Effect of artificially accelerated aging on the vigor of Metasequoia glyptostroboides seeds
2020-05-19HuanLiuYanfangZhuXiaLiuYuJiangShimingDengXunruAiZhijunDeng
Huan Liu · Yanfang Zhu · Xia Liu,2,3 · Yu Jiang · Shiming Deng,4 ·Xunru Ai · Zhijun Deng
Abstract Metasequoia glyptostroboides is an endangered relict plant native to China that has been widely introduced into many countries and areas around the world.However,its seeds germinate at a very low percentage.Consequently,population regeneration by seed is low under natural conditions, which probably contributes to the endangered status of this plant species. The present study aimed to describe the aging mechanism of M. glyptostroboides seeds. Our objective was to elucidate causes of the low germination rate in an effort to enhance potential for conservation of the species. We used germination tests,relative electrical conductivity and malondialdehyde content determination, ultrastructural observation of embryo cells, and analysis of superoxide dismutase, catalase,ascorbate peroxidase, and dehydroascorbate reductase activities during accelerated aging treatment. We found that M. glyptostroboides seeds have a low level of vigor and poor ability to maintain vigor, which is probably associated with the inefficiency of its enzymatic antioxidative system.
Keywords Dawn redwood · Seed aging · Relative electrical conductivity · Antioxidative system ·Ultrastructure
Abbreviations
ANOVA Analysis of variance
APX Ascorbate peroxidase
AsA Ascorbate peroxidase
CAT Catalase
CM Cytoplasmic membrane
CW Cell wall
DC Druse crystal
DHA Dehydroascorbate
DHAR Dehydroascorbate reductase
DW Dry weight
G Glyoxysome
GCC Globoid crystal cavity
GR Glutathione reductase
GSH Glutathione
GSSG Oxidized glutathione
H2O2Hydrogen peroxide
IS Intercellular space
ISTA International rules for seed testing
LB Lipid body
MDA Malondialdehyde
MDHAR Monodehydroascorbate reductase
Mt Mitochondrion
N Nucleus
·OH Hydroxy radicals
1O2Singlet oxygen
P Probability
PB Protein body
PPFD Photosynthetic Photon Flux Density
RH Relative humidity
ROS Reactive oxygen species
S Starch granule
SE Standard error
SG Soft globoid
S-N-K Student-Newman-Keuls
SOD Superoxide dismutase
Introduction
Metasequoia glyptostroboides (Taxodiaceae), dawn redwood (also called a ‘‘living fossil’’), is a well-known endangered plant native to China that was once distributed widely in the northern hemisphere, including middle and eastern Eurasia and North America;it was thought to have become extinct millions of years ago (Yang 1998, 1999).As one of the greatest botanical discoveries of the twentyfirst century,living individuals of M.glyptostroboides were discovered in southwestern China(Ma and Shao 2003). At present,wild trees only grow in an area of about 1000 km2to the west of Hubei and Hunan provinces and east of Chongqing City in China. Some 98% of all wild trees are concentrated in a narrow belt of approximately 600 km2in Lichuan, Hubei Province, China (Wen et al. 2001; Wang et al. 2004; Ma et al. 2006; Li et al. 2012).
As a landscape tree, M. glyptostroboides has been introduced into other areas of China outside of its natural habitat and to more than 50 countries and areas throughout the world,from Scandinavia in the north to New Zealand in the south (Satoh 1999). If only considering the individual numbers and distribution range, M. glyptostroboides is undoubtedly one of the most successfully conserved endangered plant species. However, M. glyptostroboides produces seeds with low vigor (Xin et al. 2004; Li et al.2012), which severely restricts its capacity for natural regeneration(Xin et al.2004),and contributes greatly to its extinction risk. Therefore, it is imperative to evaluate its seed vigor and assess how this vigor is lost over time, as this could help to reduce the risk of extinction by improving seed vigor maintenance and seedling establishment.
The process of seed vigor loss over time is usually referred to as seed deterioration or seed aging(Gove 1965).Aging begins once a seed reaches physiological maturity(Negreiros and Perez 2004).Along with the aging process,seeds continue to deteriorate and gradually lose vigor and quality,and eventually,viability(Kapoor et al.2011;Sano et al.2016).Seeds from various plant species,populations,and environments deteriorate at different rates (Goel and Sheoran 2003; Nagel and Borner 2010; Donà et al. 2013;Sano et al. 2016). Naturally, if the deterioration rate of a seed is rapid, then the natural regeneration and conservation of a plant species is threatened (Sano et al. 2016).
Typically, an artificial accelerated aging treatment or controlled deterioration treatment under high temperature and humidity followed by germination tests and/or electrical conductivity determination is used to investigate seed deterioration(Rajjou et al.2008;Barreto and Garcia 2017).
Seed deterioration, together with the aging process, is affected by factors associated with seed structure and biochemical processes(Shaban 2013).Seed deterioration is related to reduction in the activity of various antioxidative enzymes(Sung 1996;Goel et al.2003;Kibinza et al.2006;Yao et al. 2012; Xin et al. 2014; Xia et al. 2015).
Molecular oxygen is relatively inactive; its basal status,i.e., triplet oxygen, cannot react with organic molecules when inactivated (Engelmann 2000). However, activated oxygen, i.e., reactive oxygen species (ROS), is destructive due to its very strong oxidation ability. ROS include,H2O2,·OH and1O2. Of the various ROS, H2O2and·OH possess the strongest activity and highest level of destructiveness (Smirnoff 1993). H2O2andare produced in many cellular reactions.
The antioxidative defense system comprises enzymatic and non-enzymatic defense systems (Sarvajeet and Narendra 2010). The non-enzymatic defense system includes various molecular antioxidants, such as AsA,tocopherol (vitamin E) and GSH, and the enzymatic defense system includes SOD, CAT, APX and DHAR(Sarvajeet and Narendra 2010).There is no or low enzyme activity in dry seeds, and the scavenging of ROS is highly dependent on molecular antioxidants, such as AsA and vitamin E (Xia et al. 2015). In contrast, in aging seeds,increased seed moisture facilitates protein synthesis, and antioxidative enzymes (including SOD, CAT and the enzymes involved in the ascorbate-glutathione cycle, such as APX, GR, MDHAR and DHAR) are activated to scavenge accumulated excess ROS(Bailly et al. 1996; Mittova et al. 2000; Sarvajeet and Narendra 2010). Of the various antioxidative enzymes, SOD, CAT and APX play primary roles (Asada and Takahashi 1987; Bowler et al. 1992;Willekens et al. 1997). The balance between SOD and APX or CAT activities is thought to be crucial for maintaining steady-state levels ofand H2O2in cells(Bowler et al. 1991), and for preventing the formation of highly toxic·OH via the Haber-Weiss or Fenton reactions(Asada and Takahashi 1987).
During seed aging at relatively high moisture levels,O-2generated from the electron transport chain is first dismutated to H2O2by SOD in the matrix (Matamoros et al.2013), and then H2O2is scavenged by CAT in the peroxisome and peroxidases such as APX (which uses AsA as the substrate) and GPX (which uses glutathione as the substrate) in the cytosol, mitochondria, peroxisomes, and apoplasts (Mittler 2002; Matamoros et al. 2006). Subsequently, oxidized AsA can be reduced by NADH-dependent MDHAR or by glutathione-dependent DHAR to be regenerated via the ascorbate-glutathione cycle,and GSSG can be reduced by GR to be regenerated via ascorbateglutathione and the GPX cycle (Mittler 2002; Matamoros et al. 2013). The ability to scavenge ROS, i.e., antioxidative potential, is important for aged seeds to withstand the oxidative stress originating from deterioration (Liu et al.2007). As seeds age and ROS production exceeds the scavenging capacity of the antioxidative defense system,oxidative injury occurs, and hence seed vigor declines,until a complete loss of seed viability occurs (Donà et al.2013). This study aimed to investigate the vigor of M.glyptostroboides seeds and its mechanism of loss or retention.
Materials and methods
Seeds
M. glyptostroboides seeds were collected in 2014 from 18 trees located in Little River village in Lichuan City,southwestern China:one of the primary natural distribution regions. After collection, immature and non-filled seeds were removed by hand. The mature and filled seeds were dried at room temperature (approximately 20 °C) at 46%RH for 72 h.When the seed moisture content decreased to(8.17 ± 0.41)% [wet weight basis, mean ± SE, n = 4;1000-seed weight, (2.06 ± 0.12) g], the seeds were stored in sealed plastic bags at 10 °C until use in subsequent experiments.
Determination of seed moisture content and 1000-seed weight
The seed moisture content was determined gravimetrically according to the ISTA chapter IX(2013).Four replicates of 100 seeds each were placed in a bottle with a known weight and weighed and then dried in an oven at 80 °C for 48 h.After cooling for 1 h in a desiccator at room temperature,the seeds were weighed again, and the seed moisture content was calculated. The seed moisture content was expressed as a percentage on a wet weight basis.
Four replicates of 1000 seeds each were weighed on an electronic balance (precision ± 0.0001 g). The 1000-seed weight was expressed as the mean ± SE.
Accelerated aging treatment
According to the method of Barreto and Garcia (2017),accelerated aging treatment was performed by exposing dry,mature seeds to 45 °C in a tightly closed drier at 100%RH for 2, 4, 6, 8, or 10 days. Then, the aged seeds were further subjected to germination tests, relative electrical conductivity, MDA content determination, antioxidative system analysis, and cell ultrastructural observation.Unaged seeds were used as the control.
Germination test
According to the method of Deng et al. (2010), four replicates of 50 seeds were incubated on two pieces of filter paper moistened with 5 mL of distilled water in 9-cm-diameter Petri dishes at different temperatures and under a 12-h light/12-h dark photoperiod (PPFD, 121 μmol m-2-s-1) for 14 d. Initially, to determine the optimal germination temperature of M. glyptostroboides, 50 unaged(control) seeds were incubated at several constant temperatures (5, 10, 15, 20, 25, 30, 35, and 40 °C) and two alternating temperatures (15/25 and 20/30 °C). The most optimal germination temperature from this pilot study(25 °C) was subsequently used in the seed aging experiment. Germinated seeds were counted each day, and then the cumulative germination percentages were calculated.Radicle protrusion was used as the criterion for germination. At the end of the germination test, the viability of healthy, ungerminated seeds was assessed using a tetrazolium test according to Moore (1973).
Electrical conductivity measurement
Four replicates of 20 dry seeds each were placed in a cuvette with 25 mL Milli-Q water (Millipore, Milford,MA, USA) at 25 °C, and then the electrical conductivity(EC0) was immediately measured using an electrical conductivity meter (Delta 326, METTLER-TOLEDO,Switzerland)according to Barreto and Garcia(2017).After being soaked for 6 h (full imbibition), the electrical conductivity of the seed leachate was measured again (EC1).Subsequently, the cuvettes with seeds and their leachate were boiled for 1 h in a water bath. After being cooled to 25 °C, the electrical conductivity was measured again(EC2). The results are presented as relative electrical conductivity (EC, %) and were calculated using the following formula:

where EC0is the electrical conductivity of the seed cleaning solution; EC1is the electrical conductivity of the seed soaking solution; EC2is the electrical conductivity of the seed boiling solution;ECis relative electrical conductivity.
Protein determination
The protein content of the extracts of excised embryos from unaged and aged seeds was determined in accordance with the spectrophotometry method of Bradford (1976)using bovine serum albumin as the calibration standard.
Malondialdehyde determination
In accordance with Hodges et al.(1999),lipid peroxidation was evaluated by determining the MDA content of the embryos. There were four replicates of 50 mg of excised embryo powder from unaged and aged seeds, and the results are expressed here as nmol g-1DW.
Enzyme extraction
Enzyme extraction was performed following Kibinza et al.(2006). Four replicates of more than 1 g of excised embryos from unaged and aged seeds were ground into powder in liquid nitrogen, and then 1 g of excised embryo powder was subsampled and further ground and homogenized in 3.5 mL of 100 mM potassium phosphate buffer(pH 7.8) containing 2 mM dithiothreitol, 0.1 mM ethylenediaminetetraacetic acid, 1.25 mM polyethyleneglycol-4000 and 20% polyvinylpolypyrrolidone. Subsequently,the homogenate was subjected to centrifugation at 15,000 g at 4 °C for 15 min, and then the supernatant was stored for determination of SOD, CAT, APX and DHAR activities.
Enzyme activity determination
SOD (EC1.15.1.1) activity was estimated based on the spectrophotometric method of Rao and Sresty (2000) by determining the ability of the enzyme extract to inhibit nitro blue tetrazolium (NBT) reduction under light, i.e.,photochemical reduction. One unit of SOD activity was defined as the enzyme activity that inhibited the photoreduction of NBT by 50% at 560 nm.
CAT (EC1.11.1.6) activity was estimated according to the spectrophotometric method of Aebi (1984) by monitoring the dynamic changes in absorbance at 240 nm over 2 min due to the decrease in H2O2extinction.CAT activity was expressed as nmol H2O2decomposed min-1mg-1protein.
APX (EC1.11.1.11)activity was estimated based on the spectrophotometric method of Nakano and Asada (1981)by monitoring the change in absorbance at 290 nm due to the oxidation of ascorbate. APX activity was expressed as nmol AsA oxidated min-1mg-1protein.
DHAR (EC1.8.5.1) activity was estimated based on the spectrophotometric method of Dalton et al. (1993) by monitoring the change in absorbance at 265 nm due to the formation of ascorbate. DHAR activity was expressed as nmol AsA formed min-1mg-1protein.
Subcellular structural observation
Embryo axes were isolated from unaged(control)and aged seeds and immediately fixed with 2.5% (v/v) glutaraldehyde at 4 °C for at least 24 h. Fixed samples were subsequently processed by rinsing with 50 mM sodium phosphate buffer (pH 7.2), after which they were fixed again with osmium tetroxide, re-rinsed with the same sodium phosphate buffer, dehydrated using a graded ethanol series, embedded in epoxy resin and sectioned into ultra-thin slices using an ultramicrotome (EM UC6, Leica,Germany). Then, the slices were stained with uranyl acetate followed by lead citrate and were finally observed and photographed using a transmission electron microscope(JEM-1400, JEOL, Japan).
Data analysis
All data were analyzed using SPSS version 12.0 (SPSS Inc., Chicago, USA). Effects of temperature on germination, changes in relative electrical conductivity, and MDA content,as well as SOD,APX,CAT, and DHAR activities during accelerated aging were analyzed using one-way ANOVA followed by the S-N-K multiple comparisons test at P = 0.05.Pearson correlation analysis was used to assess correlation among all parameters measured in this work:germination percentage, relative electrical conductivity,MDA content, as well as SOD, CAT, APX, and DHAR activities. Germination courses of the seeds for each storage time in the accelerated aging experiment were fitted to a logistic curve from which the germination rate (as time taken to reach 50%of the final germination percentage,i.e.,T50, Deng et al. 2010) was calculated. Changes in germination over time during the seed aging experiment were alsofitted to a logistic curve regression, and a linear regression was used tofit changes in the germination rate and the relative electrical conductivity with time in the accelerated aging experiments. To stabilize the variances,germination percentage and relative electrical conductivity data were arcsine-transformed prior to statistical analysis.All charts were plotted using GraphPad Prism for Windows Version 5.01 (GraphPad Prism 2007). Unless otherwise noted, all statistical comparisons were made at an alpha level of 0.05.
Results
Effects of temperature on germination
The optimal germination temperatures for mature M.glyptostroboides seeds included the constant temperature range from 15 to 30 °C and the two alternating temperatures, 15/25 °C and 20/30 °C, under the 12-h light/12-h dark photoperiod (Fig. 1). Thus, the subsequent germination tests were all conducted at 25 °C under a 12-h light/12-h dark photoperiod.
Effects of accelerated aging on germination
Along with the increasing artificial accelerated aging duration, the final average germination percentage decreased following an inverse S-shaped curve(R2= 0.96;Fig. 2a), and the time taken to reach 50% of final germination percentage (T50, used to represent the germination rate) increased linearlly (r = 0.96, P <0.0001; Fig. 2b).
Effects of accelerated aging on the cytoplasmic membrane
The relative electrical conductivity of leachates from M.glyptostroboides seeds increased linearlly (r = 0.94,P <0.0001) along with the increasing accelerated aging duration (Fig. 3).

Fig. 1 Mean germination percentage of mature M. glyptostroboides seeds after a 14-day incubation period at several constant/alternating temperatures under a 12-h light/12-h dark photoperiod (PPFD,121 μmol m-2 s-1). Values are the mean ± SE of four replicates of 50 seeds each. Values indicated by different lowercase letters differed significantly (S-N-K, P = 0.05)
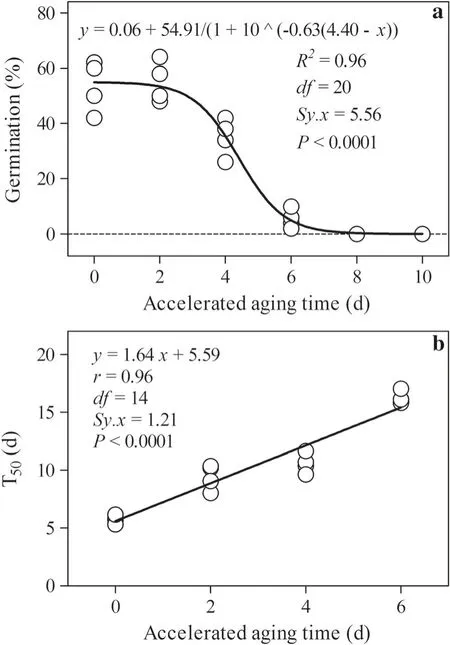
Fig. 2 Changes in germination percentage(a)and germination speed(b) of M. glyptostroboides seeds at 25 °C under the 12-h light/12-h dark photoperiod(PPFD,121 μmol m-2 s-1)after being subjected to accelerated aging for different durations at 40 °C and 100%RH.T50,the time taken to reach 50% of final germination percentage; R2 correlation index,r correlation coefficient,df degree of freedom,Sy.x residual standard deviation, P probability

Fig. 3 Changes in the relative electrical conductivity of leachates from M. glyptostroboides seeds after accelerated aging for different durations at 40 °C and 100% RH. r correlation coefficient, df degree of freedom, P probability
Changes in MDA content during accelerated aging treatment
MDA content changed significantly along with the increased accelerated aging treatment duration. It was characterized by an initial increase,followed by a decrease and another increase(Fig. 4).The maximum MDA content was measured in the seeds that had been treated for 4 days,and the lowest MDA content was measured in the seeds that had been treated for 8 days(Fig. 4).The MDA content of the seeds that had been treated for 2 days was not significantly different from that of the control group (Fig. 4).MDA content of the seeds that had been treated for 4 days was significantly greater than that of seeds treated for 2 days but was not significantly different from that of the control group (Fig. 4). MDA contents were similar for seeds treated for 8 days and those treated for 6 days Fig. 4). After 10 days of accelerated aging treatment,MDA content increased did not differ from that of seeds treated for 2 and 4 days or from that of the control group(Fig. 4).
Changes in SOD, CAT, APX, and DHAR activities during accelerated aging treatment
As the duration of the accelerated aging treatment increased, SOD activity exhibited a significant decreasing trend (Fig. 5a), and CAT activity changed significantly,characterized by,an initial increase followed by a decrease(Fig. 5b). APX activity did not change significantly(Fig. 5c), whereas DHAR activity did and indicated an initial decrease followed by an increase (Fig. 5d).
SOD activity of seeds treated for 2 days was significantly lower than that of the control group and was significantly higher than that of seeds treated for 4 days,which exhibited the lowest value (Fig. 5a). SOD activity levels were similar for seeds treated for 4,6,8,and 10 days(Fig. 5a).
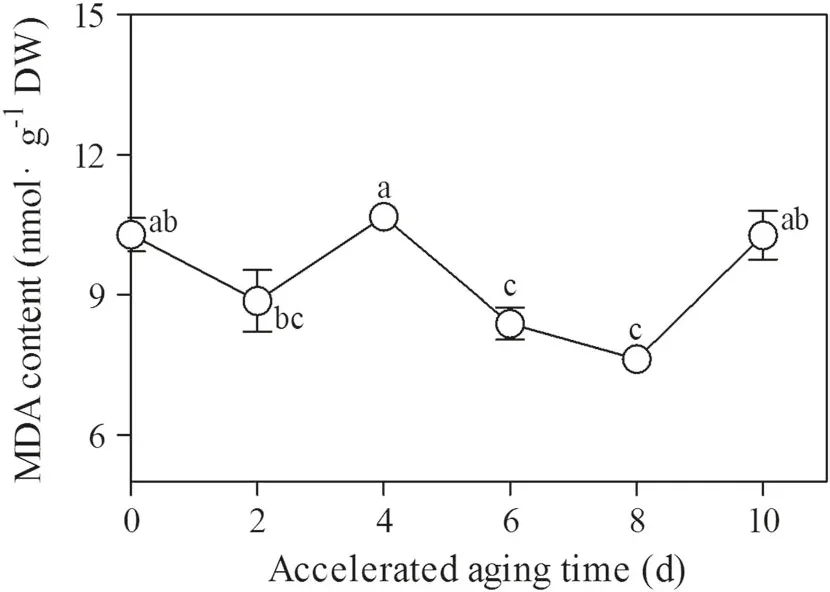
Fig. 4 Changes in MDA content in the embryonic root cells of M.glyptostroboides seeds after accelerated aging treatment for different durations at 40 °C and 100% RH. Values indicated by different lowercase letters differed significantly (S-N-K, P = 0.05)
The highest CAT activity was measured in seeds treated for 4 days. This value was significantly higher than that of the control group and that of seeds treated for 6 and 10 days, but was similar to that of seeds treated for 2 and 8 days(Fig. 5b).The lowest CAT activity was measured in seeds treated for 6 days;however,this value was similar to that of the control group and that of seeds treated for 10 days (Fig. 5b).
The lowest DHAR activity was recorded for seeds treated for 2 days. This value was significantly lower than that of the control group and the other treated seeds(Fig. 5d). DHAR activity of seeds treated for 4 days was significantly higher than that of seeds treated for 2 days and was significantly lower than that of seeds treated for 6,8, and 10 days. However, DHAR activity levels of all treated groups were similar to that of the control group(Fig. 5d).DHAR activity was similar for seeds treated for 6 and 8 days. These values were significantly lower than those of seeds treated for 10 days and significantly higher than thoses of the control group and the other treated seeds(Fig. 5d). Highest DHAR activity was recorded for seeds treated for 10 days and was significantly higher than that of the control group and all other treated seeds (Fig. 5d).
Germination was negatively correlated with elative electrical conductivity(r = - 0.852,P <0.01)and DHAR activity (r = - 0.851, P <0.01), and was positively correlated with MDA content (r = 0.387) and SOD activity(r = 0.600, P <0.01). Relative electrical conductivity was negatively correlated with SOD activity (r = - 0.609,P <0.01) and APX activity (r = - 0.383) and positively correlated with DHAR activity (r = 0.800, P <0.01).MDA content was positively correlated with APX activity(r = 0.353) and SOD activity was negatively correlated with CAT activity (r = - 0.393) but positively correlated with APX activity (r = 0.347, P <0.05, Table 1).
Changes in subcellular structure during accelerated aging treatment
Structures recorded in the control group were: normal embryonic root cells with a large variety oforganelles,including a distinct cytoplasmic membrane; a small,irregularly shaped nucleus without an apparent nucleolus;numerous approximately round or oval protein bodies with or without crystalline materials; small, dense lipid bodies either encircling other organelles such as protein bodies or appressed against the cytoplasmic membrane; and a number of amyloplasts with large starch granules (Fig. 6a).Mitochondria and glyoxysomes were not observed but were apparent in the embryonic root cells of aged seeds(Fig. 6b, c).

Fig. 5 Changes in SOD (a), CAT (b), APX (c), and DHAR(d) activities of embryonic root cells of M. glyptostroboides seeds after accelerated aging treatment for different durations at 40 °C and 100% RH. Values indicated by different lowercase letters differed significantly (S-N-K, P = 0.05)

Table 1 Correlation analysis among germination, relative electrical conductivity, MDA content, and SOD, CAT, APX, and DHAR activities(one-tailed test)
Structures recorded in seeds aged for 2 and 4 days were:the cell walls of the embryonic root cells were folded, and there were many small intercellular spaces between neighboring cells because they were separated from each other; the cytoplasmic membrane appeared normal; the nucleus was slightly enlarged and approximately round with an apparent nucleolus; there were fewer but larger lipid bodies compared with the control; there were fewer and smaller protein bodies with more and larger crystalline materials in comparison with the control; numerous mitochondria and a few glyoxysomes were recorded; and the glyoxysomes were adjacent to the mitochondria and lipid bodies (Fig. 6b, c).
Structures observed in seeds aged for 6 days were:large intercellular spaces; a distinct cytoplasmic membrane; no nucleus;larger and fewer lipid bodies;and more and larger crystalline materials in protein bodies compared with those in Fig. 6a-d.
In the seeds aged for 8 days, the cells began to break down, and the cell walls of only a few cells were essentially intact; the cytoplasmic membranes were indistinct;and all organelles had broken down (Fig. 6e).
In the seeds aged for 10 days,further degradation of the cells was noted, and most of the cells were indistinguishable (Fig. 6f).
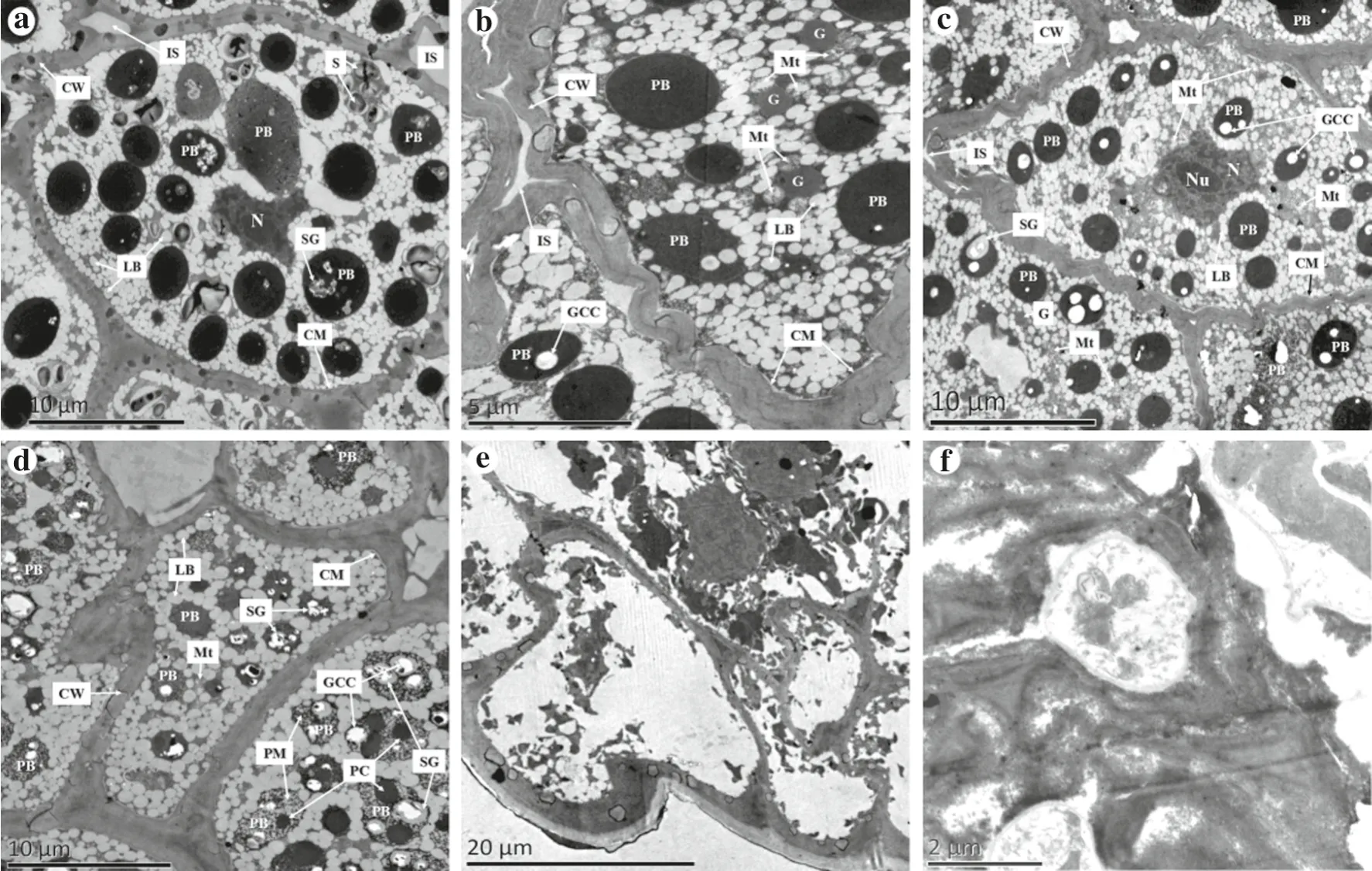
Fig. 6 Changes in the ultrastructure of embryonic root cells of M.glyptostroboides seeds after accelerated aging treatment for different durations at 40 °C and 100% RH. a Control; b aging treatment for 2 d; c aging treatment for 4 d; d aging treatment for 6 d; e aging treatment 8 d; f aging treatment 10 d. CW cell wall, IS intercellular space, PB protein body, N nucleus, LB lipid body, CM cytoplasmic membrane,S starch granule,Mt mitochondrion;G glyoxysome,GCC globoid crystal cavity, SG soft globoid, DC druse crystal
Discussion
Previous research showed that the average germination percentage of freshly harvested M. glyptostroboides seeds was only(33 ± 3)%(Li et al.2012),which is equivalent to that of seeds of two other endangered conifers, namely Cupressus gigantea (35%; Wang et al. 2005) and Cathaya argyrophylla (31%; Cao et al. 2010). It is alsofar lower than that of the seeds of two non-endangered conifers,
Pinus pinea (Sidari et al. 2008) and P. halepensis
(Olofinboba and Kozlowski 1982) at 100% and 84.3%,respectively. In the present research, the average germination percentage of fresh, mature M. glyptostroboides seeds only reached (56.5 ± 5.6)% (all ungerminated seeds rotted), even at the optimum temperature level (Fig. 1).Therefore, these results appear to suggest that M. glyptostroboides seeds naturally have low germination potential and vigor, which may contribute to poor seedling recruitment under natural conditions.
Accelerated aging experiments can be used to evaluate and predict the aging and persistence of seeds in the field(Long et al. 2008). M. glyptostroboides seeds exhibited poor capacity for maintenance of viability, with the average germination percentage decreasing from(53.5 ± 4.6)% in the control group to (5.5 ± 1.7)% after 6 days of accelerated aging treatment at 45 °C and 100%RH (Fig. 2a). This finding may explain the poor seedling recruitment in the field. In contrast, under the same accelerated aging conditions, the average germination percentage of macaw palm (Acrocomia aculeata) seeds decreased from approximately 90% in the control group to approximately 80% (Barreto and Garcia 2017), and that of hybrid cucumber (Cucumis sativa) seeds decreased from 100 to 88% (Krainart et al. 2015) after a 12-day aging treatment.
During aging,seeds progressively deteriorate as a result of ROS production, lipid peroxidation, cytoplasm membrane injury, and changes in the activity of antioxidative enzymes (Hu et al. 2012; Xin et al. 2014; Wang et al.2015). ROS have multiple conflicting roles in plant physiology, e.g., in the positive regulation of biological processes (Bailly et al. 2008; Miller et al. 2008) such as seed germination (El-Maarouf Bouteau and Bailly 2008), and the negative regulation of seed vigor during aging (Bailly 2004) as a result of the oxidative damage of various biomolecules, which ultimately leads to cell death (Bailly et al. 2008; Munné-Bosch et al. 2011). Conversely, the antioxidative defense system, especially antioxidative enzymes,can scavenge excess ROS to a certain extent and can therefore protect slightly aged seeds from oxidative damage (Xia et al. 2015). Consequently, the efficiency of the antioxidative enzyme system is strongly indicative of seed vigor. In the present research, the antioxidative enzyme system of M. glyptostroboides seeds was almost completely broken down during aging, and only CAT and DHAR appeared to play a minor role in protecting the aged seeds from oxidative damage.The activity of CAT initially increased and then decreased (Fig. 5b), which indicated that CAT could play a protective role in the initial stage of aging. The activity of DHAR significantly decreased and then progressively increased with aging treatment(Fig. 5d)and was significantly negatively correlated with the average germination percentage of the age-treated seeds(P <0.01, Table 1). As DHAR demonstrates some protective role,it could be used as an index of seed vigor in M.glyptostroboides. The activity of APX did not change significantly during the aging treatment (Fig. 5c), which suggests that APX did not have any effect against aging.The activity of SOD rapidly decreased in the initial stage of aging and was then maintained at the lowest level without any significant change (Fig. 5a). SOD activity was significantly positively correlated with the average germination percentage of aged seeds (P <0.01, Table 1), which suggests that SOD did not have any protective role in oxidative damage and could be used as an index of seed vigor in M.glyptostroboides.In the antioxidative enzyme system,each enzyme acts in sequence and SOD is the most important among all antioxidative enzymes because it controls the initial stage of the chain reaction involved in ROS scavenging, being responsible for the dismutation of O-2to H2O2(Matamoros et al. 2013). Subsequently, H2O2is completely removed by CAT from the water-water cycle,the ascorbate-glutathione cycle, and the GPX cycle (Mittler 2002). Therefore, the change in SOD activity during the aging of M. glyptostroboides seeds indicated rather poor seed vigor. When the antioxidative defense system cannot efficiently scavenge excess ROS produced during aging,membrane lipid peroxidation followed by membrane injury occurs(Sharma et al.2012).These responses can be evaluated by determining the MDA content (product of lipid peroxidation) and the relative electrical conductivity of seed leachates(Goel et al.2003).In the present research,the MDA content of M. glyptostroboides seeds initially decreased and then increased with aging treatment, which was contrary to the general CAT activity trend, but there was a hysteresis effect in terms of time.The MDA content was at its minimum after 6 days of treatment, while CAT activity peaked after 4 days. This hysteresis effect may explain why the correlation between MDA content and CAT activity was not significant (r = 0.106, P >0.05,Table 1). Conversely, relative electrical conductivity was significantly negatively correlated with average germination percentage (r = -0.852, P <0.01), SOD activity(r = -0.609, P <0.01), and APX activity (r = -0.383,P <0.05), and significantly positively correlated with DHAR activity (r = 0.800, P <0.01) in the age-treated seeds. Therefore, it seems that the relative electrical conductivity can also be used as an index of the vigor level in M. glyptostroboides seeds. Interestingly, there was no apparent trend in the MDA content along with the increasing duration of the accelerated aging treatment(Fig. 4), but MDA content was significantly positively correlated with average germination percentage(r = 0.387)and APX activity(r = 0.353).Here,APX probably acted as a signaling molecule in response to oxidative damage.The positive correlation between MDA content and average germination percentage could not be well explained at present and warrants further study.
Seed deterioration induced by aging can also be observed at the subcellular structural level (Xia et al.2015). In the present research, as aging progressed, the lipid bodies degraded and tended to merge together into a larger lipid group (Fig. 6d), similar to the observations reported by Walters et al. (2004). In addition, the protein bodies degraded, and the crystalline substances within them increased in number and size (Fig. 6c, d).This result differs from that of Walters et al. (2004) and may be attributed to the varying degrees of aging; ultimately, the whole cell degraded completely(Fig. 6e,f).This may have occurred because seed respiration increased with aging treatment under high temperature and humid conditions,and hence the organelles involved in respiration and lipolysis, such as mitochondria and glyoxysomes, were observed and increased during the middle stage of aging(Fig. 6c). The lipid bodies decreased in number and merged into a larger group as a result of degradation. In addition, the production of new organelles, such as mitochondria and glyoxysomes,requires numerous proteins and amino acids that originate from proteins; therefore, the number of protein bodies decreased.In contrast,the change in the subcellular structure was not consistent with the change in seed viability observed during the aging process.For example,the subcellular structure of seeds subjected to 6 days of aging treatment was intact, whereas the seed viability was almost completely lost, decreasing to(5.5 ± 1.7)%.
Conclusions
Seed vigor of M.glyptostroboides was low with a naturally low average germination percentage. The capacity to maintain vigor was also poor in comparison with other seeds. This can probably be attributed to its inadequate enzymatic antioxidative system, which might account for the low survival rate of this species.
AcknowledgementsThis work was supported by the Research Project of Hubei Provincial Department of Education (Q20141902). We thank LetPub (www.letpub.com) for providing linguistic assistance during the preparation of this manuscript.
Compliance with ethical standards
Conflict of interestThe authors declare that they have no conflict of interest.Huan Liu and Yanfang Zhu contributed equally to this work.
杂志排行
Journal of Forestry Research的其它文章
- Do increasing respiratory costs explain the decline with age of forest growth rate?
- At what carbon price forest cutting should stop
- Mapping the risk of winter storm damage using GIS-based fuzzy logic
- Effects of seed moisture content, stratification and sowing date on the germination of Corylus avellana seeds
- Comparison of seed morphology of two ginkgo cultivars
- De novo assembly of the seed transcriptome and search for potential EST-SSR markers for an endangered, economically important tree species: Elaeagnus mollis Diels
