min 97%; 5.0-8.9 min 92%; 9.0-13.0 min 89%;13.6-15.5 min, 65%; and 16.0-18.0 min, 90. Column temperature 30 °C, injection volume 5 μL, detector wavelengths 270,290,310 and 324 nm.The extracts of the samples were before injection into the column filtered by a nylon membrane filter (0.45 μm).
Phenolic compounds were identified based on retention times and spectral data with corresponding standards. The quantification was based on a standard curve of each identified phenols. Calibration curves for the phenolic standards were prepared by diluting each phenols standards in methanol(80%).The obtained values of phenols content(mg/g, dry wood) are given as means ± standard deviations (n = 3).
Most of identified phenols had a detection limit in the range 0.116 μg/mL to 0.953 μg/mL. The exceptions were ferulic (1.470 μg/mL), sinapic (3.115 μg/mL), and cinnamic acid (0.065 μg/mL).
Results and discussion
The UHPLC-PDA profile was recorded by series of peaks of different phenolics. Ten of these peaks were identified.This kind of systematic approach to identification provides the full range of analytical data needed to screen complex natural matrices, e.g. a complex matrix of wood.
Many variable parameters influence the course of conventional solvent extraction, in our case extraction by Randal. In this research, the most influential experimental conditions in the extraction of phenolics from ten tropical woody plants-(a) water and ethanol/water mixtures in different concentrations (20%, 40%, 60%, 80% and 96%),(b) ratio (w/v) sample/solvent (1:20, 1:30, 1:40), and(c) temperature of extraction (50, 80, 110 °C)-were evaluated to optimize the extraction efficiency. These variables have a decisive influence in the extraction process.Their optimal choice determines not only the kinetics of release of phenolic compounds from wood (extraction yield), but also the antioxidant properties of the extract.The presented research was performed in several optimization steps in sequence, the solvent concentration,sample/solvent ratio, and extraction temperature. These were determined on the basis of the amounts of phenolics determined in the wenge wood extracts. In the subsequent steps, the remaining wood samples were extracted according to the most effective approach determined for wenge wood; hydrolysis was also performed.
Solvent composition
Plant phenols have the character of polar compounds and their extraction is more suitable with mixtures oforganic solvents (methanol, ethanol, acetone, etc.) with water rather than the use oforganic solvents alone. The most used mixtures are methanol or ethanol and water(Markom et al. 2007).

Fig. 1 Samples of tropical woods-original photo provided by the Faculty of Forestry and Wood Technology, Department of Wood Science (Baar et al.2012, 2016) (only for color reproduction on the Web)
The Randall method was used to examine the extraction efficiency of water and various water/ethanol mixtures.Constant extraction parameters were: time (30 min), temperature (80 °C), and a sample/solvent ratio of 1:20. The quantitative results presented in Fig. 2 show differences in phenol recoveries obtained with aqueous mixtures of ethanol (up to 96%, v/v). Pure water was superior to any mixture of ethanol/water for the majority of the phenolics measured.It is likely that the hydrophilic substitutes of the carbohydrate nature which are bound to phenolic substances thus increased the effectiveness of the extractivity.So pure water proved to be the most suitable for the extraction of tropical wood phenolic compounds by Randall method and was used in the next. Moreover, it facilitated a more efficient pre-concentration step involving the SPE cartridges prior to UHPLC analysis, as there was no evaporation.
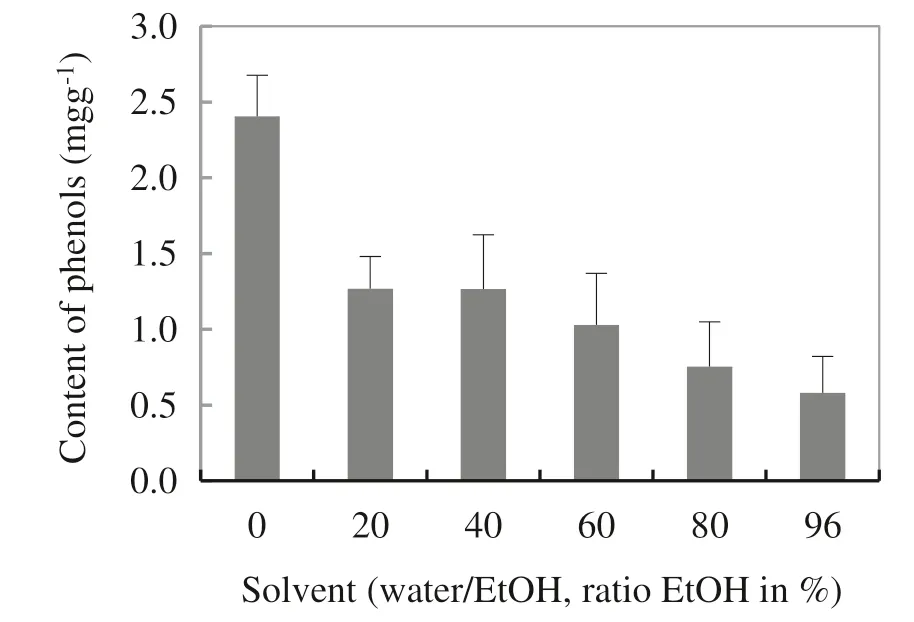
Fig. 2 Varying the concentration of ethanol and its influence on extraction effectiveness

Fig. 3 Determination of the optimum ratio of sample weight to solvent volume for the extraction of phenolic compounds
Sample to solvent ratio and temperature optimization
Figure 3 presents the interaction ratio during water extraction.The extraction yield of phenols was highest at a sample-to-solvent ratio of 1:30. At a ratio of 1:20 the amount of water was too small,at a ratio of 1:40 too large.At a sample-to-solvent ratio of 1:30, the solubility of phenolic compounds in water was the best in terms of its quantity (Prasad et al. 2009).
The temperature of phenol extraction should be chosen depending on the type of matrix. Slightly elevated temperature (e.g. 40 °C for olive leaves) generally improves the release of phenols into water(Japon-Lujan et al.2006),however, higher temperatures (e.g. 60 °C for soy beverages)may already cause degradation of phenols(Rostagno et al. 2007). In order tofind a suitable temperature for a wood matrix,extraction at three different temperatures was examined: 50 °C, 80 °C and 110 °C. The choice of temperature corresponds to border temperature (100 °C) when there are no thermic changes in wood(Fengel and Wegener 1984).It was observed that the highest contents of phenolic compounds in extracts were obtained with water at an extraction temperature of 80 °C. Higher temperatures can cause degradation of phenolic compounds and thus reduce extraction yields (Fig. 4).
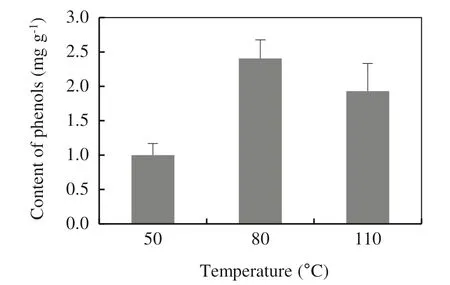
Fig. 4 Optimization of extraction temperatures
Final approach and hydrolysis
The extraction conditions using the Randall method were determined as follows: extraction agent distilled water,30 min as a time of extraction, 80 °C as a temperature of extraction, and 1:30 as an extraction ratio (sample/water;w/v).
Phenolic compounds can usually be found in various conjugates, most often as esters or glycosides, so preparation of samples involved alkaline and acidic hydrolysis as a necessary step to release bound phenols.It is well known that the hydrolysis of glycosidically linked phenols in order to release the corresponding aglycone, may be suitable for the determination of phenolic substances bound in a natural matrix(Hertog et al.2000;Robards and Antolovich 1997).In the procedure of extraction optimization, the hydrolysis step was omitted because the phenolics were analyzed as derivatives. The hydrolysis was afterward employed successfully as one of the last steps.
Alkaline and acidic hydrolysis was performed by the addition of 2 M natrium hydroxide or 2 M hydrochloric acid in the ratio 1:1. The procedure of hydrolysis was altered according to Mattila and Kumpulainen(2002). The temperatures (a water bath of ambient temperature, 80 °C and 100 °C)and duration(15 min,30 min,1 h and 4 h)of both steps of hydrolysis were chosen after a succession of experiments. For completeness of hydrolysis reactions,samples were moved to a dark place for 15 min. After finishing alkaline hydrolysis, samples were adjusted to pH ~2.

Fig. 5 Tropical woods-before and after hydrolysis
In addition, the quantitative contents and qualitative compositions of phenolics in the extracts without and with hydrolysis were compared. The hydrolysis step has been shown to increase extraction efficiency in relation to phenols by more than two-fold. Figure 5 shows the results of UHPLC analysis in the hydrolyzed extracts of ten tropical wood. Phenolic compounds obtained from hydrolysates were at very significant levels in comparison with free derivates. The result of hydrolysis depends on the nature of the acid or base (strength, concentration), on the type of saccharide, on the type of glycosidic linkage(usually -O-, but may be -N- or -S-) and the position of the glycosidic bond in relation to the aglycone. The effect of the configuration of the semi-acetal cycle of saccharide(furanose,pyranose)cannot be neglected.Also important is the current distribution of electron density at the reaction center; carbohydrate derivatives (e.g., uronides) are more resistant to hydrolysis than unaltered mono- or oligosaccharides (Hertog et al. 2000; Robards and Antolovich 1997).
Nine phenolic substances belonging to two groups of compounds were successfully identified by chromatography in vermelho wood according to the retention times and spectral characteristics of their peaks viewed against those of standards.Seven acids:sinapic,gallic,ferulic,caffeic,pcoumaric, syringic and protocatechuic; two aromatic aldehydes: 4-hydroxybenzaldehyde and vanillin (Fig. 6).Randall extraction with distilled water (sample/solvent ratio 1:30, time 30 min, temperature 80 °C) yielded the highest content of sinapic acid. The quantity (dry matter wood) of the most frequently occurring phenolic compounds ranged from 0.03 mg/g(protocatechuic acid)to the 8.12 mg/g (gallic acid) and 7.91 mg/g (sinapic acid).
Total polyphenols content and antioxidant capacity

Fig. 6 Phenolic compounds in vermelho wood-before and after hydrolysis [cinnamic acid (1), sinapic acid (2), ferulic acid (3), pcoumaric acid (4), vanillin (5), syringic acid (6), caffeic acid (7),vanillic acid (8), 4-hydroxybenzaldehyde (9), 4-hydroxybenzoic acid(10), 3,4-dihydroxy benzaldehyde (11), protocatechuic acid (12),gallic acid (13)]
The chemical composition of the matrix (wood) provides an extract comprising a mixture of substances of different chemical behavior that is based on the presence of functional groups and their polarity.This is reflected in the fact that the results of different determinations depend on the methods used. This disadvantage was eliminated by using test the radical scavenging capacity, a test based on the reaction of a stable ABTS radical with antioxidant compounds present in the extract (Paulova et al. 2004). In this study,the decolorization rate of the radical ABTS was proportional to the amount of antioxidants in the extract.

Fig. 7 Correlation between antioxidant activity (Y, AC) and total polyphenols contents (X, GAE); Y = 0.0085 * X, p = 0.0000;R2 = 0.5562(Y-intercept:not significant).Clusters are type of woods.No.1,2,3,4,5,6,7,8,9,10 are Vatairea guyanensis;Hymenolobium petraeum; Dinizia excels; Afzelia Africana; Hymenaea courbaril;Manilkara bidentata; Intsia spp. (I. bijuga, I. palembanica); Astronium fraxinifolium; Millettia laurentii; Microberlinia brazzavillensisrespectively
The antioxidant capacity was determined in the first step of the process of optimizing the extraction process and choosing the solvent composition. The results obtained were in good agreement with the UHPLC-PDA content of phenolic substances.
In each optimization step,both antioxidant capacity and total polyphenols content were measured.From Fig. 7,it is clear that data collected for antioxidant capacity are in accordance with total polyphenol content. Nevertheless,the correlation was not found for the content of particular phenolic substances determined by UHPLC. The probable cause is that the spectrum of chromatographically identified substances did not include all antioxidant substances falling within the TPC parameter.In general,an increase in the total content of phenolic substances also increases the antioxidant activity of the extract. As the results show,phenolic substances extracted from wood have a major contribution to the level of antioxidant activity. However,in the case of tiger wood, a portion of the phenolic compounds did not contribute to the antioxidant activity. For wenge and merbau woods, the antioxidant activity was probably connected with some other form of compounds than the phenolic kind.
Conclusions
Simple Randall extraction together with an additional SPE cleaning procedure and acid/alkali hydrolysis were applied to optimize the extraction conditions(percentage of ethanol solvent,extraction temperature,and extraction time)for the isolation of phenolic compounds from ten tropical woody plants. Of the extraction parameters tested, solvent composition and extraction temperature were the most significant variables. Water as the extraction solvent, an extraction temperature of 80 °C,an extraction ratio of 1:30,and an extraction time of 30 min were the most efficient conditions for the extraction of phenolic compounds from tropical woods. The optimized conditions determined in this study could be applied to any kind of woody plant for the solvent water extraction of simple phenolic compounds.This method is useful as it allows the effects of variables and their interactions on extract and phenol yields to be observed. Under the abovementioned optimum conditions,wenge, merbau, and angelim pedra woods were found to exhibit the highest levels of antioxidant capacity and total phenolics contents.
AcknowledgementsWe would like to thank the Faculty of Forestry and Wood Technology, Department of Wood Science for providing wood samples. The authors would also like to thank the companies MAGNUM Parket a.s. and ToTEM s.r.o. for the supply of materials used in the experiment.We would like to thank Mr.Matthew Nicholls for manuscript improvement and English correction.
杂志排行
Journal of Forestry Research的其它文章
- Do increasing respiratory costs explain the decline with age of forest growth rate?
- At what carbon price forest cutting should stop
- Mapping the risk of winter storm damage using GIS-based fuzzy logic
- Effects of seed moisture content, stratification and sowing date on the germination of Corylus avellana seeds
- Comparison of seed morphology of two ginkgo cultivars
- De novo assembly of the seed transcriptome and search for potential EST-SSR markers for an endangered, economically important tree species: Elaeagnus mollis Diels
