Portal stent with endovascular brachytherapy improves the efficacy of TACE for hepatocellular carcinoma with main portal vein tumor thrombus
2020-05-10TianLiChongLiuJinTongHeKaiDaSuiZhouBoZhangDuoHongHongYingSuHaiBoShao
Tian Li, Chong Liu, Jin-Tong He, Kai-Da Sui, Zhou-Bo Zhang, Duo Hong, Hong-Ying Su,Hai-Bo Shao
Department of Interventional Radiology, the First Hospital of China Medical University, Shenyang 110001, China
Hepatocellular carcinoma (HCC) with portal vein tumor thrombus (PVTT) yields poor prognosis with a median overall survival(OS) of 2.7-4 months [1] . Once PVTT develops in the main portal vein, the sudden appearance of portal hypertension often leads to fatal complications, including esophagogastric variceal hemorrhage,refractory ascites, and liver failure. Transarterial chemoembolization (TACE) has been recommended for advanced HCC patients by China Liver Cancer Group due to its benefit of OS compared with using sorafenib alone [2] . For the treatment of PVTT, portal stent with endovascular iodine-125 brachytherapy (PSEIB) was recently reported to be effective because it relieved portal hypertension rapidly and controlled PVTT effectively with an improved OS of 9.3-12.5 months [3 , 4] . The combination of PSEIB and TACE had been applied in some studies [3 , 4] . Nevertheless, these studies failed to demonstrate the effect of PSEIB on TACE results. Herein we aimed to present a case how PSEIB improves the efficacy of TACE for HCC patients with main PVTT.
All procedures performed in this study were in accordance with the ethical standards of the institutional review board. A 47-year-old male patient with a 20-year history of hepatitis B presented with abdominal distention for one week before he visited the institutional liver disease clinic. He had no other disease histories. An enhanced computed tomography (CT) scan revealed a 4 × 5 × 7 cm mass in the left lobe and a 3-cm mass in the right lobe with the left portal thrombus extending into the main portal vein ( Fig. 1 A). Both tumors were dramatically enhanced in the arterial phase and exhibited “washed out”characteristics in the vein phase. Hyper-metabolism was present in both tumors [max standard uptake value (SUV max ): 18.2] and portal thrombus (SUV max :17.4) in positron emission tomography (PET)/CT images, indicating malignant characteristics ( Fig. 1 B). The alpha fetoprotein (AFP)value of the patient was greater than 1210 IU/mL (maximum detecting threshold), and liver function evaluation classified the patient as Child-Pugh A. A clinical diagnosis of BCLC C-stage HCC was made based on HBV history, image characteristics, AFP value and liver function. The patient refused percutaneous biopsy for pathology diagnosis. An institutional liver cancer multidisciplinary team (MDT) discussed possible treatments. Sorafenib was recommended as the first line treatment; however, the patient refused it because sorafenib was not covered by his medical insurance. After informed consent was obtained, a conventional TACE with an emulsion of 40 mg epirubicin and 15 mL lipiodol was administered.However, a large amount of lipiodol entered the portal branches instead of deposition in the tumors and the thrombus, although no obvious aterioportal shunt (A-P shunt) was noted on angiography ( Fig. 1 C&D). One-month follow-up CT revealed unsatisfactory lipiodol deposition in the tumors ( Fig. 1 E). After discussions among MDT and approval by the institutional review board, a portal vein stent plus iodine-125 seed strand was placed to prevent possible complications from portal hypertension and control tumor thrombus. Angiography revealed complete obstruction of the main portal vein and a large amount of varices ( Fig. 2 A). A 14 ×60 mm self-expandable stent (S.M.A.R.T.R○, Cordis, Califonia, USA) and 18 iodine-125 seeds (CIAE-6711, 25.9 MBq with a half-life of 59.4 days,Atom-hitech, Beijing, China) that were sealed in a segment of 4-french catheter (TERUMO, New Jersey USA) were placed across the thrombus from the main to right portal vein. Forward portal blood flow was rebuilt, and the varices disappeared ( Fig. 2 B). Two weeks later, a second TACE was administered which was much more effi cient: the drug was well deposited in the left-lobe tumor without flowing into portal branches (the drug-delivering catheter was located at the same position as the first TACE) ( Fig. 3 A&B). CT images of the 3-month follow-up revealed dense lipiodol deposition in the tumor ( Fig. 4 A), and the sum of the tumors’ diameters was reduced from 12 cm to 8 cm. According to mRECIST evaluation criteria, a partial response was observed. In the following treatments,6 times of additional TACE (based on the presence of arterial-phase enhancement) were performed according to the follow-up images.The indirect portal angiographies continually revealed patency of the stent and fluency of blood flow in the main and right portal vein ( Fig. 4 B). Finally, the progression-free survival (PFS) was 23 months, and the OS was 28 months.

Fig. 1. Images before and during the first TACE and follow-up. A: An enhanced CT image of portal-phase shows the wash-out mass (arrow) in the left lobe of liver, with thrombus in the left (pound) and main (asterisk) portal vein; B: PET/CT image shows hypermetablism in both tumor and portal thrombus; C: Hepatic angiography in TACE shows “tumor staining”in both lobes (arrows) and portal thrombus (asterisk); D: Post-TACE single shot image shows lipiodol flowing into the portal branches throughout the liver instead of depositing in tumors; E: The follow-up CT one month after the first TACE shows unsatisfied dotted lipiodol deposition (arrow heads).
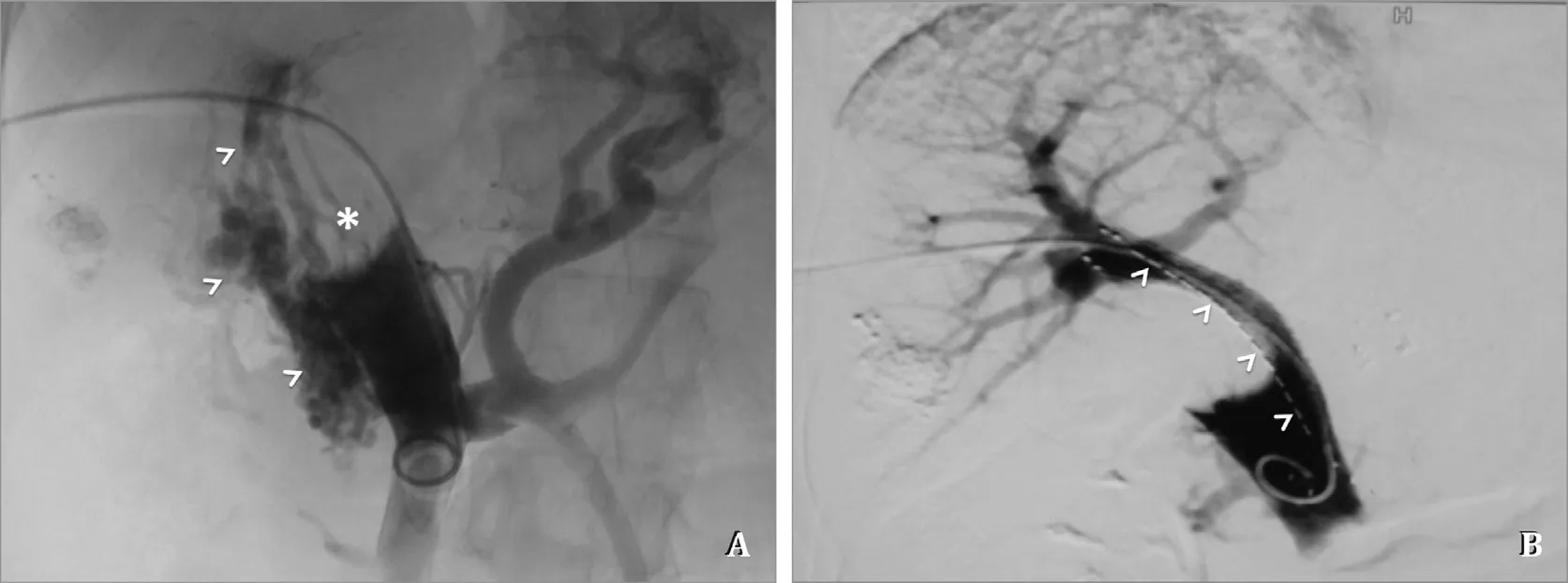
Fig. 2. Images in the procedure of PSPEIB. A: Direct portal angiography shows complete obstruction of main portal vein by thrombus presenting as filling defect (asterisk),with formation of collateral vessels (arrow heads); B: The portal blood flow is rebuilt by PSPEIB. The arrow heads shows the iodine-125 seeds strand.
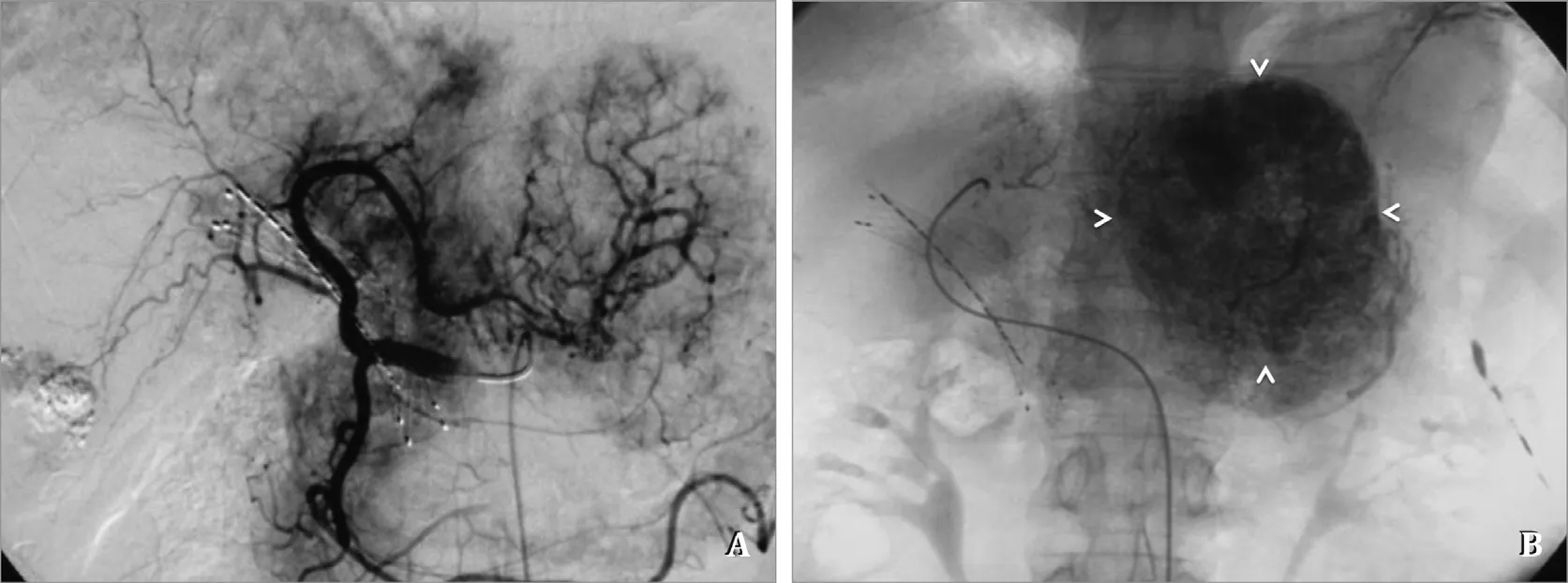
Fig. 3. Second TACE procedure after PSPEIB. A: Hepatic angiography still shows tumor staining; B: Post-embolization single shot image shows lipiodol deposition (arrow heads) was much better than the first TACE (compared to Fig. 1 E).

Fig. 4. Images after PSPEIB and the second TACE. A: The follow-up CT 2 months after the second TACE shows satisfied lipiodol deposition (arrow heads); B: The indirect portal angiography image shows fluent blood flow in the portal vein (arrow heads).
A-P shunt is commonly involved in advanced HCC with an incidence as high as 61% when combined with PVTT [5] . PVTT can cause an A-P shunt by both reducing portal flow and placing the tumor vasculature in the thrombus. For conventional lipiodol TACE,the drug-lipiodol emulsion is approximately 50μm in size, which easily passes through A-P shunts into portal branches instead of depositing in HCCs, leading to failure of TACE. In this case, no direct A-P shunt was observed in hepatic angiography, but we did notice the vessel plexus around the portal vein as shown in Fig. 1 D.Then, lipiodol passed into portal branches through these transvasal routes.
Transhepatic arterial embolizations with ethanol, gelfoam, biogel, or coils have been used to block A-P shunts [6] . Nevertheless, these embolization materials blocked the A-P shunt as well as tumor-feeding arteries, which potentially decreased the efficacy of TACE. External beam radiation therapy (EBRT) was also used to improve TACE in HCC patients with PVTT and A-P shunt [5] , which was generally time-consuming and could excessively radiate normal liver. As a result, the ideal period for TACE treatment might be lost in some patients. China Food and Drug Administration approved iodine-125 radioactive particles for the treatment of solid malignant tumors (lung, liver, bone, etc.) and luminal malignant tumor (esophagus, bile duct, trachea, etc.) [7] . PSEIB was employed recently for the treatment of PVTT and achieved satisfactory clinical results. Nevertheless, our case for the first time demonstrated that the efficacy of TACE was greatly improved by PSEIB in such a short period. Unlike EBRT, brachytherapy can continuously deliver radiation in a local PVTT region and provide a high dosage of accumulative radiation (466.2 MBq in this case), controlling PVTT and preventing the stent from obstruction by tumor growth. As the PVTT shrinks, the A-P shunt was greatly alleviated after PSEIB, and the tumor exhibited satisfactory lipiodol deposition in this case.We also concluded that a good PFS (23 mon) and OS (28 mon) for this advantage-stage HCC patient was attributed to the enhanced efficacy of TACE improved by PSEIB.
CRediT authorship contribution statement
Tian Li:Data curation, Formal analysis, Writing - original draft.Chong Liu:Conceptualization, Writing - review & editing.Jin-Tong He:Conceptualization, Writing - review & editing.Kai-Da Sui:Conceptualization, Writing - review & editing.Zhou-Bo Zhang:Conceptualization, Writing - review & editing.Duo Hong:Investigation, Writing - original draft.Hong-Ying Su:Investigation, Writing - original draft.Hai-Bo Shao:Conceptualization, Funding acquisition, Supervision, Writing - review & editing.
Funding
This study was supported by grants from the National Natural Science Foundation of China ( 81771944 ) and the National Hi-Technology Research and Development Program ( 863 Program )( 2012AA022701 ).
Ethical approval
The consent for publication was obtained from the reported patient.
Competing interest
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- MEETINGS AND COURSES
- Liver transplantation for intrahepatic and perihilar cholangiocarcinoma: Current and future
- Pancreatic schwannoma: Imaging features and pathological findings
- Rare giant asymptomatic skull metastasis from intrahepatic cholangiocarcinoma
- Lifesaving transarterial embolization using absorbable gelatin sponge particles for massive bleeding of ruptured metastatic hepatic melanoma
- Lumen-apposing metal stent deployment for walled-off necrosis using semi-free hand technique from the intestine
