Transarterial chemoembolization versus percutaneous microwave coagulation therapy for recurrent unresectable intrahepatic cholangiocarcinoma: Development of a prognostic nomogram
2020-05-10YanSosonJonGuiJuanLuoYiBinRnBaoHuaZanYonJiZanFnSnQinBaoCnCnJunSuiHonYanWanQianXiaLiCn
Yan G , Soson Jon , Gui-Juan Luo , Yi-Bin Rn , Bao-Hua Zan ,Yon-Ji Zan , Fn Sn , Qin-Bao Cn , Cn-Jun Sui , Hon-Yan Wan ,Qian Xia , Li Cn
a School of Public Health, Shanghai Jiaotong University School of Medicine, Shanghai 200025, China
b Department of Liver Surgery, Renji Hospital, Shanghai Jiaotong University School of Medicine, Shanghai 200127, China
c International Co-operation Laboratory on Signal Transduction, Eastern Hepatobiliary Surgery Institute, Second Military Medical University, Shanghai 200438, China
d National Center for Liver Cancer, Shanghai 201805, China
e Biliary Tract Department II, Eastern Hepatobiliary Surgery Hospital, Second Military Medical University, Shanghai 200438, China
f Department of Hepatobiliary Surgery, Eastern Hepatobiliary Surgery Hospital, Second Military Medical University, Shanghai 200438, China
g Biliary Tract Department I, Eastern Hepatobiliary Surgery Hospital, Second Military Medical University, Shanghai 200438, China
h Department of Special Medical Care & Liver Transplantation, Eastern Hepatobiliary Surgery Hospital, Second Military Medical University, Shanghai 200438, China
i Department of Oncology, Shanghai Cancer Center and Institutes of Biomedical Sciences, Shanghai Medical College, Fudan University, Shanghai 200032,China
ABSTRACT
Keywords:
Bile duct cancer
Biliary malignancy
Cholangiocarcinoma
Locoregional therapy
Nomogram
Introduction
Intrahepatic cholangiocarcinoma (ICC) is the second most common primary liver cancer after hepatocellular carcinoma that arises from the bile duct within hepatic parenchyma [1] . The incidence of ICC has been increasing over the past 3 decades and the rising trend is still ongoing [2] . Surgical resection remains the standard of care, since liver transplantation after neoadjuvant therapy is limited to a subset of patients with early-stage or locally advanced ICC with adjuvant treatment [3 , 4] . Despite surgical approach, reported rates of postresection recurrence are approximately 60%-80% [5 , 6] .Therefore, recurrence treatment has emerged as an important issue.
Locoregional therapies, including transarterial chemoembolization (TACE), percutaneous microwave coagulation therapy (PMCT),radiofrequency ablation, and high-dose external beam radiation,are considered options with reasonable effectiveness for unresectable and localized recurrent ICC, whereas gemcitabine and platinum-based chemotherapy is the standard treatment option for advanced ICC with extrahepatic involvement [7-12] . Among the modalities for recurrent ICC without extrahepatic involvement,TACE and PMCT are frequently applied in real-world, and the selection between TACE and PMCT are commonly based on tumor size [13] . However, there is no statistical information regarding identification of significantly supportive factors for modality selection, except for repeat resection that many patients are not candidates, either for medical or anatomic reasons [14] .
In pursuit of evaluation on effectiveness of locoregional modalities for recurrent ICC, we conducted this study to provide outcomes and prognostic factors of TACE and PMCT. In addition,predictive nomograms were developed and validated to support individualized survival prediction.
Patients and methods
Study patients
Pathologically confirmed mass-forming ICC who received postresection TACE or PMCT between May 2008 and December 2015 at two hospitals (Eastern Hepatobiliary Surgery Hospital and Renji Hospital) were screened. Patients who received resection as an initial treatment, unresectable tumor at recurrence, and had complete demographic, clinical, and follow-up information were included in the present study. In addition, patients who received postresection adjuvant treatment prior to TACE or PMCT, had combined hepatocellular-cholangiocarcinoma, extrahepatic metastasis,Eastern Cooperative Oncology Group performance status>1, and relapse-free survival (RFS) or overall survival (OS)<1 month were excluded from further analyses. Recurrence of the tumor was defi ned as detection of new lesions confirmed in imaging studies,including computed tomography (CT) and/or magnetic resonance imaging (MRI) by the interpreting radiologists with or without elevation of serum tumor markers. Finally, a total of 275 patients with recurrent ICC (183 TACE patients, 92 PMCT patients) were identifi ed and included in the analyses. Among them, 92 PMCT patients were 1:1 matched to 92 TACE patients using the propensity score matching [15] . The patients were divided into training and validation groups according to the time frame (training group, May 2008 to December 2012; validation group, January 2013 to December 2015) for development of predictive nomograms. This study was conducted in accordance with the transparent reporting of a multivariable prediction model for individual prognosis or diagnosis(TRIPOD) statement and theDeclarationofHelsinki[16] . All the Institutional Review Boards approved the protocol. Consent for publication was waived due to the retrospective nature of this study.
Data collection and modality selection
Standard demographic and clinicopathological data were collected, including age, sex, serologic examinations, and primary tumor characteristics from prospectively collected ICC database.Serum alpha-fetoprotein (AFP), carbohydrate antigen 19-9 (CA19-9), and carcinoembryonic antigen (CEA) levels, tumor size, number,and differentiation, vascular invasion, lymph node metastasis, and extent of resection depict data at the time of surgical resection.Alcohol consumption, smoking, hypertension, diabetes, fatty liver,liver fluke, cholelithiasis, and viral hepatitis status were defined positive when either of first visit (surgical resection) or second visit (recurrence treatment) reported positivity. Hepatitis B virus(HBV) infection was defined as seropositive for hepatitis B surface antigen. All prognostic variables were collected at the time of primary resection. Modality decisions were made at the institutional liver tumor board. For cases with intrahepatic locoregional recurrence, both TACE and PMCT were recommended for patients without extrahepatic metastasis who were not candidates for repeat hepatectomy. In addition, the choice between TACE and PMCT were generally guided by the treating clinicians.
Protocol of TACE and PMCT
For either of TACE or PMCT to be performed, patients were required to have unresectable tumor, Child-Pugh class A or B, white blood cell count ≥ 3.0 × 109/L, platelet count ≥ 50 × 109/L,and normal kidney function. Visceral angiography was performed to evaluate arterial blood supply to liver and to confirm patency of portal vein. Distal superselective catheterization of the tumor-feeding hepatic artery was followed. Hepatic artery infusion chemotherapy, chemolipiodolization, and embolization were performed using 5 mL of iodized oil emulsion (Lipiodol; Guerbet Laboratories, Aulnay-sous-Bois, France) with 500 mg of 5-fluorouracil,10 mg of hydroxycamptothecin, and 20 mg of epirubicin. When a subsequent application of TACE was required, it was done once every 2 months until complete disappearance of the viable tumor.However, usually one TACE was performed according to the unnecessary circumstance (disappearance of the tumor), liver function deterioration, and recurrence of the tumor [17] .
PMCT was performed using a FORSEATM MW delivery system(Qinghai Microwave Electronic Institute, Nanjing, China), which is composed of MTC-3 microwave generator with 2450 MHz frequency, power output of 1-100 W, 14-gauge cooled shaft antenna(18 cm shaft coated with Te flon, 3 cm exposed antenna with 1.5 cm active tip coated with polytetra fluoroethylene terminus),and flexible low-loss coaxial cable. The power output setting of 80-100 W was used routinely. The electrode was introduced at the distal margin for coagulation. The treatment was carried out until the tumor was completely covered by gasification zone under ultrasound monitoring. The treatment was performed once every month until the tumor was completely unobservable; for tumors with equal to or less than 5 cm, one ablation was usually preplanned, which involved most cases. When more than one ablation was required, the time interval for the second ablation was 1 month [18] . Recurrence treatments were local alone without combining with systematic therapy.
Follow-up and end points
Patients underwent clinical evaluation, routine blood test, liver function, tumor markers, and image tests (CT and/or MRI) 1 month after treatment, every 3 months for the first 2 years, and every 6 months thereafter. Only lesions detected by CT or MRI were diagnosed as recurrence by the interpreting radiologist and confi rmed by the treating physician. Patients alive without evidence of relapse was censored at last follow-up. The primary end point of the study was OS; time from TACE/PMCT treatment to death due to any cause. RFS was defined as time from TACE/PMCT treatment to diagnosis of recurrent ICC.
Statistical analysis
The data were presented as mean ±standard deviation (SD),median (interquartile range [IQR]), orn(percentage). Baseline demographic, clinical, and tumor-associated characteristics were compared between TACE and PMCT groups using Student’st-test and Chi-square test. Cumulative event rates were evaluated using the Kaplan-Meier method and were presented using the Graph-Pad Prism version 6 (GraphPad Software Inc. San Diego, USA) [19] .Hazard ratio (HR) and 95% confidence interval (CI) was calculated using the Cox proportional hazards model [20] . A propensity score was calculated to account for potential characteristic bias. All covariates potentially associated with treatment outcome were included in the logistic regression model, including age,HBV infection, tumor markers, tumor size and number, differentiation, extent of resection, vascular invasion, lymph node metastasis, cholelithiasis, diabetes, smoking, and active alcohol use. Each PMCT patient was matched to a TACE patient using nearest neighbor matching that minimizes difference of propensity score. All patients were matched with a lower than 10% of difference. To evaluate the adequacy of the propensity score, covariate balance was assessed within quartiles of the propensity score, which revealed to be well balanced. Predictive nomograms were developed using the Cox multivariate analysis-indicated prognostic factors, which were validated by receiver operating characteristic curves (ROC)with area under curve (AUC) values. All statistical analyses were performed using SPSS version 20.0 (SPSS Inc., Chicago, IL, USA) and the R project version 3.4.2 ( https://www.r-project.org ).
Results
Patient characteristics
The median age of our cohort was 55 years (range 20-85) and consisted of 57.1% male patients ( Table 1 ). Of the 275 patients,183 (66.5%) were prescribed TACE and 92 (33.5%) were prescribed PMCT. HBV infection was the most common etiology in all training and validation groups. Only 1 patient had HCV infection, who is in the training cohort of PMCT group. Less than a half of the patients showed alcohol consumption (P= 0.986), smoking(P= 0.189), hypertension (P= 0.797), diabetes (P= 0.760), fatty liver (P= 0.724), liver fluke (P= 0.217), cholelithiasis (P= 0.724),and cirrhosis (P= 0.285) in each subgroup without statistical differences between TACE and PMCT groups. The only significant differences were found in CA19-9 (P= 0.014) and CEA (P= 0.012)levels. Mean platelet counts were within normal range, whereas the level of CA19-9 was elevated in subgroups. Tumor size for TACE training and validation groups were 6.0 ±2.7 cm and 6.5 ±3.7 cm, whereas it was slightly smaller in PMCT training(5.9 ± 2.4 cm) and validation (5.7 ± 2.4 cm) groups without statistical significance. The majority of patients had well or moderate differentiation (72.7%), especially in the PMCT validation group(100%). Lymph node metastasis was present in 15 (10.9%) patients in TACE training cohort, whereas it was 13 (18.1%) in PMCT training cohort. A majority of patients received minor resection at the first time of surgery in all subgroups.
Survival outcomes
In the overall patients, TACE was significantly more favorable compared to PMCT in both OS (HR = 0.71; 95% CI: 0.50-0.97;P= 0.034; Fig. 1 A) and RFS (HR = 0.71; 95% CI: 0.49-0.97;P= 0.032; Fig. 1 B). The 1-, 3-, and 5-year survival rates and median survival months are shown in Table 2 .
Propensity score matching
To minimize characteristic bias, we conducted a matched analysis using propensity score. Ninety-two PMCT patients were matched to 92 TACE patients with an excellent balance of relative clinical variables. After matching according to the clinicopathological characteristics, TACE still revealed to be significantly more favorable compared to PMCT in both OS (HR = 0.69; 95% CI: 0.47-0.98;P= 0.041; Fig. 1 C) and RFS (HR = 0.69; 95% CI: 0.47-0.99;P= 0.047; Fig. 1 D).
Independent prognostic factors
All significant prognostic factors identified from the univariate analysis were enrolled in the multivariate analysis ( Table 3 ).On the multivariate analysis for TACE group, factors affecting survival included tumor size of larger than 5 cm (HR = 1.777; 95%CI: 1.062-2.973;P= 0.029), poor differentiation (HR = 5.979; 95%CI: 3.588-9.964;P<0.001), and major resection (HR = 1.763; 95%CI: 1.058-2.936;P= 0.029). In the PMCT group, poor differentiation (HR = 5.199; 95% CI: 2.701-10.0 07;P<0.0 01), HBV infection (HR = 0.337; 95% CI: 0.167-0.682;P= 0.002), cholelithiasis (HR = 3.736; 95% CI: 1.208-11.552;P= 0.022), and lymph node metastasis (HR = 2.641; 95% CI: 1.282-5.441;P= 0.008) were found to be independently associated with survival. However, the recurrence-free interval between initial resection and recurrence of the tumor revealed no significance in both TACE (HR = 0.986; 95%CI: 0.951-1.022;P= 0.446) and PMCT (HR = 0.985; 95% CI: 0.938-1.033;P= 0.528) groups in the univariate analyses.
Development of the predictive nomograms for TACE and PMCT
The identified independent prognostic covariates for each TACE and PMCT were applied to build predictive signatures using the Cox hazard proportional model, which were visualized as nomograms. For survival estimation of recurrent ICC without extrahepatic metastasis after TACE, resection type (minor versus hemi or extended hepatectomy), tumor differentiation (well or moderate versus poor), and tumor size ( ≤ 5 cm versus>5 cm) were applied ( Fig. 2 A). For evaluation of predictive accuracy, ROC curves were developed for calculation of AUC values. The AUC revealed to be good for the TACE nomogram (AUC = 0.84; Fig. 2 B). In addition, the PMCT nomogram was built based on 4 covariates, including HBV infection, tumor differentiation, cholelithiasis, and lymph node metastasis ( Fig. 2 C). The predictive performance of the PMCT nomogram (AUC = 0.87) also revealed to be good ( Fig. 2 D).
Validation of the predictive nomograms for TACE and PMCT
To validate predictive performance of the nomograms, ROC curves were developed using the independent validation groups.The ROC curve for both TACE and PMCT nomograms demonstrated to be satisfactory (AUC = 0.77 and 0.70; Fig. 3 A and B). According to the categorization by the optimum thresholds, the TACE validation group was stratified into high-risk (n= 30, 65.2%) and low-risk(n= 16, 34.8%) groups. The high-risk group for TACE revealed anHR of 4.982 (95% CI: 2.048-8.569;P<0.001; Fig. 3 C). Among 20 patients in the PMCT validation group, 3 (15.0%) and 17 (85.0%) patients were of high- and low-risk groups, respectively. The Kaplan-Meier curve revealed a significant difference between the highand low-risk groups for PMCT (HR = 4.694; 95% CI: 2.220-203.0;P= 0.010; Fig. 3 D).
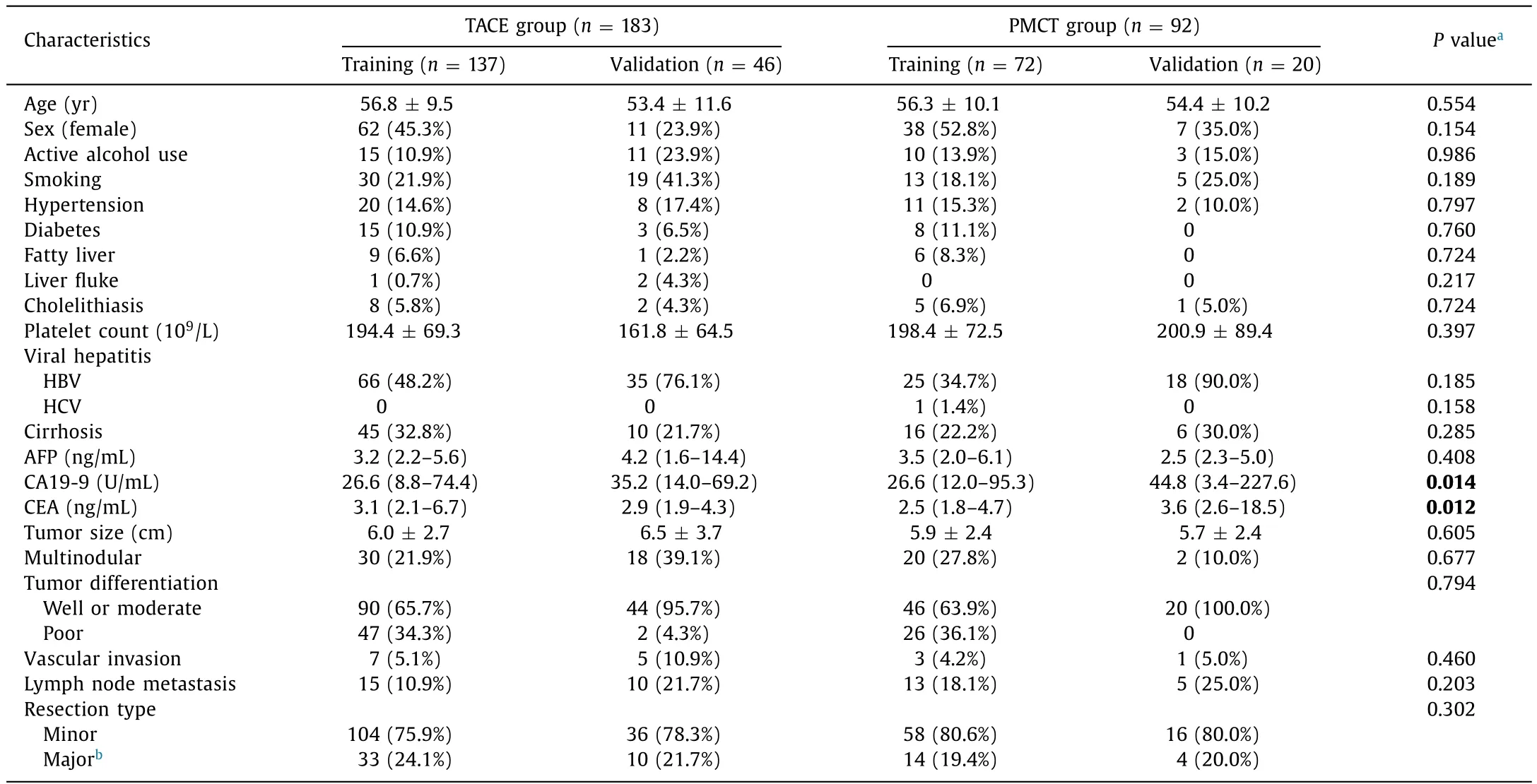
Table 1Baseline demographic and clinical characteristics (comparison between TACE and PMCT groups).
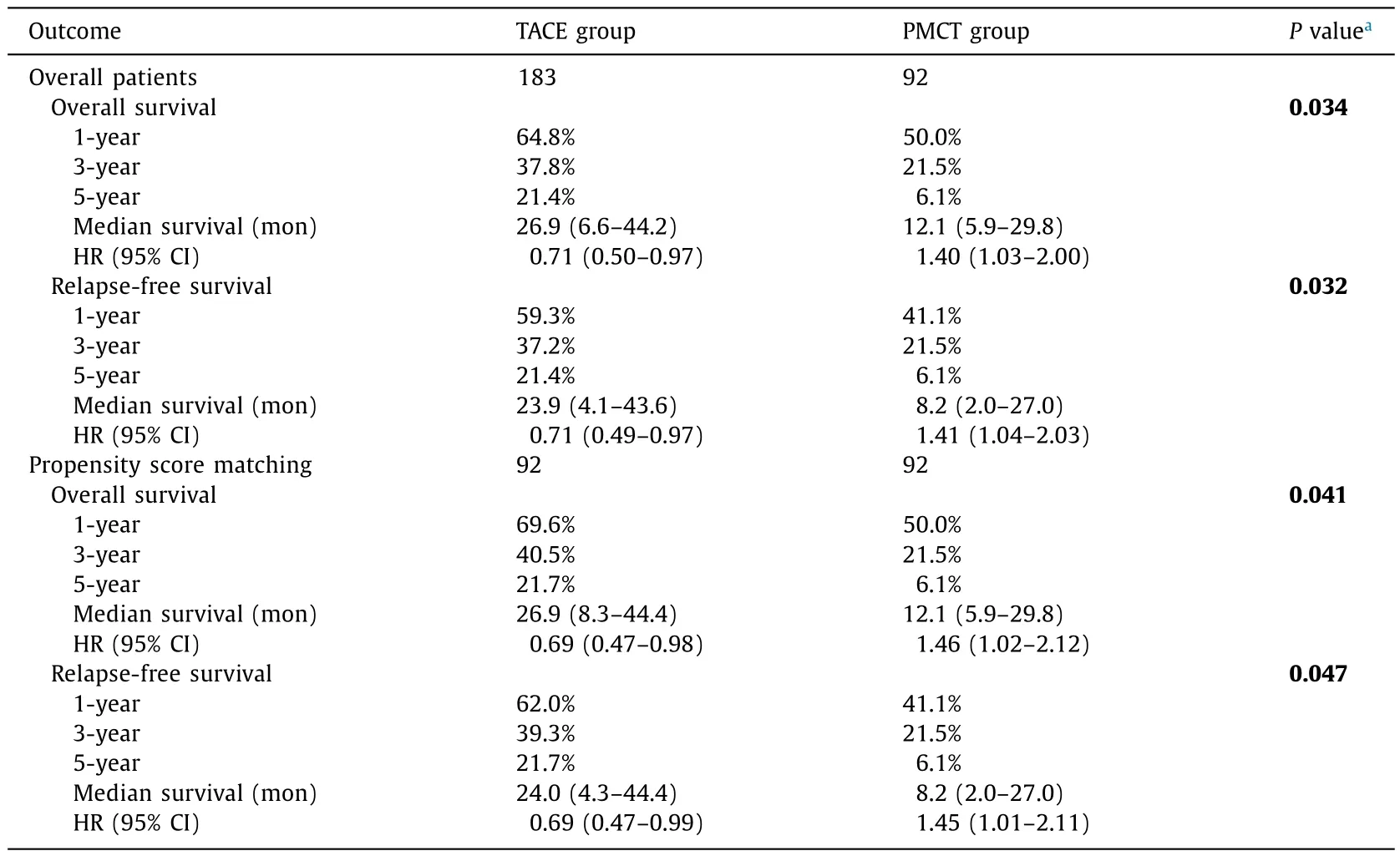
Table 2Summary of outcomes (comparison between TACE and PMCT groups).
Discussion

Fig. 1. Kaplan-Meier curves for estimation of survival outcomes. ( A) TACE versus PMCT (OS) in overall patients. ( B ) TACE versus PMCT (RFS) in overall patients. ( C) TACE versus PMCT (OS) after propensity score matching. ( D) TACE versus PMCT (RFS) after propensity score matching. Numbers in the brackets are the number of cumulative censored patients.
TACE and PMCT are the two common approaches for treatment of unresectable localized liver cancers, but the effectiveness has not been evaluated and compared for recurrent ICC [21] . In our study, TACE provided higher survival benefits than PMCT in both overall and propensity score matched groups. However, PMCT has also been reported to provide survival benefits, but with low effectiveness in achieving extensive necrosis for large tumors [22] .Therefore, we further explored significant prognostic factors for TACE and PMCT, respectively. Unexpectedly, the prognostic factors for TACE and PMCT were different, which led us to develop predictive nomograms for individualized evaluation. Use of the two nomograms may be helpful in selection between TACE and PMCT.In addition, patients with low-risk for TACE may spare the evaluation for PMCT. Conversely, patients with high-risk for TACE require to be further evaluated for PMCT.
In the present study, most recurrences were clustered within 1 year after TACE or PMCT treatment in nomogram-stratified patients at high-risk. However, low-risk patients had a coherently low recurrence. Therefore, the predictive nomograms may also be informative in setting individualized follow-up schedules for ICC patients after TACE or PMCT; patients with high-risk may require tighten follow-up for at least 3 years, and regular follow-up may be enough for patients with low-risk.
Although the time period revealed no significant prognostic impact when stratified according to the early and late cases, the late cases of PMCT group showed an HR of 0.573 (95% CI: 0.298-1.102;P= 0.095), suggesting that there may be a significant learning curve when enlarge sample size. In addition, no signi ficant difference was found in treatment selection according to the time period. In previous years, repeat hepatectomy, radiation, and chemotherapy were considered treatment modalities for recurrent ICC, whereas liver-directed locoregional therapies, including TACE,PMCT, radioembolization (yttrium-90), and external beam radiation are frequently applied for recurrent ICC without extrahepatic metastasis in recent years [23-25] . However, comparative therapeutic outcomes of these liver-directed locoregional treatments remain unclear, rendering us to call for future large-scale trials to verify. Moreover, recent advances in targeting therapy, such as infi gratinib (selective inhibitor of fibroblast growth factor receptor)and AG-120 (inhibitor of mutant isocitrate dehydrogenase 1), may additionally improve prognosis of recurrent ICC when combined with TACE or PMCT [26] .

Fig. 2. Development of predictive nomograms for TACE and PMCT. ( A ) A predictive nomogram for TACE built basing on independent prognostic factors. ( B) A ROC curve for description of sensitivity and specificity of the TACE nomogram. ( C) A predictive nomogram for PMCT built basing on independent prognostic factors. ( D) A ROC curve for description of sensitivity and specificity of the PMCT nomogram.
To date, number of estimative systems have been reported for patients with ICC, including our previous works [27 , 28] .Most of them are applicable to evaluate effectiveness of surgical resection [29] . However, none of them were developed specifically for TACE and PMCT in a recurrence setting. Therefore, we still call for large-scale clinical trials to validate our results.
We noted that there are no definite guidelines for treatment of localized intrahepatic recurrent ICC, except for radiation therapy suggested to be a palliative treatment to relieve symptoms and improve the quality of life [30] . The reason for this may be due to the traditional fixed-conception that ICC has abundant stromal components to promote lymph node metastasis, which is one of the representative hallmarks of ICC [31] . It should be noted,but the most common pattern of recurrence is intrahepatic locoregional recurrence in patients with ICC worldwide, accounting for 60%-80% [32 , 33] . In addition, Doussot et al. [34] reported that recurrence within 2 years after resection involved the liver in 82.7%patients with ICC. However, studies focusing on the evaluation of locoregional treatments are relatively little. In a clinical setting,only tumor size is often decisive of selection between TACE and PMCT that patients with smaller tumor ( ≤3 cm) are treated with PMCT, whereas those with larger tumor (>3 cm) are treated with TACE. Therefore, more comprehensive identifications on effectiveness of treatment modalities and factors that significantly affect effectiveness of the treatments seem necessary to improve prognosis of intrahepatic recurrent ICC after resection.
Different prognostic factors for each treatment type found in this study are noteworthy. Tumor size, tumor differentiation and major resection were independent prognostic factors in the TACE group, whereas tumor differentiation, HBV infection,cholelithiasis, and lymph node metastasis were identified in the PMCT group only. According to our experience, large tumor size requires relatively frequent major resection, and these are considered to be associated with tumor progression at the time of resection. Regarding locality of TACE treatment, advanced progression of the tumor may have contributed to intrahepatic micro-metastasis that could not be detected by imaging studies,leading to recurrence of the tumor and unfavorable prognosis.In addition, HBV-associated ICC and cholelithiasis-associated ICC are the common etiologic subtypes with prognostic difference potentially due to biological malignant invasiveness rather than the extent of tumor progression [35] . Furthermore, HBV-associated ICC demonstrated to be a distinct subtype with low incidence of lymph node metastasis after resection [36] . Therefore, the extent of tumor progression may be the main factor affecting outcomes of TACE in patients with recurrent ICC, whereas the etiologic subtype seems to be associated with prognosis of patients undergoing PMCT.
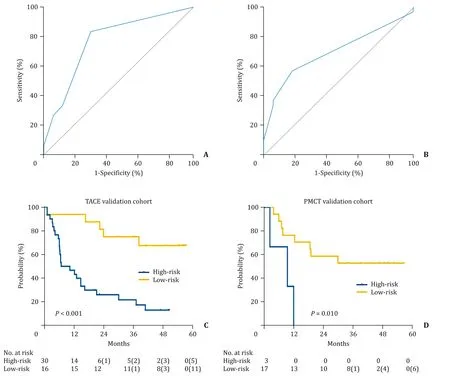
Fig. 3. Validation of the predictive nomograms. ( A) Receiver operating characteristic (ROC) curve for validation of the TACE nomogram. ( B) ROC curve for validation of the PMCT nomogram. ( C) Validation using Kaplan-Meier estimation of the PMRI-categorized TACE risk groups. ( D) Validation using Kaplan-Meier estimation of the PMRI-categorized PMCT risk groups. Numbers in the brackets are the number of cumulative censored patients.

Table 3Multivariable analyses of significant prognostic factors.
Despite initial and novel findings, the underlying limitations of this study should be noted. This study focused on localized recurrent ICC cases who received TACE or PMCT only. Evaluation on other locoregional treatments along with TACE and PMCT is required. In addition, evaluation on combined application of locoregional treatment and adjuvant treatment for cases with both intrahepatic and extrahepatic recurrence is also necessary. Furthermore, regarding rare incidence of intrahepatic recurrent ICC, the predictive models were derived and validated in a limited number of patients. Future validation studies are necessary.
In conclusion, TACE revealed to be a better treatment modality in terms of the OS and RFS compared to PMCT. However, there was a disparity in prognostic factors of TACE and PMCT, and the developed nomograms may be helpful in treatment decision making. We call for future prospective validation studies to validate our data for the nomograms to be applied in clinical practice.
CRediT authorship contribution statement
Yang Ge:Conceptualization, Formal analysis, Funding acquisition, Investigation, Methodology, Visualization, Writing - original draft.Seogsong Jeong:Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Visualization, Writing - original draft.Gui-Juan Luo:Data curation, Formal analysis,Investigation, Methodology, Resources, Writing - original draft.Yi-Bin Ren:Conceptualization, Data curation, Investigation, Resources,Writing - review & editing.Bao-Hua Zhang:Conceptualization,Data curation, Investigation, Resources, Writing - review & editing.Yong-Jie Zhang:Conceptualization, Data curation, Investigation, Resources, Writing - review & editing.Feng Shen:Conceptualization, Data curation, Investigation, Resources, Writing - review& editing.Qing-Bao Cheng:Conceptualization, Data curation, Investigation, Resources, Writing - review & editing.Cheng-Jun Sui:Conceptualization, Data curation, Investigation, Resources, Writing- review & editing.Hong-Yang Wang:Conceptualization, Methodology, Supervision, Writing - review & editing.Qiang Xia:Conceptualization, Methodology, Supervision, Writing - review & editing.Lei Chen:Conceptualization, Methodology, Supervision, Writing -review & editing.
Funding
This study was supported by grants from the National Natural Science Foundation of China ( 81902939 ) and Startup Fund for Young Teacher from Shanghai Jiaotong University (KJ3-0214-18-0022).
Ethical approval
This study was approved by all the Institutional Review Boards.Consent for publication was waived due to the retrospective nature of this study.
Competing interest
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Neoadjuvant chemoradiotherapy before resection of perihilar cholangiocarcinoma: A systematic review
- Hepatobiliary&Pancreatic Diseases International
- Human microbiome is a diagnostic biomarker in hepatocellular carcinoma
- Current practice of anticoagulant in the treatment of splanchnic vein thrombosis secondary to acute pancreatitis
- Enhanced recovery after surgery program in the patients undergoing hepatectomy for benign liver lesions
- Assessment of biological functions for C3A cells interacting with adverse environments of liver failure plasma
