Pancreatic schwannoma: Imaging features and pathological findings
2020-05-10HnWngBingBingZhngShenFnWngJingJioZhongJinMingZhengHunHn
Hn Wng , Bing-Bing Zhng , Shen-Fn Wng , Jing-Jio Zhong , Jin-Ming Zheng ,Hun Hn , *
a Department of Pathology, Eastern Hepatobiliary Surgery Hospital, Second Military Medical University, Shanghai 200438, China
b Department of Pathology, Changhai Hospital, Second Military Medical University, Shanghai 200433, China
c Department of Urology, Changhai Hospital, Second Military Medical University, Shanghai 200433, China
d Department of Imaging, Changhai Hospital, Second Military Medical University, Shanghai 200433, China
TotheEditor:
Schwannoma is an infrequent tumor originating from Schwann cells of the peripheral nerve sheath and was first reported as a true neoplasm originating from Schwann cells in 1910 [1] . The majority of schwannomas are detected as a solitary tumor from the peripheral nerves of the face, neck, trunk, extremities, or retroperitoneal region. Based on a previous study [2] , merely 3% of schwannomas are retroperitoneal, accounting for approximately 4% of all retroperitoneal tumors. Furthermore, pancreatic schwannomas that stem from either parasympathetic or sympathetic fibers of the pancreas are especially rare. Schwannomas are generally encapsulated, and over 90% are benign [3] . Considering its benign biological behavior, it is essential to accurately diagnose schwannomas in order to apply reasonable surgical methods and postoperative surveillance. Herein, we report four cases of pancreatic schwannoma in our center to update the data on the clinicopathological traits about this type of tumor.
Between April 2016 and December 2017, four consecutive patients underwent surgical resection at Changhai Hospital in Shanghai, China, and the data were retrospectively evaluated. All patients were ultimately diagnosed with pancreatic schwannoma based on the modified WHO definition [4] . We extracted preoperative images and surgical records from the relevant medical records. The pathological reports and macroscopic and microscopic appearances, as well as immunohistochemical features were reviewed and identified by two experienced pathologists (Wang H and Han H). The follow-up information was censored through December 2018. A Leica ASP300S Fully Enclosed Tissue Processor(Leica, Darmstadt, Germany) was used to dehydrate the surgical specimens for 12 h after sampling. Thereafter, the specimens were embedded in paraffin, and 4-μm thick sections were cut to stain with hematoxylin and eosin (HE) for routine histopathological examination. Immunohistochemical slices were stained with an automated immunostainer (Leica BOND-MAXTM, Darmstadt, Germany). The antibodies used in this study are shown in Table 1 ,and all are produced by Abcam (Cambridge, UK).
The baseline characteristics of all patients are shown in Table 1 .One male and three females were included. The age ranged from 44 to 55 years, with a median age of 51.5 years. One patient had mild clinical symptoms of abdominal pain, and the other 3 were discovered haphazardly. The imaging findings showed that all patients had solitary tumors. The median tumor size was 3.5 cm(range 2.0-5.8). Two were located in the pancreatic head and body, one in tail, and one in uncinate. Magnetic resonance imaging showed that the tumors of patient 2 and patient 3 compressed the neighboring vessels, and all the masses had well-defined margins( Fig. 1 A). In addition, there were no calcification and dilatation of the pancreatic duct. Referring to the imaging reports, the patients were diagnosed with solid pseudopapilloma, mucous cystadenoma,serous cystadenoma, and neurogenic tumors (possible pancreatic schwannomas), respectively. Endoscopic ultrasound-guided fineneedle aspiration (EUS-FNA) was performed on two patients. Only one patient received local resection after a pathological diagnosis of frozen sections. All patients recovered gradually after the initial operative treatment without complications. During the regular follow-up, neither recurrence nor metastasis occurred in any patient. The median follow-up interval was 16 months (range 13-33).
The pathological gross appearance demonstrated two solid masses, one was a solid cystic mass, and one had a multilocular and cystic cut surface containing yellowish clear liquid and gelatinous substances ( Fig. 1 B). Microscopically, the tumors were mainly composed of spindle-shaped cells. Typical histological morphology included an organized hypercellular component (Antoni A areas)and a hypocellular component with loose myxoid stroma (Antoni B areas). The cells in the Antoni A area were arranged in bundles and fences, and the cells in the Antoni B area were star-like. Tumor cells had abundant cytoplasm and light eosinophilic staining,as well as no obvious nuclear atypia or mitotic figures ( Fig. 1 C). A clear boundary was observed between tumor tissue and the adjacent pancreas in all patients. The spindle-shaped cells were immunopositive for S-100 ( Fig. 1 D), vimentin, ABC,β-tubulin, and SOX-10. In addition, the cells were negative for NGFR, SMA, Dog-l,EMA, and Ki-67.
Schwannomas are mesenchymal neoplasms derived from Schwann cells of peripheral nerve sheaths and do not contain neuroganglion cells [5] . As such, the pancreas is an atypical site of origin for schwannomas, which arise from either the parasympathetic or sympathetic nervefibers passing through the pancreas.Pancreatic schwannoma still lacks a clear differential diagnosis, so it is prone to misdiagnosis in clinical practice. Given its noninvasive biological behaviors, simple enucleation is usually sufficient if pathology is conclusive before surgery [6] . However, based on the latest literature review, few patients (12%) underwent local excision because of an imprecise diagnosis [7] . Consequently, further experience about pancreatic schwannomas is necessary to assist in its pre- and postoperative management.
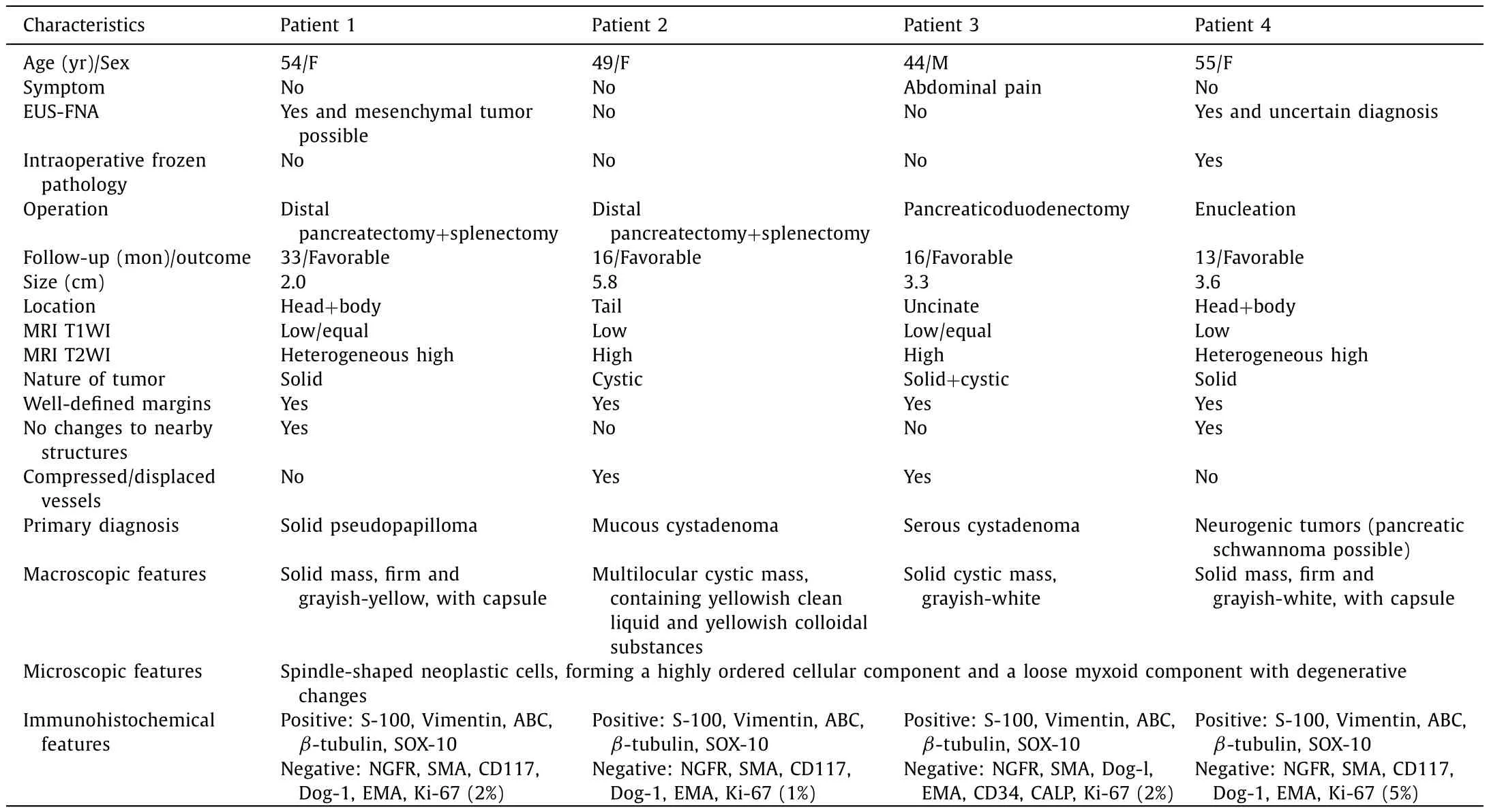
Table 1Baseline characteristics of 4 patients.
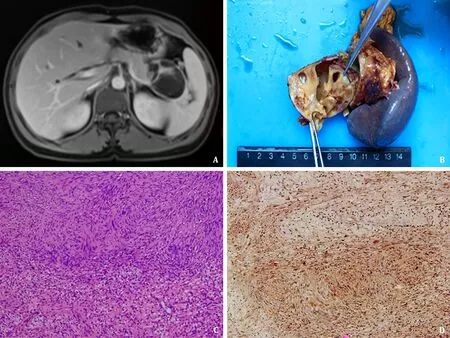
Fig. 1. A: Imaging findings showed the mass had a well-defined margin and compressed the vessel; B: Pathologic gross specimen showed the mass had a multilocular cystic surface; C: The tumor cells arranged as interlacing and palisading patterns (hematoxylin and eosin staining, original magnification ×100); D: Immunohistochemical studies indicated S-100 was positive (streptavidin peroxidase conjugated method, original magnification ×100).
We analyzed four cases of pancreatic schwannomas diagnosed in our center. We found that this kind of tumor was easily misdiagnosed as pseudopapilloma or cystadenoma to some extent. Remarkably, a recent study [8] reported the successful experience of using EUS-FNA to diagnosis pancreatic schwannoma. In our study,two patients underwent EUS-FNA due to the suspicion of imaging diagnosis, while no one obtained a certain diagnosis. Because of the diagnosis based on tissue acquired by EUS-FNA is not conclusive, distal pancreatectomy was performed in one patient. Interestingly, examination of frozen sections was conducted on other patient, and a presumptive diagnosis was achieved during operation. Hence, this patient underwent an enucleation. Considering the possibility of schwannoma enlargement, we think that pancreatic biopsy plus conservative treatment may be insufficient for this rare tumor due to its limitation of obtaining only a small amount of tumor tissue and depending on the experience of pathologists.Accordingly, in addition that EUS-FNA is recommended to assist in diagnosis of pancreatic schwannoma, when the diagnosis based on EUS-FNA is not clear, intraoperative freezing is a useful supplement. Since our suggestions are based on case experience,combined using of EUS-FNA and intraoperative frozen section examination to assist in the diagnosis of pancreatic schwannoma is valuable to be verified with a large, multicenter research.
For pathological diagnosis, it is crucial to differentiate the correct diagnosis and to judge the biological behavior of a tumor. Microscopically, classical pancreatic schwannomas appear to consist of two distinctive areas: Antoni A and Antoni B areas.The Antoni A area is featured by a hypercellular region of closely packed, long bipolar cells (spindle cells) arranged in interlacing and palisading fashions. Verocay bodies without mitoticfigures can also be observed [9] . Conversely, loose hypocellular regions exhibiting degenerative changes such as hemorrhage, calcification,hyalinization, xanthoma infiltration, and cyst formation are characteristic of the Antoni B area. Immunohistochemically, pancreatic schwannomas diffusely and strongly stain positive for S-100 protein, vimentin, ABC,β-tubulin, and SOX-10. Meanwhile, spindle cells stain negative for NGFR, SMA, CD117, Dog-1, EMA, CD34, and CALP [8] . Regarding the biological behavior of pancreatic schwannoma, a previous study demonstrated that a tumor size larger than 6.90 cm, vascular encasement, or visceral invasion should elicit suspicion of malignant transformation [7] . These parameters should be applied into the imaging and pathological diagnosis of pancreatic schwannoma. Interestingly, we found that the expression of Ki-67 was low in our four cases, yielding a great prognosis,which was consistent with that in a previous study [10] . Although it is a limitation that the follow-up period of these four patients was relatively short, the absence of short-term recurrence still indicated the effectiveness of Ki-67 in judging the malignancy of this tumor to some extent. For this reason, we recommend Ki-67 to classify the biological behavior of pancreatic schwannoma.
In conclusion, the detection of pancreatic schwannoma is extremely rare. Over the past four decades, only 71 cases of pancreatic schwannoma have been reported in the English literature [7,9,11,12] . Although multiple imaging modalities are currently available, it is challenging to make an accurate diagnosis before operation. Based on our experience, the combination of EUS-FNA and intraoperative freezing is helpful to determine the surgical scope of a pancreatic schwannoma. Immunohistochemistry can facilitate in determining the final diagnosis and help evaluate the malignancy of the tumor.
CRediT authorship contribution statement
Han Wang:Writing - original draft, Data curation, Formal analysis, Investigation.Bing-Bing Zhang:Writing - original draft, Formal analysis.Shen-Fan Wang:Data curation.Jing-Jiao Zhong:Data curation.Jian-Ming Zheng:Conceptualization, Writing - review & editing.Huan Han:Conceptualization, Funding acquisition,Methodology, Writing - review & editing.
Funding
This study was supported by a grant from the Youth Start-up Fund Project of Changhai Hospital (CH201823).
Ethical approval
This study was approved by the Ethics Committee of Changhai Hospital of Second Military Medical University. Consent for publication was obtained from all the patients.
Competing interest
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- MEETINGS AND COURSES
- Liver transplantation for intrahepatic and perihilar cholangiocarcinoma: Current and future
- Rare giant asymptomatic skull metastasis from intrahepatic cholangiocarcinoma
- Portal stent with endovascular brachytherapy improves the efficacy of TACE for hepatocellular carcinoma with main portal vein tumor thrombus
- Lifesaving transarterial embolization using absorbable gelatin sponge particles for massive bleeding of ruptured metastatic hepatic melanoma
- Lumen-apposing metal stent deployment for walled-off necrosis using semi-free hand technique from the intestine
