Low-temperature alteration of uranium-thorium bearing minerals and its significance in neoformation of radioactive minerals in stream sediments of Wadi El-Reddah, North Eastern Desert, Egypt
2020-04-26····
· · · ·
Abstract The stream sediments of Wadi El Reddah(North Eastern Desert, Egypt) are geochemically and mineralogically investigated. Their content of radioactive and other heavy minerals is mainly represented by thorite, uranothorite, zircon, monazite, xenotime, columbite, fergusonite,and unknown rare earth elements (REEs) bearing minerals as well as cassiterite. Special emphasis on REE content of thorite,uranothorite,zircon and xenotime has been done to correlate them with the increase of uranium contents in these sediments. The key evidence for the presence lowtemperature alteration processes includes the presence of some zircon crystals as remnants after complete dissolution of the overgrowth zircon in severe acidic environment,the sulphur content, biogenic minerals, occurrence of unusual minerals as cassiterite pore filling in zircon,variation in the REEs content from the surrounding granites to the stream sediments and the abundance of monazite in the surrounding granites. Most minerals are partially and/or completely altered, which indicated by the pseudomorphism of zircon by xenotime, thorite, and uranothorite.
Keywords Zircon · Thorite · Xenotime · Radioactive minerals · Low-temperature alteration · Wadi El-Reddah
1 Introduction
Wadi El Reddah is one of the most important valleys in the northeastern Eastern Desert of Egypt. This wadi is located at the northeastern peripheral sector of Gabal Gattar batholiths.These batholiths(Fig. 1)are of the granitic type and constitute a part of the basement rocks of the northern part of the Eastern Desert, Egypt (El Rakaiby and Shalaby 1988).
Zircon along with Thorite is often the principles sink for Th, U, Y and heavy rare earth elements (HREE). In peralkaline and peraluminous granites of A-type affinity,Thorite may contain 10–20% of U and 20–40% of Th contents of the host rock(Bea 1996).Thorite is particularly susceptible to low-temperature alteration following lattice disruption induced by alpha-radiation, as well as the formation of hydrated secondary thorite (thorogummite)(Lumpkin and Chakoumakos 1988; Farges and Calas 1991).
Zircon is a common accessory mineral in nature;occurring in a wide variety of sedimentary, igneous, and metamorphic rocks. A significant proportion of Th and U may occur structurally in zircon, whereas Th4+(r = 1.02 A˚) and U4+(r = 0.97 A˚) substitute for Zr4+(r = 0.80 A˚). Ions of other minor elements may also occur structurally in zircon. For example, Y3+(r = 0.92 A˚) may substitute for Zr4+and P5+(r = 0.35 A˚) for Si4+(r = O-42 A˚). In fact, the coupled substitution, Y3++ P5+—Zr4++ Si4+can be quite extensive (Ahrens et al. 1967).So, zircon forms isostructural solid solutions with, for example, hafnon (HfSiO4), coffinite (USiO4), thorite(ThSiO4), and xenotime–(Y) (YPO4) (Finch and Hanchar 2003; Hoskin and Schaltegger 2003; Fo¨rster 2006).

Fig. 1 Location and Regional Geological Map of G. Gattar batholith and its Environments. Modified after El Rakaiby and Shalaby (1988)
Radioelement content of zircon may lead to its metamictization (Speer et al. 1981). Metamictization occurs as a result of α-particle emission and recoil after radioactive decay of a U or Th atoms.The recoiled particle will travel a distance equivalent to several unit cells (Onstott et al. 1995) damaging the crystallographic lattice of the host mineral. Metamictization must also be considered as a mechanism favoring porosity formation,particularly in minerals rich in radiogenic actinide elements. These damaged zones are susceptible to dissolution and removal by fluids, leaving behind pores. Tomaschek et al. (2003) recognized the involvement of a fluid phase in the formation of porous and mineral inclusion-bearing (mainly xenotime and an unknown Y–REE–Th silicate phase)zircon crystals from blueschist rocks of Syros, Greece.
Two fundamentally different processes have been proposed to account for the formation of altered areas in natural zircon (Geisler et al. 2007; Putnis 2002, 2009;Soman et al. 2010). These processes are: (1) A so-called interface-coupled dissolution reprecipitation process where a dissolution–reprecipitation front is thought to migrate into the crystal with both reactions to be temporally and spatially coupled at an inwardly moving reaction interface,and (2) A diffusion-controlled recovery-recrystallization process that operates only in severely radiation-damaged zircon when it comes in contact with an aqueous solution.
Monazite and xenotime crystals are generally preserved in the regolith and relatively accumulate before being exported in streambed sediments. They are prone to relative accumulation within the weathering profile leading to potential accumulation of REY–Th–U. The reactive accessory minerals, broken down during the first stage of weathering,are then able to release 86%of Th and Y,90%of REE (except Eu) and 93% of U (Braun et al. 2018).
This study aims to clarify the effect of low-temperature alteration processes that affect the stream sediments of Wadi El Reddah and consequence change in its content of radioactive minerals. This will help greatly in the further exploration of uranium in these sediments and define the relationship between the occurrence of uranium in both the Gabal Gattar younger granites and the surrounding stream sediments of Wadi El Reddah.
Whole-rock geochemical data indicate that these rocks are post-collisional syenogranites, mildly alkaline subsolvus and alkaline hypersolvus granites originated from metaaluminous to slightly peraluminous and alkaline magma(El-Sayed et al.2003;Moussa et al.2008;Ali et al.2016). The mineral chemistry of these granites revealed that circulation of hydrothermal solutions or fluids produces physicochemical changes in Gattar granite through which they circulate.This is what is commonly referred to as hydrothermal alteration. These fluids set off chemical reactions, which tend to approach equilibrium through processes of dissolution and precipitation of new mineral assemblages. The mineralizing solutions may be uraniumbearing of hypogene and supergene origin fluids which percolate on the granites and leach of uranium. Then,mobilization of uranium within the granite and deposition of uranium and fluorite mineralization along the fractures where the planes of these structures provided easy channels for the passage of uranium-bearing solutions and suitable structural trap for uranium accumulations (Mourad 2011).
Most of stream sediment samples exhibit increase in trace elements which show enrichment in Ni, Cu, Pb, Zn,Cd, Ta, Nb, Zr, Y, Rb, Cs, Ga, Hf, Sn, Th, Tl, U, W, Mo,Bi,As,Be,Li,Sb and Au whereas they are depleted in Co,Sr,V,Ag,Cr and Sc.Pb concentration increases as a result of the alteration of Pb minerals from the surrounding granites including kasolite,cotunite,coronadite and galena.Zr, Hf, and Y are relatively immobile and essentially concentrated in the accessory minerals (zircon and its alteration product branirite, xenotime, thorite and uranothorite). High contents of U, Th, Y, Nb, and Ta may be related to the presence of fergusonite (Y, REE) (NbO4),xenotime Y(HRE) (PO4), ferrocolumbite, Yttrocolumbite,columbite and monazite(Ce,La,Nd,Th)PO4as indicated from the mineralogical studies (Ebian et al. 2018).
2 Geological setting
Wadi El Reddah extends in an N–S direction and represents a semi-closed basin as it has only one very narrow outlet in its northern tip while the other parts of the Wadi collect flood from the internal tributaries along granites and other rock types. The drainage system of Wadi El Reddah reveals the presence of three sub-basins along its course;these are the southeastern, central and northeastern subbasins (Fig. 2). Each of these sub-basins collects its sediments and floods from the different rocks that exposed around the semi-circular southern margin of this wadi.This will help too much in prospecting these remote and highly elevated points, especially in Gattar granites. Wadi El Reddah is truncated northward by the main course of Wadi Bali. The floor of Wadi El Reddah and their tributaries in Gabal Gattar area are generally covered by thick(25–30 m) recent sediments. They are represented by gravelly(boulder-pebble size)to sandy size unconsolidated sediments of fluvial origin.

Fig. 2 Wadi El Reddah and its course subdivisions and the exposed rock types(where H.S.R.is Hammamat sedimentary rocks,M.Vol.is metavolcanics and M.G. is monzogranite of Gabal El Reddah)
Wadi El Reddah is mainly surrounded by scattered exposures, in a chronological sequence, of metavolcanics and metagabbro-diorite complex in addition to the long sector of Hammamat sedimentary rocks, monzongranites of Gabal El Reddah,perthitic leucogranites of Gabal Gattar as well as swarms of post-granitic dykes.
The metavolcanics is mainly represented by scattered and disconnected exposures at the extreme northern and northeastern flanks of Wadi El Reddah.It is represented by metabasalt, metabasaltic andesite, metaandesite and metadacite as well as well foliated meta-pyroclastics.
The metagabbro–diorite complex,which has an intimate association with the previously mentioned metavolcanics,is located at the southeastern part of Wadi El Reddah. It is intruded and cut by arms and off-shoots from the north by Gabal El Reddah monzogranites and from the south and east by the perthitic granite of Gabal Gattar. The complex carries frequently scattered xenoliths of metavolcanics,metaporphyrites, and metapyroclastics. It has a composition ranging from melano-gabbro to diorite and quartz diorites with different grain size.
The Hammamat sedimentary rocks are well exposed along Wadi El Reddah at its western flank forming an intrusive contact with the Gattarian granites. These exposures are the southern extension of the main Gabal Umm Tawat basin present along Wadi Bali. These sediments include the polymictic conglomerate, greywacke, and siltstone.They are of molasses type,and were deposited in intermountain basins;as a result of rapid uplift and erosion in freshwater braided stream sediments and alluvial environment(Grothaus et al.1979)during the Late Proterozoic.
Gabal El Reddah monzogranite is located along the eastern flank of Wadi El Reddah constitute one circular rounded granitic mass. This type of granite represents an early phase of younger granites ‘‘G2-granites’’ (Hussein et al. 1982). It intrudes the metavolcanics exposures at its northern contact sending off-shoots and apophasis to it and also intrudes the metagabbro-diorite complex at its southern contact taking abundant huge xenoliths of it. This granite is of medium to coarse-grained whitish pink monzogranites or adamalite where the percentage of the K-feldspar slightly exceeds that of plagioclase.
Gabal Gattar younger granites represent very high rough topographic scenery in this area of North Eastern Desert(1969 m above sea level). It has a sharp intrusive contact against the other rock types in the area as well as the acidic dykes. It is only cut and traversed by the younger basic dyke swarms. This granite is a very good potential for uranium prospect where up to 24 uranium occurrences were discovered at its northern sector. It is of typical medium grained, hard and massive pink granite with extensive wall rock hydrothermal alterations along its fractures. These granites were classified as G3-granites according to the classification of the Egyptian granites(Hussein et al. 1982).
Post granitic dyke swarms are spread all over the area and represented by bi-modal acidic (older) and basic(younger) dyke swarms where the intermediate dykes are very rare. They cut all the above-mentioned rock units along NE–SW parallel directions and can be traced for hundreds of kilometers.
3 Sampling and analyzing
Forty-one stream sediment samples were collected from Wadi El Reddah (Fig. 3) by digging holes at different depths(190–230 cm)using the stone cutter and the shovel.Each sample (~7 kg) was sieved to get sand-sized sediments, after that, the magnetite was separated by hand magnet meanwhile the other heavy minerals were separated using bromoform. Counting of heavy minerals was carried out using a binocular microscope.Some selected an unidentified heavy mineral species were investigated by the Environmental Scanning Electron Microscope (ESEM).Average chemical analyses of three country rock samples and ten stream sediment samples sulphur were carried out in ACME Analytical Laboratories of Vancouver, Canada for major oxides, trace and rare earth elements by ICPemission spectrometry (ICP-ES) and ICP-mass spectrometry (ICP-MS). Detection limits for major oxides and trace elements are 0.001–0.04 wt%and 0.01–0.5 ppm;respectively. The analytical precision, as calculated from replicate analyses, is 0.5% for major elements and varies from 2 to 20% for trace elements.
4 Results
4.1 Mineralogy and mineral chemistry of U-Th bearing minerals
4.1.1 Zircon
Zircon occurs as short or long euhedral prismatic and/or bipyramidal translucent to opaque crystals. It possesses various colors(pale yellow,reddish brown,reddish orange and colorless)(Fig. 4a).The occurrence of euhedral zircon crystals suggests its magmatic origin. Occasionally, zircon grains show slight to moderate roundness and terminations of the pyramidal and/or prismal faces (Fig. 4). This fact most probably related to late magmatic corrosion or late hydrothermal effect(Armstrong 1922;Esmail 2016).Some grains of the studied zircon show secondary and multiple growths (Figs. 4, 5, 6, 7). The Th/U ratio in Wadi El Reddah zircon noticeably varies from 0.48 to 15.59 with an average of 2.74. In most cases, igneous zircon is characterized by lower Th/U ratio in the range of 0.28–1.17(Heaman et al. 1990). Finally, the increase of U and Th in some spots within the same zircon crystals from Wadi EL Reddah indicates local metamictization at the ‘‘blurred’’zones (Nasdala et al. 1996; Surour and El-Feky 2003).
Some zircon grains are porous as a product of alteration.These pores contain black inclusions of thorite, uranothorite, xenotime, cassiterite and unknown REE-bearing mineral (Figs. 4, 5, 6). Plotting of the investigated zircon samples on the Zr-Hf-(U, Th, Y, HREE) ternary diagram(Fig. 8) revealed that zircon could be discriminated into two types according to its origin i.e. the magmatic zircon(MZ) and metasomatic hydrothermal zircon (MHZ). In these samples, Zr is extensively and simultaneously substituted by Hf, (U, Th), and (Y, HREE) beyond the compositional boundary with some samples lie in the compositional gap. This fact indicates the existence of a compositional gap between the isomorphous series of zircon—hafnon, zircon—(thorite, coffinite) and zircon—xenotime. In other words, the inclusion of Hf into the zircon structure is seemingly delimited in the presence of actinides (Th, U) and lanthanides (HREE) and Y. lanthanides (HREE) and Y (Abdalla et al. 2009).
The SEM profiles across some zircon grains clearly display very characteristic cryptic chemical zoning despite the traversed crystals are optically unzoned or very slightlyzoned (Figs. 4, 5, 6).The scan line of the qualitative elemental analyses along a zircon grain(from core to rim and in the opposite side) revealed the heterogeneous distribution of Zr, U, Th, P, Y, REES, and Si within this grain(Figs. 4, 5, 6 and Table 2). However, the distribution of these elements in an individual grain is mostly irregular and no systematic core-to-rim evolution is observed.

Fig. 3 Landsat image showing the sampling sites along wadi El Reddah

Fig. 4 Binocular photomicrograph, BSE images and EDX analyses of the separated zircon grains and their inclusions. c–f show zircon inclusions (c thorite, d uranothorite, e unknown REE-bearing mineral and f cassiterite)
4.1.2 Thorite
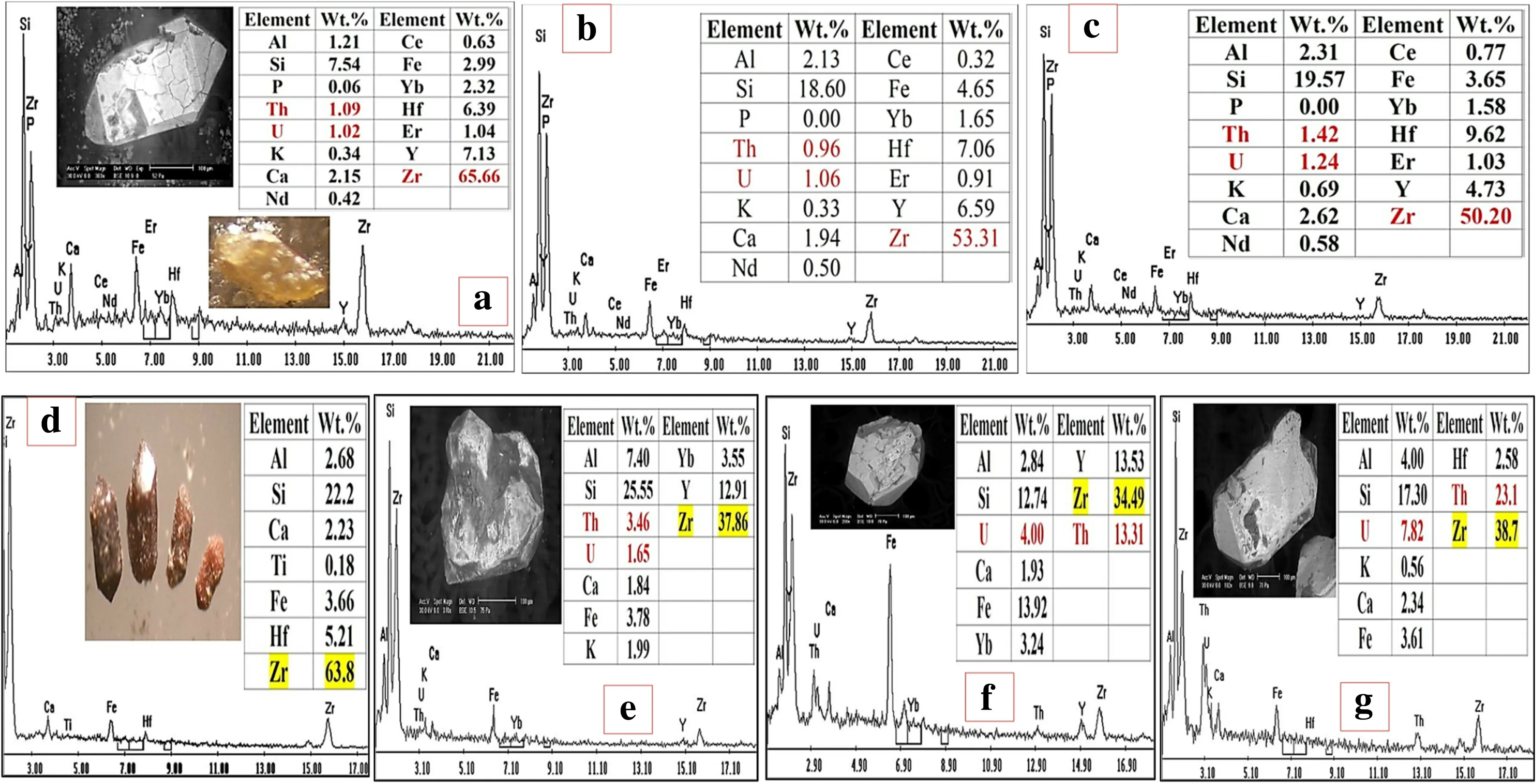
Fig. 5 Binocular photomicrographs BSE images and EDX analyses of the metamict zircon grains. e–g show U, Th and REE distributions in zircon
Thorite occurs as discrete brown to brownish black grains vary in size from less than 70 μm up to 400 μm in diameter. Sometimes, it resembles zircon, as small, square prismatic grains with pyramidal ends (Fig. 9). The qualitative results of EDAX indicate that the major elements in thorite are Th (60.35 wt%), Si(12.74 wt%),Y (9.55 wt%)and U (8.86 wt%). Also, minor amounts of Fe (2.6 wt%),Ca (3.27 wt%) and Al (2.61 wt%) were reported as substitutions. Occasionally, it is present as inclusions within the dark patchy alteration domains in zircon host grains(Fig. 4c). Thorite inclusions are small (<10 μm), anhedral,and often intergrown with a Y–REE–Th silicate phase(Fig. 4e). Solid solution between thorite, zircon and xenotime are also recorded in different proportions as Zrrich thorite, Zr-, Y-, P-, and heavy REEs-rich thorite, Th-,P-rich zircon,Y-,P-,and heavy REEs-rich zircon (Fig. 5).X-ray diffraction (XRD) confirms the presence of solid solution between Thorite, uranothorite and zircon(Fig. 10).

Fig. 8 Zr–Hf–(U, Th, Y,HREE) ternary diagram for zircon compositions in Wadi El Reddah stream sediments. The solid line represents an interpretative boundary that limits the compositional gap between the two zircon series.See the text for the shown trends magmatic zircon (MZ), and metasomatic hydrothermal zircon (MHZ)

Fig. 9 a Binocular photomicrograph and EDX analysis for the separated brownish black grains of thorite. b–e BSE images and EDX analyses for solid solution between thorite and light REE, xenotime, zircon-xenotime and zircon respectively

4.1.3 Uranothorite
It occurs as pale to dark yellow–brown grains that are generally translucent to opaque.They are found as massive grains of anhedral to subhedral and granular forms,having a characteristic vitreous or resinous luster (Fig. 11). The obtained qualitative EDX data indicated that the major elements in uranothorite are Th (47.93–60.76 wt%), Si(10.49–11.72 wt%)and U(10.31–19.13 wt%).Also,minor amounts of Fe (0.87–4.41 wt%), Y (4.35–12.75 wt%), Ca(1.09–2.65 wt%)and Al(2.24–3.95 wt%)were reported as substitutions in uranothorite. The back-scattered images showed that uranothorite sometimes found as a inclusion in zircon (Fig. 4d).
Sometimes, the presence of Zr and Yb in the composition of uranothorite (Fig. 11d and Table 2) confirms the isostructural association of zircon and xenotime with uranothorite. Such association suggests the limited solid solution series, which probably due to the low coupled substitution between Zr4+,REEs3+and Th4+or U4+(Chen et al. 1996; Massonne and Nasdala 2003; El Balakssy 2010; Draz 2017).
U and REEs distribution profiles in the studied uranothorite revealed the presence of the following four categories:

Fig. 11 a BSE image, binocular photomicrograph and EDX analysis of pale to dark yellow brown uronothorite grains. b–d BSE images and EDX analyses for the separated uranothorite grains
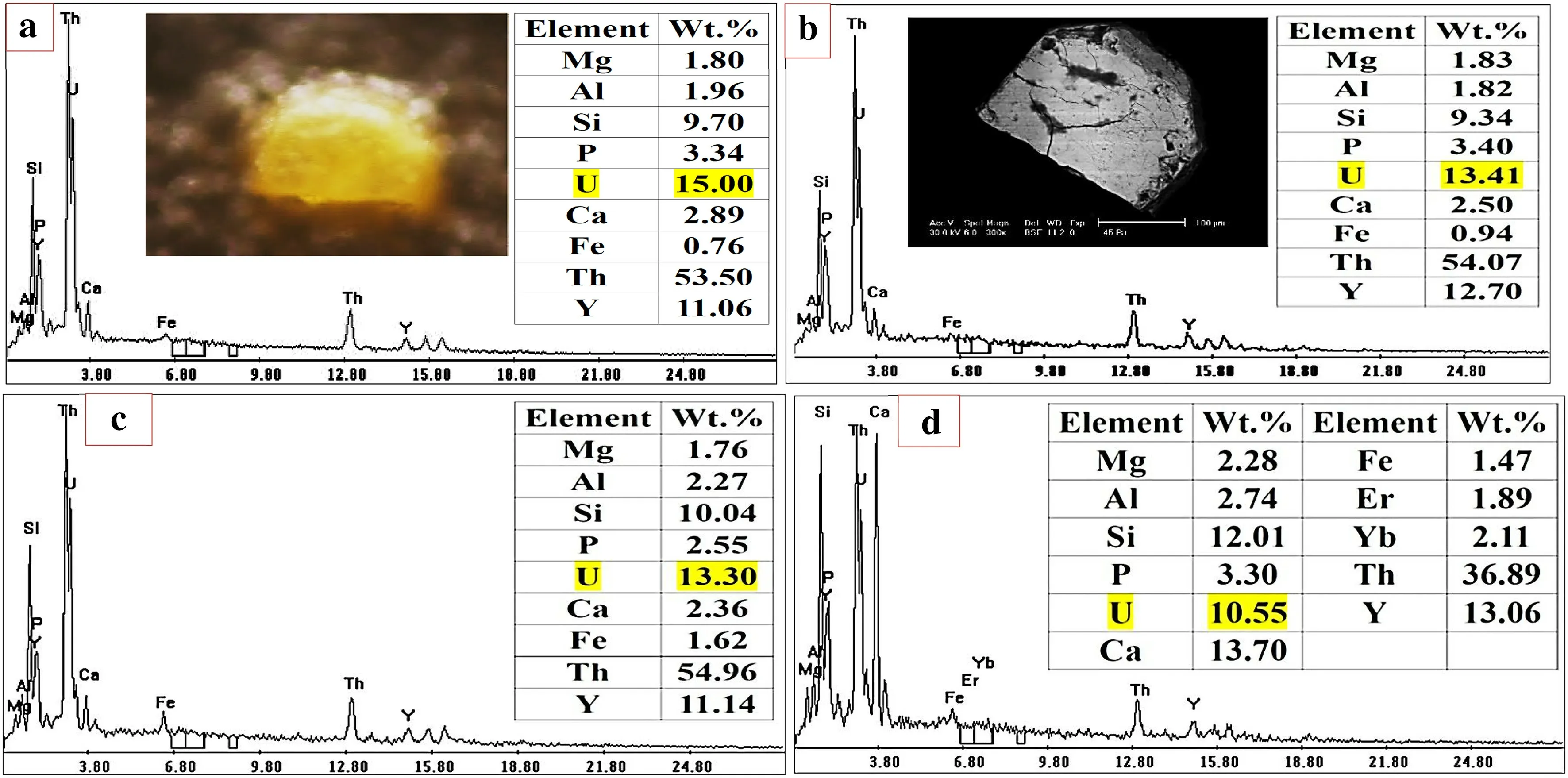
Fig. 12 Binocular photomicrograph,BSE image and EDX mineral analyses,of uranothorite profiles across the grain.a At center,b at rim,c at opposite rim and d on cracks
1. Uniform distribution from core to the rim in one side(increasing), in contrast to the other side (decreasing)mainly related to the diffusion of these elements in one side with respect to the other (Fig. 12).
2. Uniform distribution from core to rim of the grain or crypto-zoning from one side to the other(increasing or decreasing),mainly resulting from the interaction of U and REEs rich solutions with uranothorite grains from core to rim or vice versa (Fig. 13).
3. Random distribution in all sides of the crystal due to the sporadic distribution of pores in uranothorite crystals.
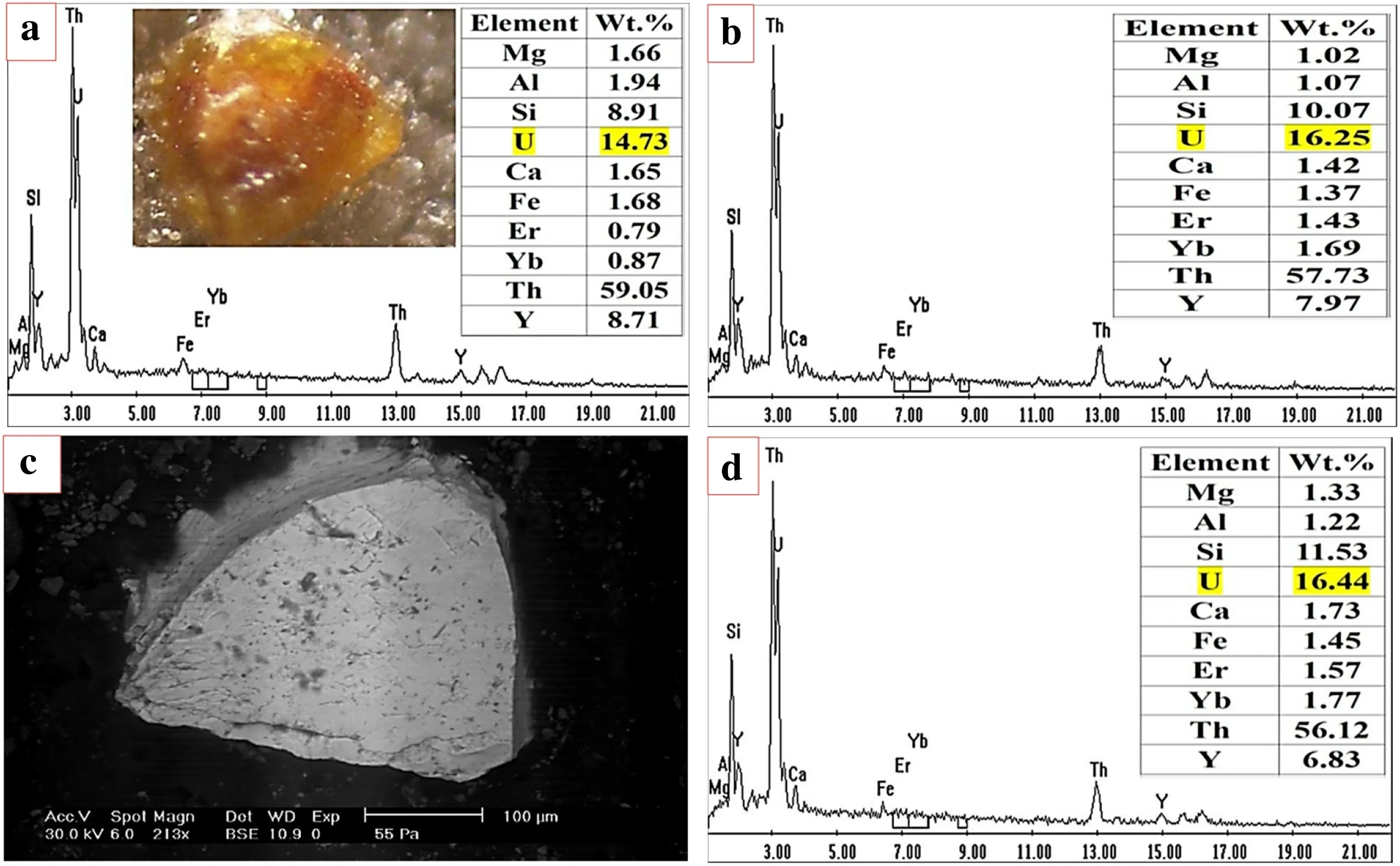
Fig. 13 EDX mineral analysis and BSE and Binocular images of Uranothorite profile across the grain, a at center of grain, b at rim and d at opposite rim
4. Nearly, constant distribution of uranium and REEs across the grain could be related to durability of Zrrich uranothorite grains to alteration.
4.2 Zircon-thorite-xenotime association
In some cases, complete dissolution of some metamictized overgrowth zircon crystals is recorded which evidenced by the presence of zircon remnants in the crystals.Also,some zircon crystals are completely transformed into a yellow variety of thorite(oragnite)and uranothorite with different proportions (Fig. 7).
In addition to solid solutions, the solutions rich in Zr,Hf, Th, U, REEs, and other elements fill the pores in the metamictized zircon and produce mineral inclusions such as thorite, uranothorite, xenotime, cassiterite and unknown REE-bearing mineral. Cavities and fissures in the studied zircon are partly or completely filled with Fe-dominant oxide/hydroxide phases, (goethite-like composition) and admixtures of Si, Al, Mg, Ti and Ca are also observed(Fig. 7).
Solid-solution ranges between thorite, xenotime and zircon are recognized in the present study (Fig. 14 and Table 2). Furthermore, the existence of sphalerite and pyrite minerals in framboidal shape, typically formed by microbial processes, are found in the studied sediments(Fig. 15), suggesting the role of sulphides and microorganisms in the reacting solutions.
The REEs average analyses of three granite and ten stream sediment samples in the study area (Table 3) indicated that the total REEs increased from 275.8 ppm in granite to 621.2 ppm in the surrounding stream sediments.Also, the heavy REEs in studied granite and the surrounding stream sediments range from 126.23 to 275.80 ppm and from 281.77 to 339.40 ppm, respectively.
5 Discussion
The identified minerals are mainly represented by thorite,uranothorite, zircon, monazite, xenotime, columbite, fergusonite, unknown REE-bearing minerals and cassiterite.Alteration of thorite, zircon in high and low-temperature solutions was reported by Abd El-Naby (2009). The highest reported U contents in thorite were reported from Ririwai granite, Nigeria (28.9 wt% of UO2; Pointer et al.1988), the Kirchberg Pluton, the Erzgebirge (23.3 wt% of UO2;Fo¨rster 2006)and the highly radioactive ultrapotassic melasyenite porphyry (≤27.6 wt% of UO2; Zˇa´cˇek et al.2009).The recorded zircon grains show partial to complete solid solution with thorite,uranothorite and xenotime.This fact may be supported by: (1) the highly metamictized nature of these minerals due to their high contents of radioelements,(2)the averages of the total REEs are higherin the stream sediments than the surrounding granite, and(3) the complete absence of monazite grains in the heavy fraction of stream sediments in spite of its abundance in the surrounding granite(Shalaby et al.2015).Zircon and other associating minerals (like xenotime, thorite, uranothorite,xenotime monazite and maybe other minerals) may react with the surrounding solutions. These solutions become more enriched with various elements (like Zr, Hf, Th, U,and REEs) and affect the previously occurring minerals with different degrees depending on the mineral durability.Zircon and thorite are the most minerals susceptible to metamictization due to their radioelement contents. So,solid solutions with different ranges could be resulted between these two minerals and produce zirconian thorite,thorian zircon, yttrian zircon, and yttrian thorite. Seifert et al. (2012) classified the minerals hosted in the zircon grains into three types:

Fig. 14 Composition of thorite and zircon plotted on the basis of SiO2–ThO2–ZrO2. The shaded field encloses the composition of thorite–zircon solid solution. See also Breiter et al. (2006),Fo¨rster(2006)and Abd El-Naby (2009)
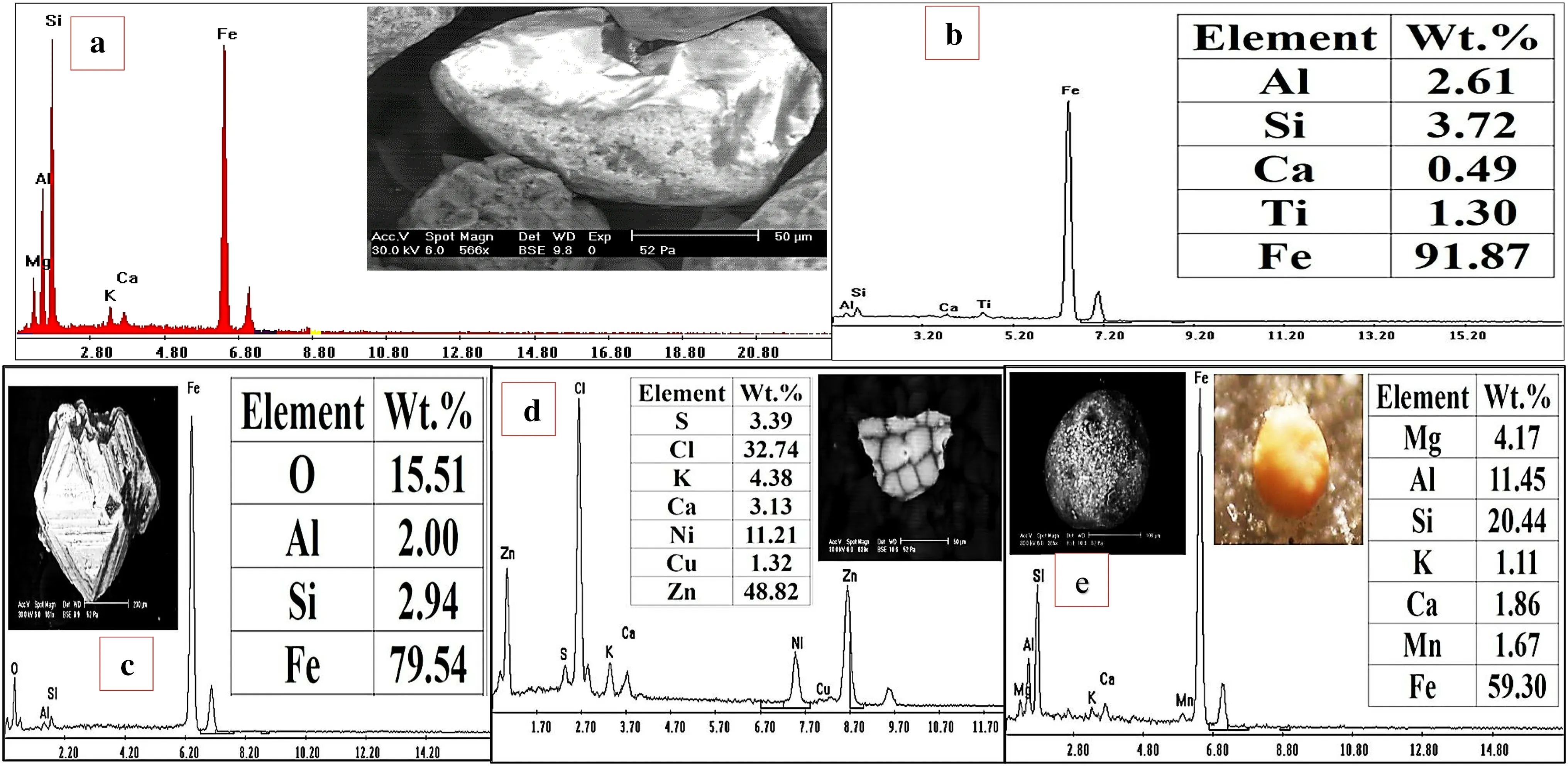
Fig. 15 a–c BSE images and EDX analyses of iron oxides and oxy-hydroxides minerals.d BSE image and EDX analyses of biogenic sphalerite.e Binocular photomicrograph, BSE image and EDX analyses of framboidal pyrite
Type-I: Primary inclusions (protogenetic and syngenetic).
Type-II:Secondary(epigenetic)inclusions.These inclusions may be formed by dissolution–re-precipitation processes using the elemental budget of the host (type-IIa) or by metasomatic addition of elements from external sources (type IIb).
Type-III: Finally, metasomatic processes postdating the formation of type-I and type-II inclusions again may alter primary and secondary inclusions.
The complex internal textures, involving (oscillatory)growth and patchy zonation patterns, the presence of secondary mineral inclusions and pore spaces are early reported during BSE studies of zircon (Nasdala et al.2009, 2010; Seifert et al. 2012). The entrance of other‘‘non-formula’’ elements into (thorite and zircon) and the removal of the original components by post-magmatic,low-temperature solutions are very probable. Metamictization and hydration lead to the volume expansion and consequent formation of the cracks.These cracks enhanced the fluid flux and related interaction processes (Pointer et al. 1988; Zˇa´cˇek et al. 2009).
It is worthy to mention that there is a clear difference between zircon types with depth;this may be resulted from changing in oxidation–reduction conditions as will be shown in the following paragraph.
5.1 Zr in the first 90 cm of the stream sediments section
In the studied zircon of W. El-Reddah area, it contains appreciable amounts of Hf, Fe, Ca, Al, Mg, U, and Th.Also,the results indicate that there is a preponderance of U over Th, which reveals that the radioactivity of zircon is related to U rather than Th. Zircon-thorite exsolusion intergrowths rarely occur in some zircon mineral grains in the form irregular masses of thorite within zircon crystals.The origin of exsolution intergrowths of thorite in zircon may be due to contemporaneous crystallization of zircon and thorite. Some zircon grains contain the inclusion of other minerals such as thorite,apatite,silicate minerals and rutile (Fig. 16).
5.2 Zr from 90 to 220 cm of the stream sediments section
Most minerals are partially or wholly altered and some minerals were dissolved resulting in their replacing by other minerals (pseudomorphism). This is indicated by replacing of zircon by xenotime, thorite and uranothorite.Results confirm the role of low-temperature alteration in changing the structure and composition of the studied mineral in the stream.
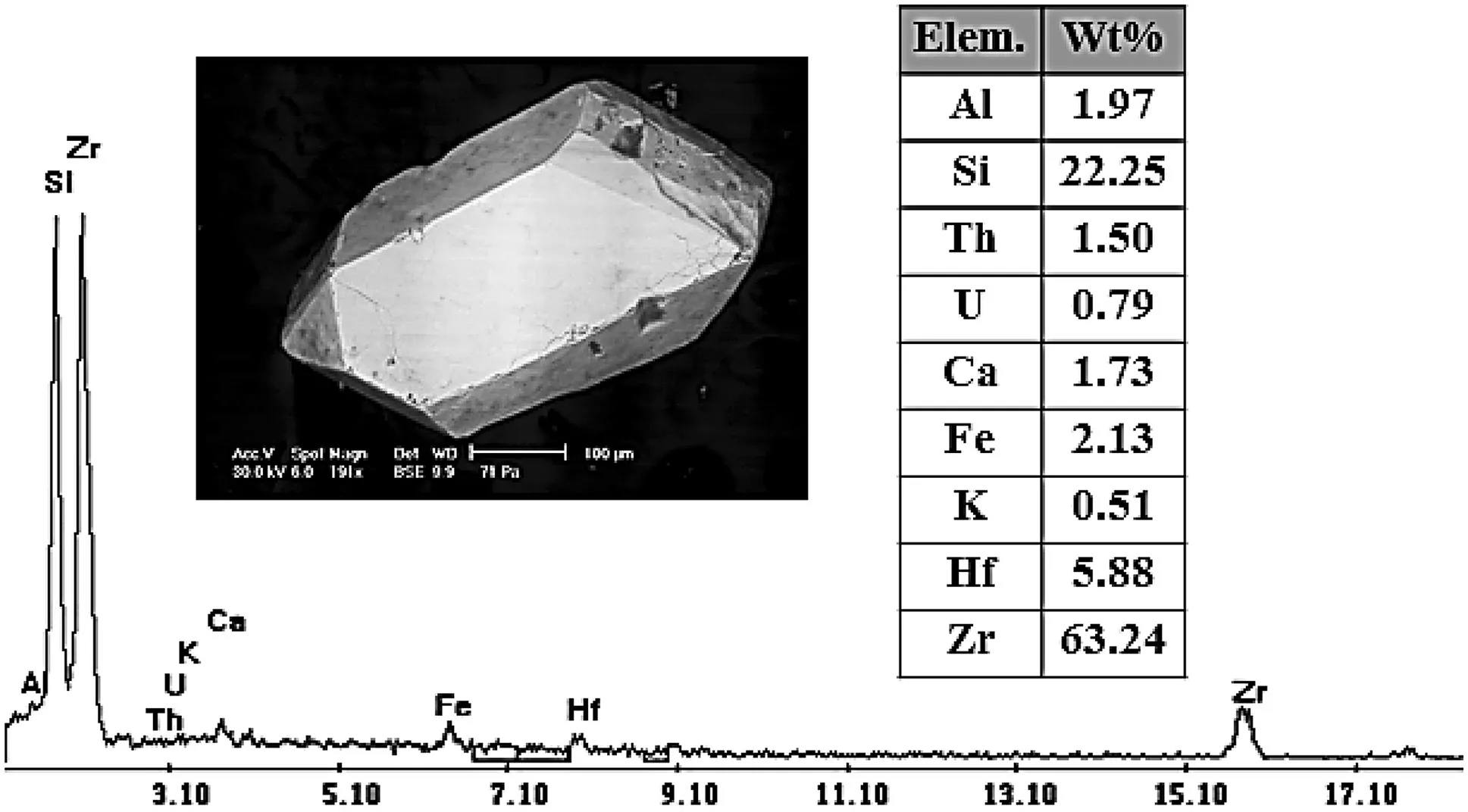
Fig. 16 EDX mineral analyses and BSE image of zircon in the first 90 cm from stream sediments of Wadi El-Reddah
Also, there is a clear difference zircon composition in the source granitic rocks (Gattar and El Reddah granites)(Table 1) and zircon in the stream sediments of Wadi El Reddah.
The Zr/Hf ratio in igneous zircon may reach to about 39 in syenite (Murali et al. 1983) and 52 in evolved A-type arfvedsonite granite (Wang et al. 2000). Irber (1999)indicated that the Zr/Hf ratio for peraluminous granite of Mideastern Germany varies between 9 and 39. He mentioned that granite with lower Zr/Hf ratio (<20) was affected by strong magmatic hydrothermal alteration. The Zr/Hf ratio in common granite is about 39 (Erlank et al.1978) and is mostly close to the chondritic ratio of 38(Andres and Grevesse 1989). The average Zr/Hf ratio in pegmatites is about 25, (Erlank et al. 1978) and shifts toward smaller ratio with increase evolution of the silicate melt. Weyer et al. (2002) recorded that the most precise chondritic ratio for Zr/Hf = 34 ± 0.3. On the other hand,Zr/Hf ratios of the studied zircon grains in Wadi El Reddah area range between 5.22 and 39.1 with an average of 14.27(Table 2). It appears that this ratio in the investigated zircon is controlled to a great extent by pore metamorphic fluids that bear Cl, F, SO42+and CO2, which are responsible for U and Th redistribution in zircon (Pidgeon et al.2000).
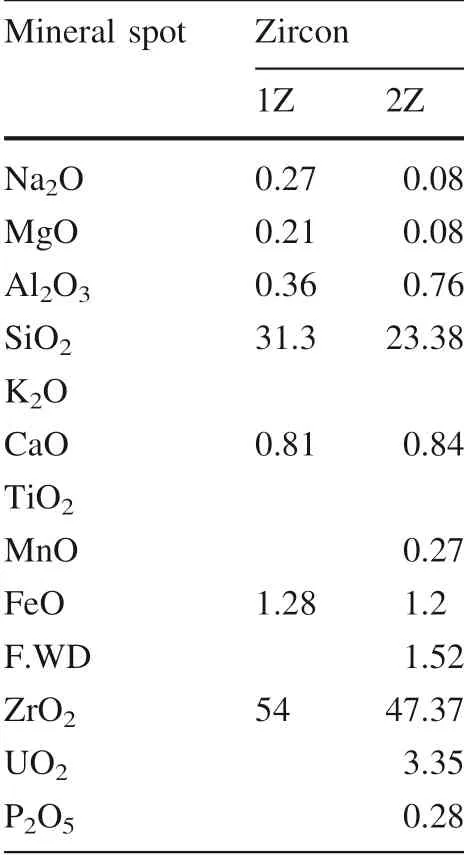
Table 1 Zircon composition in the source granitic rocks. After Shalaby et al. (2015)
5.3 Evolution of Zr/Hf ratio and other nonformula elements in zircon
The concentration of Hf in zircon is variable and depends on the magmatic evolution(Wang et al.2000).Hf contents in the studied zircon of the studied stream sediments range from 1.09 to 21.61 wt% with 6.52 wt% as an average. On the other hand, Zr/Hf ratios range from 3.14 to 39.01 and averaging 13.63, suggesting that the studied zircon is mainly related to the surrounding granitic rocks and their associating pegmatite pockets. The diagram Zr/Hf versus Hf of Cerny et al.(1985)(Fig. 17a),indicate that the zircon originated from the surrounding Gattar alkali feldspar granite and El Reddah monzogranite plots in the normal granitic domain, whereas zircon of the pegmatites associating these rocks plots in domains of granitic and pegmatitic rocks. The pegmatitic affinity of hafnian zircon, is known from F-rich granitoids (Wang et al.1992, 1996, 2000). However, increased contents of the incompatible trace elements (ITEs) as P, together with Fe,Al and Ca,in addition to the weak or ill-defined correlation between P and these elements could be explained by partial leaching of some elements during metamictization and entrance of other nonformula elements (Fig. 17b–d).
Zircon can crystallize under pressure (P)–temperature(T) conditions ranging from those in the mantle to the low P and T pertaining to diagenesis. Low-temperature zircon growth, including examples from sedimentary environments, has been well documented in the literature (Geisler et al.2003a,b;Rasmussen 2005;Hay and Dempster 2009;Hay et al.2010).Metamictization in zircon results from αdecay events in the natural radioactive decay of238U,235U,and232Th and their daughter products. Specifically, the high energy α-particle produces hundreds of atomic displacements with a range of ~10 nm, and the energetic recoil nucleus, with a range of ~20 nm, produces localized damage with about 1000 atomic displacements (Weber et al. 1994). The physical changes in zircon that result from radiation damage include an increase in unit-cell dimensions and decreases in density and hardness(Holland and Gottfried 1955; Speer et al. 1981). The different interference colors frequently observed in thin sections of zircon are known to be due to differing levels of a-decay damage. Increasing radiation damage decreases birefringence until the fully metamict crystal is optically isotropic(Sahama 1981). Petrographic studies indicate that the partially damaged portion of zircon appear as a glassy form while the completely damaged portions appear as opaques surrounded by iron oxides.
Referring to the geological history of the region, it is very important to mention that the Gattar–Dokhan area is considered as the rift-shoulder during the time of the Red Sea Rifting tectonics. In addition, Esh El mallaha Range was considered as a high up-lifted or tectonically raised elongated block or island within the Miocene Red Sea paleo-shoreline. When mapping the area of Red Sea coast between 27°00′and 27°10′N several scattered Eocene and Miocene exposures are recorded along the Red Sea coast,between Esh El mallaha and the Red Sea hills and even near the Red Sea hills. The nearest exposure of the Eocene–Miocene succession is located 7 km away from the entrance of W. El Reddah (the studied area).This may give a clue about the location of the shoreline and basin depocentre. The presence of these exposures of Miocene carbonates and evaporites near and close to the basement(close to G. Salat Bali) indicates that the seawater invades and cover most of the promontraiy areas of the basement and may extend through the main engulfed wadies like W.Bali and W. El Reddah (El Kholy et al. 2017). In addition to the unusual Y/Ho ratios and w-type tetrad in the studied sediments rare earth pattern (Ebian et al. 2018). High sulphur contents (Table 3) indicate the role of chemical weathering by the effect of an acidic solution, the role of seawater and microorganisms (Fig. 15) lead to partial or complete dissolution of zircon crystals (Fig. 7) resulting formation of new mineral associations and isomorphism between zircon, thorite, uranothorite and xenotime. Bojanowski et al. (2012) reported that zircon was dissolved from the concretions, transported in fluids, and reprecipitated in voids. Most major and trace elements have been mobilized during chemical weathering and microorganisms due to dissolution or replacement of the main components and accessory minerals, and new formation of mineral phases.
The chemical analyses of the studied stream sediments and their surrounding granites indicate that sulphur contents reach up to 4000 ppm. The high contents of sulfide minerals (pyrite and sphalerite) in these stream sediments resulting in high contents of SO3–which may increase acid concentration and enhance leachability of Zr, Hf, Th, U and REEs with other elements from the recorded mineral association. Due to the occurrence of significant amounts of dissolved sulfide and sulfide minerals, the redox pairs SO42-/HS-, and the heterogeneous redox pairs SO42-/pyrite have been included (Noseck et al. 2009). Furthermore, the existence of sphalerite and pyrite minerals in framboidal shape,typically formed by microbial processes,in the studied sediments is suggesting the role of sulfides and microorganisms in the reacting solutions.

Table 2 Qualitative EDAX analyses of major, trace and rare earth element contents of the studied zircon

Table 2 continued
The presences of fluorine and carbonate-rich solutions are also probably due to fluorite and calcite occurrences in the studied sediments. The mobility of (Y + REE) in(SO4)2-or (CO3)2-fluids is possible, although the degree of solubility is generally lower than for F or Cl(Haas et al.1995; Hetherington et al. 2008). The effects of acidic solution in the complete dissolution of some crystals of highly metamictized overgrowth zircon (Fig. 7a) or thepresence of its remnant indicate the role of these solutions in redistribution of various elements in the studied mineral association. During this process, U could be released from the crystal lattices and metamictization was largely caused by nucleic recoil of U and Th alpha decay. The bombardment of the crystal lattice by large nuclei progressively distorts and eventually destroys the crystal lattice(Holland and Gottfried 1955; Chakoumakos et al. 1987; Murakami et al. 1991; Abd El-Naby 2009). This conclusion is supported by precipitation U, Zr and Y rich thorite, U-rich xenotime and thorite during reduction condition. Zˇa´cˇek et al.,(2009)concluded that the entry of Ca,Fe,Al,S,As,Ti and other‘‘non-formula’’elements into thorite or zircon and the removal of the original components by post-magmatic, low-temperature fluids is highly probable. The interaction between the metamict mineral and hydrothermal fluid results in a change of the chemical composition,whereby the primary substitution mechanisms are obscured.
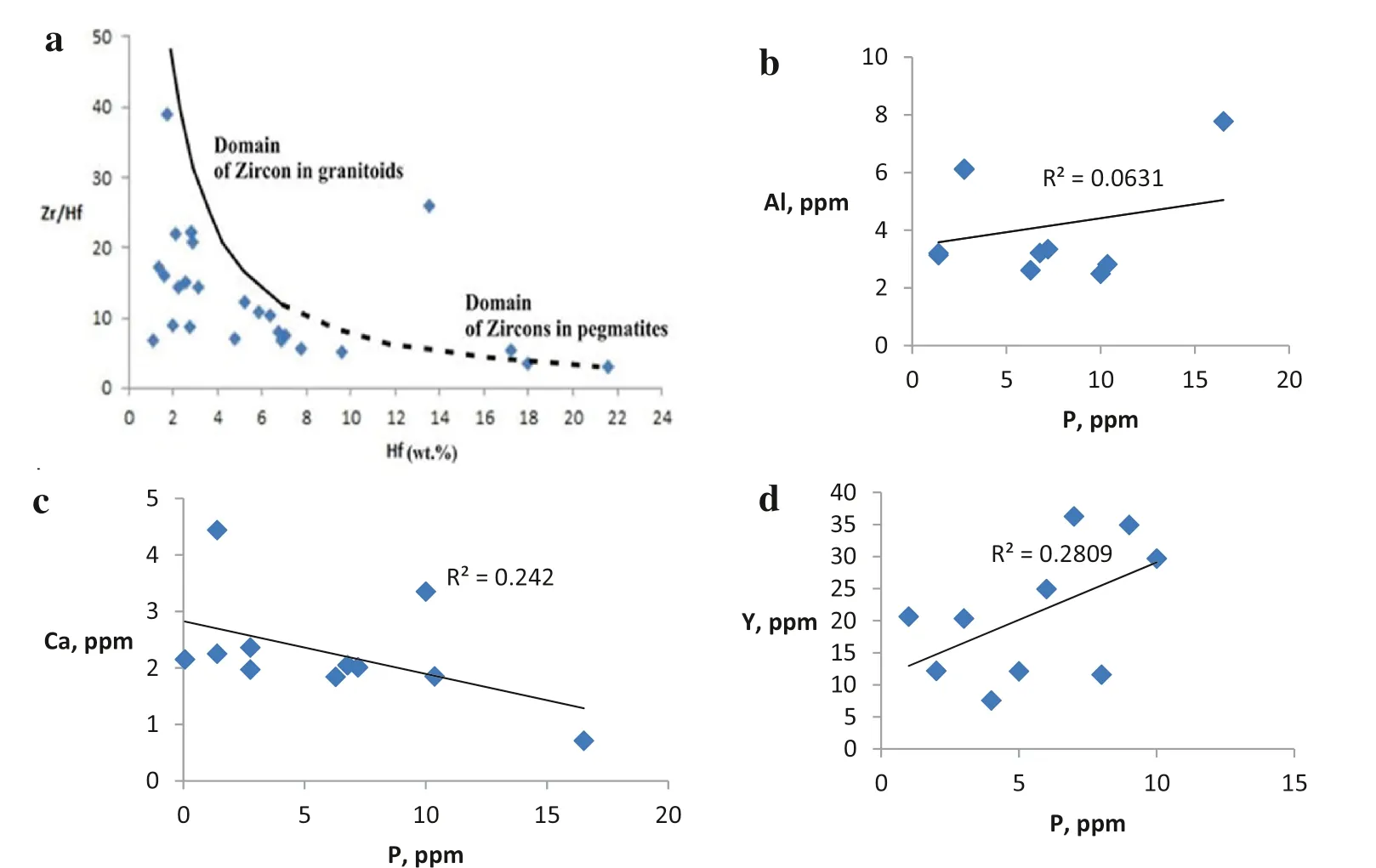
Fig. 17 a Plot of the composition of zircon in the studied stream sediments, in terms of Zr/Hf versus concentration of Hf. The curve represents the theoretical fractionation of Zr/Hf in zircon.The domains of zircons in granitoids and pegmatites,indicated by solid and dashed lines, respectively, are modified after Cerny et al.(1985).b–d PAl, P-Ca and P-Y binary diagrams in zircon of the studied stream sediments respectively
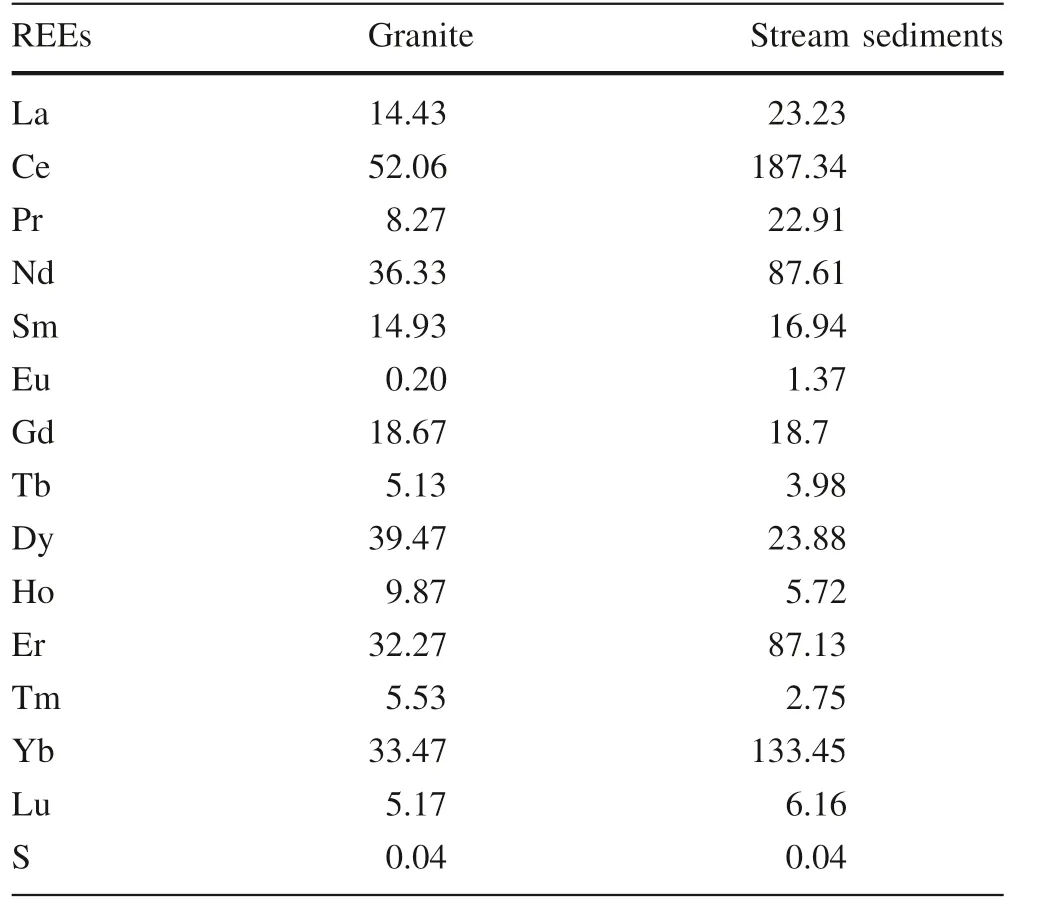
Table 3 Average contents of rare earth element(REE)in the studied granite and the surrounding stream sediments
Metamictization and hydration lead to volume expansion and consequent formation of cracks. These cracks enhanced the fluid flux and related interaction processes,indicating the probability of low-temperature alteration processes. El-Feky (2011) reported that primary uranium mineralization occurs in temperature ranges reach up to 230 °C where Helmy(1999)recorded that the temperature may reach up to 150 °C for secondary uranium mineralization, suggesting low-temperature alteration processes.Shalaby et al. (2015) indicated the role of H2O and F-bearing fluids in alteration of essential and accessory minerals. They mentioned that transformation of uranium element from solution or fluids into the crystal lattice of zircon occurs as U4+in an early stage event. The role of supergene alterations by oxidized meteoric water that flowed down along faults and fracture zones of the host rocks resulted in weathering of the host granite and leaching of uranium. The sources of heat causing hydrothermal remobilization of uranium are the decay of radioelements in the granite and the tectonism (basaltic dykes), which may have been sufficient to produce a convective circulation of fluids in host granites. The foregoing discussion confirms the role of low-temperature alteration(supergene) in changing the structure and composition of the studied mineral whether in the granitic sources or in the stream sediments.

Fig. 18 a–c BSE images and EDX analyses of thorite, light REE with Monazite and solid solution between thorite and xenotime respectively.d Binocular photomicrograph and EDX analyses of spherical apatite. e BSE image and EDX analyses of Thorite inclusion in spherical apatite
Not only, the minerals alteration is the main cause for the discussion of the low-temperature alteration processes but for the following reasons:
Firstly,calculations of La/Y ratios indicate that this ratio lesser than unity in all samples suggesting an acidic environment. In addition to that, the presence of Ce-positive anomaly and Eu-negative anomaly values indicate severe changes in the oxidation–reduction conditions that enhance low-temperature alteration processes. Oxidation–reduction conditions changes also occur by the effect of micro-organisms and this is indicated by the presence of framboidal pyrite and biogenic sphalerite.
The LREE enrichment in the studied stream sediments is relative to the surrounding granitic rocks. Moreover, the nearly complete absence of monazite grains in the stream sediments through their presence in the surrounding hard rocks are indicated by petrographic and mineralogical studies.This is may have resulted in the complete dissolution of refractory monazite grains in low-temperature alteration processes.Su et al.(2017)reported that chemical weathering is responsible for the change of zircon geochemistry in the weathering products. The evidence of these processes is indicated by occurring of monazite and REE minerals as pore filling in the patchy parts of zircon and Thorite grains suggesting partial or complete dissolution of monazite(Fig. 18) and also by the presence of Thorite inclusion in spherical apatite. The complete isomorphism between xenotime, thorite, uranothorite and zircon crystals are mostly observed in the studied stream sediments.Zr/Hf ratios of the studied stream sediments are lesser than their corresponding international values. Also, the non-chondritic Y/Ho, Nb/Ta,Sr/Eu and U/Th ratios indicate the role of alteration processes in these unusual ratios. All these ratios indicate the role of seawater and hydrothermal alteration processes in the mobilization of major,trace and REE elements(Ebian et al.2018).So, physic-chemical changes and the assistant role of microorganisms play the main role and confirm the incidence of low-temperature alteration processes.
6 Conclusions
The main reasons for expecting low-temperature alteration processes are the presence of some overgrowth zircon crystals completely dissolute leaving only their remnants.In addition,the presence of dark patchy domains filled with unusual mineral in this media like cassiterite as pore filling in zircon crystals indicating partial dissolution.La/Y ratios than unity in all samples suggesting an acidic environment,in addition,Ce-positive anomaly and Eu-negative anomaly values indicate severe changes in the oxidation–reduction conditions that enhance low-temperature alteration processes. However, unusual Zr/Hf, Y/Ho, Nb/Ta, Sr/Eu and U/Th ratios indicate the role of alteration processes in these changes. The abundance of monazite crystals in the granitic rocks surrounded the studied stream in contrast to their rarity in the studied stream, also the occurrence of biogenic minerals could be considered as clear evidence for the presence of low enhance low-temperature alteration processes. The study indicates the presence of near uranium mineralization in the studied sediments.There is also the probability for the presence of Sn, Mo, Au and Pb mineralization in Gattar granite and the studied sediments.Also, the role of microorganisms in stream sediments mineralization needs further studies. Wadi El Reddah is a promising area so we recommend our Authority to carry out a project of heap leaching for U production according to availability and ease of U obtaining.The probability for the presence of more U-occurrences in Gattar and El-Reddah granites are clarified by increasing radioactivity around these peaks. The probability of finding REE and U mineralization at the contact with basement rocks at higher depths due to change in pH conditions.
AcknowledgementsAuthors thank the Nuclear Materials Authority,Egypt for the facilities used in this work.
Compliance with ethical standards
Conflict of interestOn behalf of all authors, the corresponding author states that there is no conflict of interest.
杂志排行
Acta Geochimica的其它文章
- Chemical structure of the Earth’s mantle defined by fast diffusion elements like helium
- A helium stratified and ingassed lower mantle: resolving the helium paradoxes
- Mechanism of accelerated dissolution of mineral crystals by cavitation erosion
- Zircon chemistry and new laser ablation U-Pb ages for uraniferous granitoids in SW Cameroon
- Zircon U-Pb age and geochemical constraints on the origin and tectonic implication of the Tuotuohe Cenozoic alkaline magmatism in Qinghai-Tibet Plateau
- Evaluation of the potential risks of heavy metal contamination in rice paddy soils around an abandoned Hg mine area in Southwest China
