Different characteristics of chronic dibutyltin dichloride-induced pancreatitis and cholangitis in mouse and rat
2020-05-10FriederikettLuiseEhlersHorstNizzeRoertJster
Friederike Lütt , Luise Ehlers , Horst Nizze , Roert Jster , *
a Department of Medicine II, Division of Gastroenterology, Rostock University Medical Center, E.-Heydemann-Str. 6, 18057 Rostock, Germany
b Institute of Pathology, Rostock University Medical Center, Strempelstraße 14, 18057 Rostock, Germany
ABSTRACT
Keywords:
Animal models
Cholangitis
Pancreatitis
Introduction
Chronic pancreatitis (CP) is a syndrome that is morphologically characterized by progressive and irreversible morphological changes of pancreatic architecture, and clinically by signi ficant abdominal pain, development of exocrine and endocrine organ failure, and reduced life expectancy [1 , 2] . In the past decades,various animal models of CP have been introduced to study the pathophysiology of the disease as well as to test experimental therapies. Briefly, they can be classified, based on their etiology, into the subgroups of mechanic, genetic, immune-based and infectious models, as well as CP caused by biological, chemical and environmental factors [3 , 4] . To this end, none of these models perfectly resembles human CP, although many of them have proven useful to study selected pathomechanistic aspects of the disease. Further restrictions include limitations of availability, requirement of advanced surgical skills, or a lack of specificity (e.g., unassociated extrapancreatic manifestations).
The organotin compound dibutyltin dichloride (DBTC) has previously been shown to induce DBTC-pancreatitis in rats after a single intravenous injection, making its use easy and convenient [5-7] . Moreover, the course of this pancreatitis can be controlled through the dose of DBTC. While 4 mg/kg body weight cause a mild acute pancreatitis with complete regeneration, doses of 6-8 mg/kg trigger a chronification of the disease with a progressive replacement of gland tissue by connective tissue (fibrosis) [5-8] . DBTC exerts its effects on the pancreas both through a biliary obstructive and a direct toxic effect on acinar cells [5-7] . In addition, inflammatory cells (macrophages and T cells) accumulate in the diseased organ and contribute to disease progression [8 , 9] .Bile duct obstruction develops as a consequence of biliary secretion of DBTC, which causes a DBTC-cholangitis with necrosis of the epithelium of small bile ducts followed by an increased proliferation of portal bile ducts [ 6 , 10 , 11 ]. The portal tissue damage also may extend to the liver, where parenchymal necrosis and infl ammatory cell invasion can occur. In summary, the DBTC-model combines relevant pathophysiological features (biliary and hematogenic effects, immunological component,fibrosis) with practical simplicity.
To enhance the value of the DBTC-model, its transfer from rats to mice would be advantageous. Given the much higher availability of genetically engineered mouse models, a transition from rats to mice would facilitate the use of DBTC in the context of defined genetic backgrounds and open new avenues for investigations into the pathophysiology of CP. Unfortunately, the anatomy of the pancreaticobiliary system significantly differs between the two species.Thus, rats are lacking a gallbladder, whereas the mouse pancreas,in contrast to rats, has multiple pancreatic ducts that vary considerably [3] . It has therefore been assumed that the DBTC-model,due to its dependence on bile duct anatomy, is only applicable in rats [4] . Published experimental evidence, however, is largely missing. Moreover, Zhang et al. recently reported a mouse strain,KM mice, where the joint application of DBTC (at 8 mg/kg; single intravenous injection) and ethanol (added at 10% to the drinking water) induced an experimental CP with pathomorphological features that largely resemble the findings in DBTC-exposed rats [12] . Their study prompted us to ask if DBTC alone might be effective as well, and if KM mice could be replaced by C57BL/6 J mice, which are commonly used as a background strain for genetic studies.
Materials and methods
Animal studies
Lewis rats and C57BL/6 J mice (both species from Charles River Laboratories, Sulzfeld, Germany) were kept under standard laboratory conditions and fed a rodent chow diet. Male individuals at an age of approximately 3 months received a single intravenous injection of DBTC at the indicated dose. Therefore, DBTC (Sigma-Aldrich, Deisenhofen, Germany) was first dissolved in 100% ethanol(2 parts), mixed with glycerol (3 parts), and injected very slowly into the tail vein as described for rats before [ 5 , 13 ]. At the indicated points after DBTC injections, animals were sacrificed under anesthesia, and blood and tissue samples were collected and stored under appropriate conditions until they were assayed. From liver and pancreas, formalin- fixed paraffin-embedded (FFPE) material and cryoconserved tissue samples were obtained. In total,23 rats (8 untreated controls, 6 individuals for time point day 7 and 9 animals for day 28; DBTC at 8 mg/kg) and 31 mice (n= 5 each for untreated controls, 6 mg/kg and 8 mg/kg DBTC, andn= 8 for the dose groups 10 mg/kg and 12 mg/kg, respectively) were used in the study.
All experiments were performed according to the guidelines of the local animal use and care committee, which also approved this study. The animals had access to water and chowadlibitum. All animals received humane care according to the German legislation on protection of animals and the Guide for the Care and Use of Laboratory Animals (NIH publication 86-23, revised 1985), and all effort s were made to minimize suffering.
Routine laboratory parameters
Activities of lipase, aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP) and levels of bilirubin were determined using standard laboratory assays.
Histology and immunohistochemistry
Routine hematoxylin and eosin (HE) staining was performed on 4-6μm sections of FFPE material and cryoconserved tissues using standard procedures. Histopathological evaluation of pancreatic and liver morphologies was performed by light microscopy. Severity of hepatobiliary injury was determined on blinded samples,employing a semi-quantitative score from 0 (healthy) to 3 (severe injury) based on the assessment of the following parameters: (a)extension of the affected area, (b) portal hyperemia, (c) portal duct epithelium damage (swelling; cell death), (d) damage to periportal hepatocytes (swelling; cell death), (e) portal/periportal infiltrates of inflammatory cells, (f) proliferation of bile ducts, and (g) increase of portal connective tissue (fibrosis).
Deparaffinated FFPE material and cryoconserved tissues were subjected to the detection of macrophages (liver and pancreas)as well as cytokeratin-7 (CK-7) (staining of bile ducts in liver tissue) as indicated in the figure legends. The following primary antibodies were utilized: anti-CD11b (macrophages in murine specimens; ImmunoTools, Friesoythe, Germany, #221591115), anti-CD163 (macrophages in rat tissues; antibodies-online, Aachen, Germany, Abx1300348), and anti-CK-7 (anti-KRT7; Merck, Darmstadt,Germany, #HPA007272). Antibody binding to murine antigens was visualized using an avidin-biotin-peroxidase complex (ABC) technique [14] , whereas rat tissues were processed employing the alkaline phosphatase anti-alkaline phosphatase (APAAP) technique as described before [13] . Cell nuclei were counterstained with Mayer’s hematoxylin.
For collagen detection in pancreas and liver tissues, cryostat sections (rats) were fixed with Bouin’s solution (Merck) for 1 h at 37 °C, followed by staining with Direct Red 80 (Merck) for 1 h, counterstaining of nuclei with Mayer’s hematoxylin and dehydration by four short incubations in ethanol and xylene (two times each). Deparaffinated samples (mice) were first stained with Weigert’s iron hematoxylin (Merck) for 8 min, followed by Direct Red 80 staining and dehydration as described above. Finally, all histological samples were embedded in Pertex (MEDITE, Burgdorf,Germany).
Statistical analysis
All data were stored and analyzed using the IBM SPSS Statistics 22.0 (IBM Corp., Armonk, New York, USA). Values are expressed as mean ±standard error (SE) for the indicated number of samples (n) per experimental protocol. Mean group differences were checked using analysis of variance (ANOVA). If data did not meet the assumptions for ANOVA, nonparametric analysis of variance was performed employing the Kruskal-Wallis test, before subgroups were tested pairwise using the Mann-WhitneyUtest.Values ofP<0.05 (Bonferroni-adjusted) were considered to be statistically significant.
Results
Clinical and laboratory findings in DBTC-treated mice
In initial experiments, mice received a single intravenous injection of DBTC at 6-8 mg/kg body weight, the same doses that are routinely used to induce experimental CP in rats [5-8] . All 5 mice with a DBTC dose of 6 mg/kg and 3 out of 5 mice with a dose of8 mg/kg reached the designated endpoint at day 28 without clinical symptoms. One mouse had to be sacrificed because of a local bleeding complication related to injection, and only one mouse was sacrificed because of reaching a humane endpoint before day 28. In summary, DBTC at 6-8 mg/kg was tolerated well by most of the animals. In contrast, 15 out of 16 mice that were treated with increased doses of DBTC (10-12 mg/kg) reached humane endpoints on days 2-4 after DBTC-injection and underwent euthanasia in accordance with German law. Thus, there was a sharp border between well tolerated DBTC-doses and highly toxic concentrations( ≥10 mg/kg).
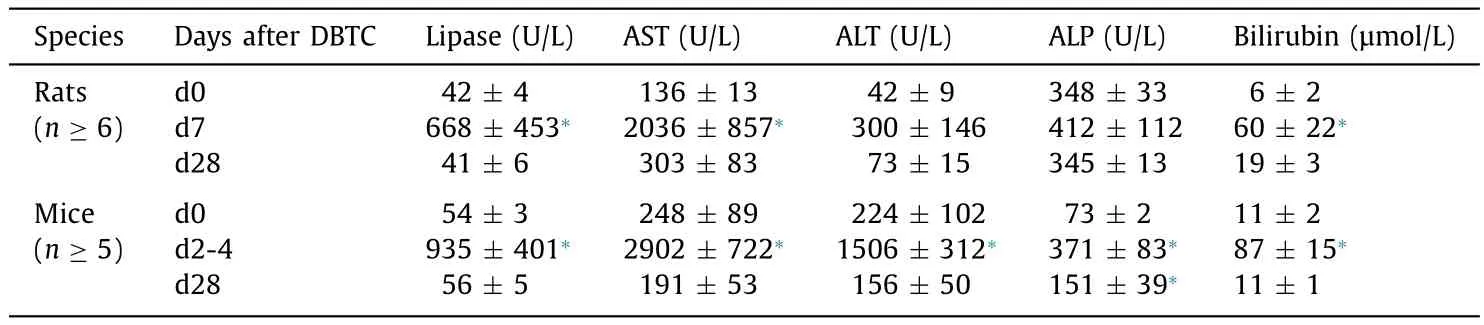
Table 1Laboratory findings in rats and mice exposed to DBTC.
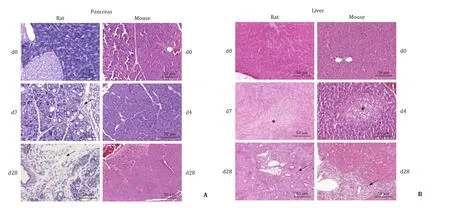
Fig. 1. Histopathology of pancreas and liver from DBTC-treated rats and mice. Rats and mice were either left untreated (day 0), or received a single intravenous injection of DBTC (photographs: rats 8 mg/kg; mice day 4: 10 mg/kg, day 28: 6 mg/kg). FFPE sections of pancreas and liver were subjected to HE staining. A: Pancreatic tissues from rats displayed the typical signs of DBTC-pancreatitis, including acinar cell damage, ductal changes, periductal and interstitial fibrosis (indicated by an arrow), and inflammatory cell infiltrates. Tissues from mice lacked major histopathological changes. B: Liver tissues from both species displayed signs of DBTC-cholangitis, with portal hyperemia and damage of the epithelium of the small bile ducts (swelling and necrosis; indicated by an asterisk). The initial phase of cholangitis (middle panels) was followed by a later phase (lower panels) with regeneration and increased proliferation of small bile ducts (indicated by an arrow).
For direct comparison of paraclinical and histological findings,we extended our studies to rats that received DBTC at 8 mg/kg and survived over the investigation periods of 7 and 28 days, respectively. Both mice and rats presented with significantly increased lipase activities and bilirubin levels at early time points after DBTC-injection ( Table 1 ). Furthermore, increases of AST, ALT and ALP activities were observed, although only in mice these differences were found to be statistically significant. However, it has to be taken into account that the mice of this subgroup, on average, obtained higher doses of DBTC ( Table 1 ) than rats, and were sacrificed because of reaching humane endpoints. At day 28 after application of DBTC at 6-8 mg/kg, in both species largely normalized enzyme activities and bilirubin levels were observed.
Histological findings
Pancreatic histopathology revealed in rats treated with DBTC at 8 mg/kg ( Fig. 1 A) and 6 mg/kg (data not shown) the typical signs of DBTC-induced pancreatitis. Specifically, severe acinar cell damage, ductal changes and pronounced inflammatory cell infiltrates at day 7 (acute stage) and extended periductal and interstitialfibrosis as well as persistent inflammation at day 28 (chronic stage) were observed. Unexpectedly, HE stains of pancreatic tissues from DBTC-treated mice did not reveal any signs of pancreatitis, independent of the time point of investigation and the dose of the drug. In contrast, liver histopathology was found to be similar in both species( Fig. 1 B). Specifically, portal hyperemia, cell swelling, necrosis and loss of small bile ducts were observed in the first week after DBTC-injection. This initial tissue injury was followed by a vivid proliferation of regenerating small bile ducts in the course of DBTC-cholangitis (as shown by CK-7 staining; Fig. 2 ). The latterfindings,however, were more common and more pronounced in rats. These observations were also confirmed by a semi-quantitative scoring of histopathological changes ( Fig. 3 ). Specific stains revealed that pancreas ( Fig. 4 A) and liver ( Fig. 4 B) from DBTC-treated rats contained dense infiltrates of macrophages at day 7 after injection. Interestingly, not only liver but also pancreatic tissues from DBTC-treated mice presented with increased numbers of macrophages in the first week after DBTC application, a finding that remained unrecognized when HE stains were evaluated ( Fig. 1 ).
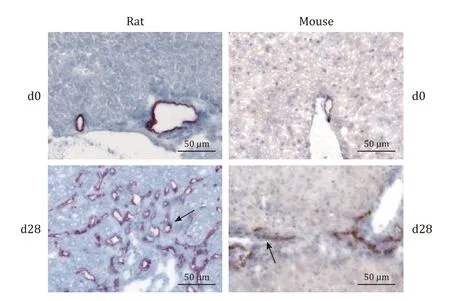
Fig. 2. Visualization of small bile ducts by CK-7-staining. DBTC-cholangitis in rats and mice was induced by a single intravenous injection of DBTC (photographs exemplify animals exposed to 8 mg/kg). After 28 days, the animals were sacrificed,and cryostat sections of liver tissue from rats (left panels) and FFPE liver sections from mice (right panels) were subjected to staining with anti-CK-7, employing APAAP (rats) and ABC (mice) techniques. Arrows point to positively stained small bile ducts.
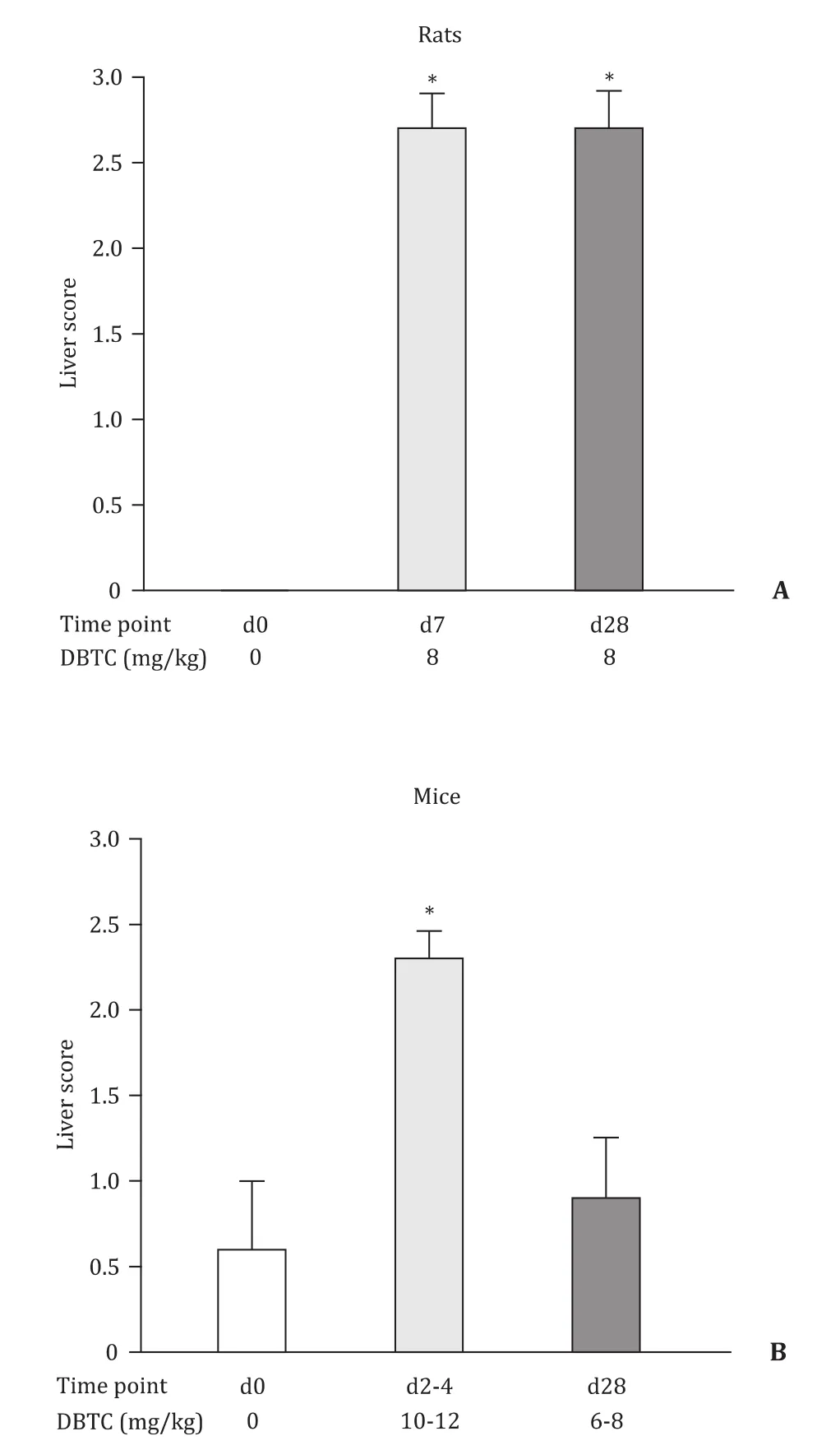
Fig. 3. Scoring of morphological findings in liver tissues from DBTC-treated rats and mice . Data are indicated as mean ± SE ( n ≥ 5); *P < 0.05, versus d0. Liver score for rats at d0 was 0 in all animals.
As expected, chronic DBTC-pancreatitis in rats was associated with deposition of collagen in areas of periductal and interstitial fibrosis ( Fig. 5 ). In pancreases from DBTC-treated mice, no increased amounts of collagen were detected. Liver tissues showed that portal and periportal areas from DBTC-exposed rats and mice contained more collagen than the respective regions of untreated controls, a finding compatible with an early stage of portal/periportal hepatic ibrosis in DBTC-cholangitis.
Discussion
Our study indicates that a direct transfer of the DBTC-model of CP from rats to C57BL/6 J mice is impossible: 4 weeks after injection of DBTC at 6-8 mg/kg, no morphological signs of chronic DBTC-pancreatitis were observed. At 10-12 mg/kg, DBTC exerted toxic effects to the hepatobiliary system that required euthanasia of the mice around day 3 after drug application. Even at these high concentrations of DBTC, however, only mild signs of acute pancreatitis (increased lipase activities, tissue in filtrates of macrophages)but no major tissue damage was observed. We therefore suggest that pancreatic tissue of C57BL/6 J mice might be more robust against toxic effects of DBTC, display a higher regenerative capacity than rat pancreas, or both. Of course, anatomic differences between the two species, as described in the introduction, have to be taken into account as well. In contrast to Zhang et al. [12] , we did not combine injection of DBTC with ethanol. We nevertheless believe that the more important difference is the factor “mouse strain”, since in this study KM mice were replaced by C57BL/6 J mice. The reason for this change was that the majority of genetic mouse models used C57BL/6 J as the background strain. A reliable and convenient CP model if it is applicable to C57BL/6 J mice would be very advantageous, since it would facilitate the functional evaluation of risk genes (or protective factors) of the disease.To this end, mouse models that fulfill these conditions and resemble the key features of human CP, including fibrosis, are rare. The probably most widely used model, cerulein-induced experimental CP, depends on repeated supramaximal stimulations of mice, over several weeks, with the secretagogue [15] , and still fibrosis remains relatively mild. An important modification of the cerulein model was introduced in 2015 by Sendler et al. [16] , who combined ligation of the midpancreatic duct with only a single supramaximal injection of cerulein to obtain severe experimental CP with extended fibrosis and fatty tissue replacement specifically in the affected part of the pancreas. This elegant model, however, requires the availability of advanced microsurgery.
Furthermore, various genetic mouse models have been suggested to cause spontaneous CP and may provide an important resource for studies that are dedicated to the evaluation of a specific genetic background. Examples include, but are not restricted to, acinar cell-specific overexpression of IL-1β[17] , expression of transforming growth factor-beta 1 in pancreatic betaislet cells [18] , or disruption of theproteinkinaseR-likeendoplasmicreticulumkinase(PERK) gene [19] (reviewed in [3 , 4] ). Still, if the phenotype is mild or the gene even plays a protective role,the availability of a potent trigger, with stronger profibrotic effects than cerulein, remains desirable. To study autoimmune pancreatitis, a rare and unique form of CP, MRL/Mp mice have proven a useful model [20-22] .

Fig. 5. Detection of collagen in tissues from DBTC-treated rats and mice (rats: 8 mg/kg, mice: 6 mg/kg). Cryostat sections (rats) and deparaffinated samples (mice) of pancreatic ( A) and liver ( B) tissues were subjected to staining with Sirius Red for collagen visualization.
While DBTC failed to induce DBTC-pancreatitis in C57BL/6 J mice under our experimental conditions, its effect on the epithelium of the small bile ducts may be of interest for follow-up studies on DBTC-cholangitis: two established models of chemically induced cholangitis and cholestatic liver disease, the trinitrobenzenesulfonic acid (TNBS) [23] and alpha-naphthylisothiocyanate(ANIT) [24] models, are restricted to rats. The drugs 3, 5-diethoxycarbonyl-1, 4-dihydrocollidine (DDC) [25] and lithocholic acid (LCA) [26] have been successfully used in mice, where they are applied through the diet. In this context, a specific advantage of DBTC might be the precise dosing by a single intravenous injection. Compared to models of spontaneous cholangitis, such as autoimmune biliary disease of non-obese diabetic (NOD).c3.c4 mice,the use of DBTC offers the possibility to control and modulate the onset of the disease [27] . The suitability of the DBTC-cholangitis model to evaluate experimental therapies for hepatobiliary diseases warrants further investigation.
Acknowledgment
We thank Mrs. Katja Bergmann for the excellent technical assistance.
CRediT authorship contribution statement
Friederike Lütt:Investigation, Methodology, Validation, Visualization, Writing - review & editing.Luise Ehlers:Investigation,Methodology, Validation, Writing - review & editing.Horst Nizze:Investigation, Methodology, Validation, Writing - review & editing.Robert Jaster:Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources,Supervision, Validation, Visualization, Writing - original draft,Writing - review & editing.
Funding
None.
Ethical approval
All animal experiments were approved by the local animal use and care committee (Landesamt für Landwirtschaft, Lebensmittelsicherheit und Fischerei Mecklenburg-Vorpommern, Rostock,Germany).
Competing interest
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Neoadjuvant chemoradiotherapy before resection of perihilar cholangiocarcinoma: A systematic review
- Hepatobiliary&Pancreatic Diseases International
- Human microbiome is a diagnostic biomarker in hepatocellular carcinoma
- Current practice of anticoagulant in the treatment of splanchnic vein thrombosis secondary to acute pancreatitis
- Enhanced recovery after surgery program in the patients undergoing hepatectomy for benign liver lesions
- Assessment of biological functions for C3A cells interacting with adverse environments of liver failure plasma
