胎膜早破患者绒毛膜羊膜炎的发生情况及NLRP3炎症小体表达与炎症因子、蛋白酶的相关性
2020-04-03蔡树梅王亚敏陈辉
蔡树梅 王亚敏 陈辉
[摘要] 目的 探討胎膜早破患者绒毛膜羊膜炎的发生情况及NLRP3炎症小体表达与炎症因子、蛋白酶的相关性。 方法 选择2017年3月~2019年2月河北省邯郸市妇幼保健院收治的胎膜早破孕妇138例,回顾性分析胎膜早破孕妇绒毛膜羊膜炎发生情况将患者分为感染组49例、非感染组89例。比较两组患者绒毛膜羊膜组织中NLRP3炎症小体的表达量,以及血清、羊水标本中炎症因子[白细胞介素-6(IL-6)、IL-8、IL-18、肿瘤坏死因子-α(TNF-α)]、蛋白酶[中性粒细胞弹性蛋白酶(NE)、基质金属蛋白酶-2(MMP-2)、MMP-9]水平的差异。采用Pearson检验评估胎膜早破合并绒毛膜羊膜炎患者NLRP3炎症小体表达量与病情相关指标的内在联系。 结果 感染组患者分娩后绒毛膜羊膜组织中NLRP3炎症小体的表达量高于非感染组(P < 0.05)。感染组患者血清及羊水标本中,IL-6、IL-8、IL-18、TNF-α的水平高于非感染组;NE、MMP-2、MMP-9的水平高于非感染组(P < 0.05)。相关性分析发现,胎膜早破合并绒毛膜羊膜炎患者绒毛膜羊膜组织中NLRP3炎症小体表达量与IL-6、IL-8、IL-18、TNF-α、NE、MMP-2、MMP-9的水平呈正相关(rNLRP3 = 0.674、0.718、0.698、0.829、0.759、0.726、0.882;rASC = 0.704、0.783、0.627、0.599、0.771、0.804、0.837;rcaspase-1 = 0.667、0.572、0.782、0.705、0.812、0.632、0.576,均P < 0.05)。 结论 高表达的NLRP3炎症小体可能是胎膜早破孕妇合并绒毛膜羊膜炎的重要机制之一。
[关键词] 胎膜早破;绒毛膜羊膜炎;NLRP3炎症小体;炎症因子;蛋白酶
[中图分类号] R714.4 [文献标识码] A [文章编号] 1673-7210(2020)02(c)-0107-04
[Abstract] Objective To investigate the incidence of chorionic amnitis in patients with premature rupture of fetal membranes and the correlation between NLRP3 expression and Inflammatory cytokines, proteases. Methods From March 2017 to February 2019, 138 cases of pregnant women with premature rupture of fetal membrane admitted to Handan Maternal and Child Health Hospital in Hebei Province were selected. The occurrence of chorionic amnitis in pregnant women with premature rupture of fetal membrane was retrospectively analyzed, and the patients were divided into infection group (49 cases) and non-infection group (89 cases). Placenta tissue expression of NLRP3 inflammatory corpuscle, inflammatory cytokines[interleukin 6 (IL-6) and IL-8 and IL-18, tumor necrosis factor-α (TNF-α)], protease[neutrophil elastase(NE), matrix metalloproteinase-2(MMP-2) and matrix MMP-9] levels in serum and amniotic fluid specimens between two groups were compared. Pearson test was used to evaluate the correlation between expression of NLRP3 inflammatory corpuscle and disease-related indicators in patients with premature rupture of membranes and chorionic amnitis. Results Expression level of NLRP3 inflammatory corpuscle in infected group was higher than that in non-infected group (P < 0.05). Levels of IL-6, IL-8, IL-18 and TNF-α in serum and amniotic fluid were higher than those in non-infected group; levels of NE, MMP-2 and MMP-9 were higher than those of non-infected group (P < 0.05). Correlation analysis showed that the expression of NLRP3 inflammasome in placental tissues of premature rupture of membranes patients combined with chorionic amnitis was positively correlated with the levels of IL-6, IL-8, IL-18, TNF-α, NE, MMP-2 and MMP-9(rNLRP3=0.674, 0.718, 0.698, 0.829, 0.759, 0.726, 0.882;rASC = 0.704, 0.783, 0.627, 0.599, 0.771, 0.804, 0.837;rcaspase-1=0.667, 0.572, 0.782, 0.705, 0.812, 0.632, 0.576, all P < 0.05). Conclusion High expression of NLRP3 inflammasome may be one of the important mechanisms of premature rupture of membranes in pregnant women with chorionic amnitis.
[Key words] Premature rupture of fetal membranes; Chorioamnionitis; NLRP3 inflammatory corpuscle; Inflammatory cytokines; Protease
絨毛膜羊膜炎是病原体感染胎盘的绒毛膜羊膜而导致的炎症性疾病,是临床胎膜早破、早产及母儿围生期感染的重要病因[1-2]。孕妇合并绒毛膜羊膜炎时,新生儿败血症、呼吸窘迫、癫痫等发病率大幅提升,显著增加胎儿及新生儿的死亡率,故早期发现绒毛膜羊膜炎并明确其严重程度在降低新生儿患病率、提升新生儿存活率方面具有重要意义[3-4]。目前临床中仍缺乏绒毛膜羊膜炎快速准确诊断的可靠手段,有研究发现NLRP3炎症小体在复发性流产[5]、子痫前期[6]等患者胎盘组织中的表达量明显增加,推测其在绒毛膜羊膜炎的发生发展中可能也扮演重要角色。本研究中检测胎膜早破合并绒毛膜羊膜炎产妇绒毛膜羊膜组织中NLRP3炎症小体的表达情况,并进一步分析其表达量与炎症因子、蛋白酶水平的内在联系,旨在明确NLRP3炎症小体在胎膜早破患者绒毛膜羊膜炎发生中扮演的角色。
1 资料与方法
1.1 一般资料
选取2017年3月~2019年2月河北省邯郸市妇幼保健院收治的胎膜早破孕妇138例作为研究对象,纳入标准:①单胎活产;②胎膜早破发生孕龄≥32周;③孕妇年龄20~45周岁;④本人签署知情同意书。排除标准:①合并梅毒、艾滋病等性传播疾病;②孕期手术史。回顾性分析胎膜早破孕妇绒毛膜羊膜炎发生情况并分为感染组49例和非感染组89例。本研究获医院医学伦理委员会批准。两组患者的基础资料比较,差异无统计学意义(P > 0.05),具有可比性。见表1。
1.2方法
1.2.1 胎盘组织中NLRP3炎症小体表达量 两组患者分娩过程中留取胎盘中的绒毛膜羊膜组织,采用荧光定量PCR法检测其中NLRP3炎症小体(NLRP3、ASC、caspase-1)mRNA的表达量。上述过程中涉及的仪器包括:Realtime PCR仪(美国伯乐公司,型号CFX96)、风光光度计(上海光谱仪器有限公司,型号V-1100P)、离心机(德国SIGMA公司,型号3-18KS)等。
1.2.2 炎症因子、蛋白酶水平检测 入组即刻留取两组患者的外周静脉血及羊水标本,分离上层澄清液后采用酶联免疫吸附法检测其中炎症因子[白细胞介素-6(IL-6)、IL-8、IL-18、肿瘤坏死因子-α(TNF-α)]和蛋白酶[中性粒细胞弹性蛋白酶(NE)、基质金属蛋白酶-2(MMP-2)、MMP-9]的水平,酶联免疫试剂盒购自武汉赛默飞世尔科技公司。
1.3 统计学方法
采用SPSS 20.0对所得数据进行统计学分析。计量资料采用均数±标准差(x±s)表示,组间比较采用t检验;计数资料采用百分率表示,组间比较采用χ2检验。相关性分析采用Pearson检验。以P < 0.05为差异有统计学意义。
2 结果
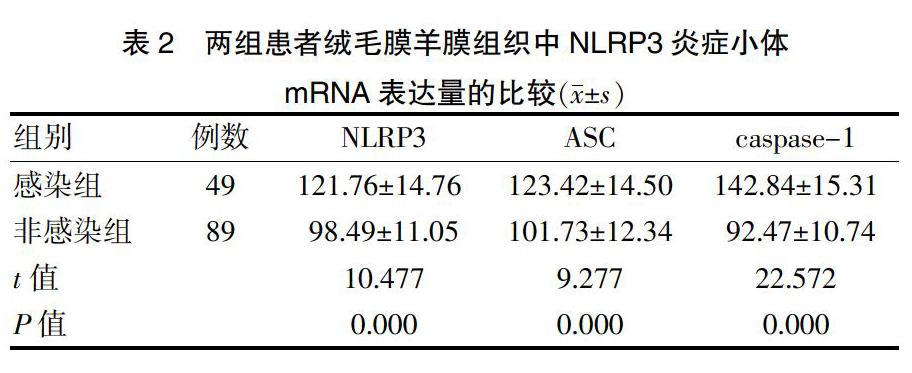
2.1两组患者绒毛膜羊膜组织中NLRP3炎症小体mRNA表达量的比较
感染组患者分娩后胎盘组织中NLRP3、ASC、caspase-1 mRNA的表达量高于非感染组,差异有统计学意义(P < 0.05)。见表2。
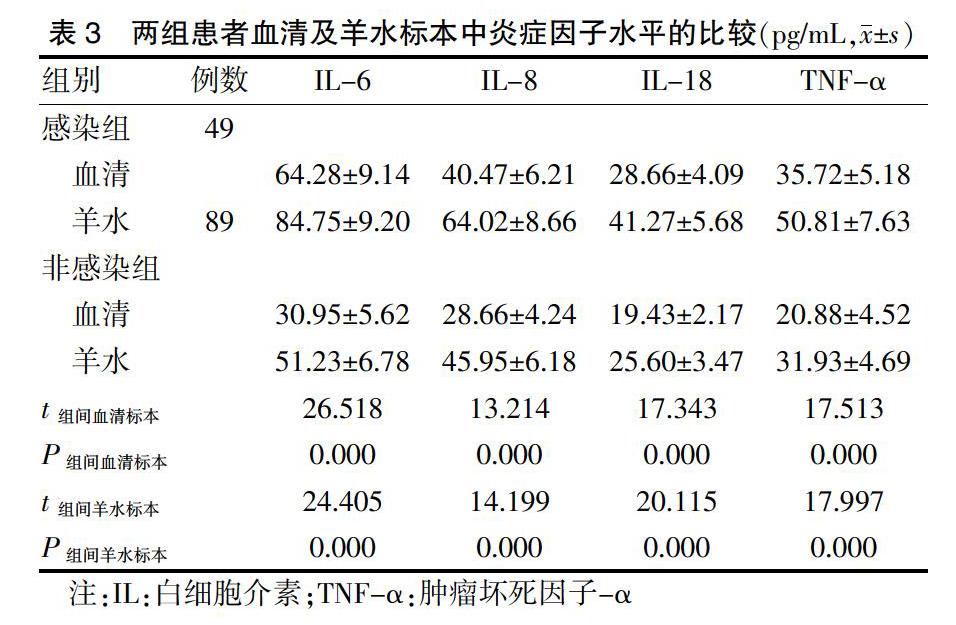
2.2 炎症因子水平检测
感染组患者血清及羊水标本中,IL-6、IL-8、IL-18、TNF-α的水平均高于非感染组患者,差异有统计学意义(P < 0.05)。见表3。
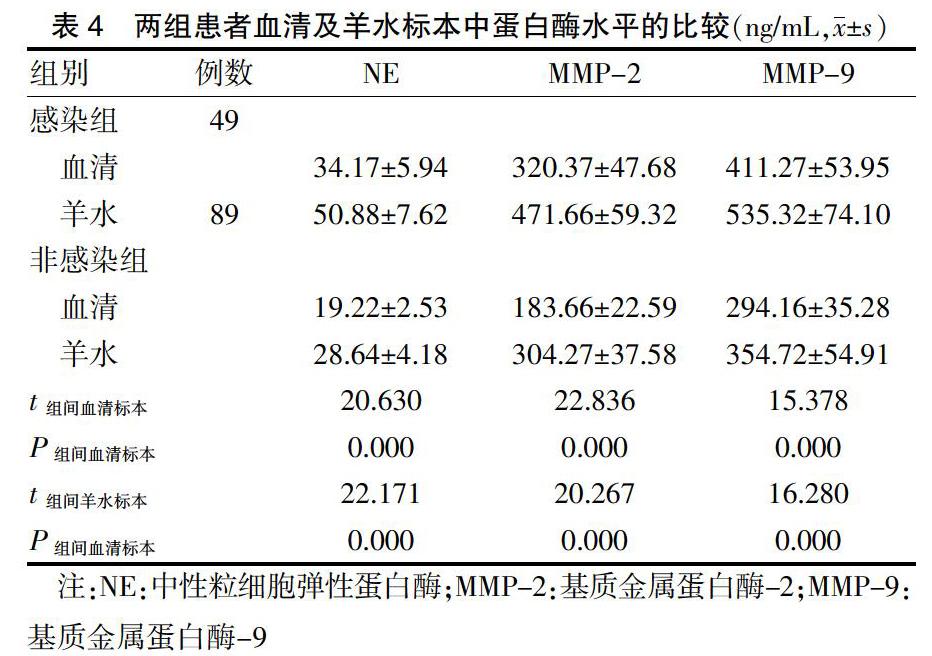
2.3 两组患者血清及羊水标本中蛋白酶水平的比较
感染组患者血清及羊水标本中,NE、MMP-2、MMP-9的水平均高于非感染组患者,差异有统计学意义(P < 0.05)。见表4。
2.4 相关性分析
Pearson相关性分析发现,胎膜早破合并绒毛膜羊膜炎患者NLRP3、ASC、caspase-1 mRNA表达量与血清及羊水标本中炎症因子IL-6、IL-8、IL-18、TNF-α,蛋白酶NE、MMP-2、MMP-9的水平均呈正相关(rNLRP3 = 0.674、0.718、0.698、0.829、0.759、0.726、0.882;rASC=0.704、0.783、0.627、0.599、0.771、0.804、0.837;rcaspase-1=0.667、0.572、0.782、0.705、0.812、0.632、0.576,均P < 0.05)。
3 讨论
绒毛膜羊膜炎是胎膜早破的主要原因,其发病机制尚未完全明确。NLRP3炎症小体是一种典型的炎症复合体,由NLRP3及下游因子ASC、caspase-1共同组成,表达于中性粒细胞、巨噬细胞中,可与多种宿主发生免疫及炎性反应[7-9]。本研究中胎膜早破合并绒毛膜羊膜炎患者的绒毛膜羊膜组织中NLRP3、ASC、caspase-1 mRNA表达量均较高,提示异常高表达的NLRP3炎症小体参与绒毛膜羊膜炎的发生。关于NLRP3炎症小体异常表达对绒毛膜羊膜炎的具体影响,以下从炎症因子、蛋白酶两方面展开研究。
绒毛膜羊膜炎作为炎症性疾病,存在多种炎症因子的异常高表达,是导致母婴组织脏器功能损伤甚至死亡的最直接因素。IL-6、IL-8、IL-18、TNF-α均是临床研究最多的炎症因子,由聚集的单核巨噬细胞分泌,随胎膜破裂时间延长、上述促炎因子的水平持续增高[10-12]。本研究中胎膜早破合并绒毛膜羊膜炎患者血清及羊水标本中上述炎症因子水平较高,且相关性分析发现此类患者绒毛膜羊膜组织中NLRP3炎症小体表达量与各个炎症因子水平呈正相关,推测NLRP3炎症小体被激活后可刺激炎症因子大量分泌并扩大患者的宫腔局部及全身炎性反应。
有研究[13-14]指出,羊水中NE水平是診断绒毛膜羊膜炎的敏感指标之一,且与该病严重程度间存在良好的相关性。MMPs具有裂解胶原及弹性蛋白的作用,MMP-2、MMP-9是其主要亚型,感染状态下局部白细胞浸润可增加其分泌[15-16]。胎膜的弹性依赖于细胞外基质的胶原,而MMP-2、MMP-9可通过改变细胞外基质微环境而调节妊娠进展的张力、容量等[17-18]。研究[19-20]发现在胎膜早破患者的胎膜组织中存在MMPs的异常高表达,在胎膜结构弱化中扮演重要角色。本研究中胎膜早破患者,合并绒毛膜羊膜炎的患者母体血清及羊水标本中NE、MMP-2、MMP-9的水平进一步增加,提示高表达的NE、MMP-2、MMP-9是胎膜早破孕妇合并存在绒毛膜羊膜炎的重要标志。相关性分析进一步指出,胎膜早破合并绒毛膜羊膜炎患者绒毛膜羊膜组织中NLRP3炎症小体表达量与NE、MMP-2、MMP-9表达量呈正相关,推测NLRP3炎症小体激活后通过刺激上述蛋白酶表达从而进一步弱化胎膜结构、增加胎膜早破风险。
综上所述,胎膜早破合并绒毛膜羊膜炎患者绒毛膜羊膜组织中NLRP3炎症小体异常高表达可能通过促进炎症因子、蛋白酶表达而参与疾病发生发展。
[参考文献]
[1] Devillard E,Delabaere A,Rouzaire M,et al. Induction of labour in case of premature rupture of membranes at term with an unfavourable cervix:protocol for a randomised controlled trial comparing double balloon catheter (+oxytocin) and vaginal prostaglandin (RUBAPRO) treatments [J]. BMJ Open,2019,9(6):e026090.
[2] Imachi Y,Hidaka N,Kai S,et al. Prolongation of Second Twin′s Delivery Until Term:A Rare Case of Delayed-Interval Delivery [J]. Clin Med Res,2019,17(1/2):37-40.
[3] Oh KJ,Romero R,Park JY,et al. The earlier the gestational age,the greater the intensity of the intra-amniotic inflammatory response in women with preterm premature rupture of membranes and amniotic fluid infection by Ureaplasma species [J]. J Perinat Med,2019,47(5):516-527.
[4] Silwedel C,Fehrholz M,Speer CP,et al. Differential modulation of pulmonary caspases:Is this the key to Ureaplasma-driven chronic inflammation? [J]. PLoS One,2019, 14(5):e0216569.
[5] 高鹏,莫春艳,龚洵,等.复发性流产患者绒毛和蜕膜中NLRP3炎症小体的差异性表达研究[J].现代妇产科进展,2018,27(10):762-765.
[6] 杨勇,张华.TXNIP激活NLRP3炎性小体在子痫前期发病中的作用[J].重庆医科大学学报,2016,41(7):658-661.
[7] Chaves MM,Sinflorio DA,Thorstenberg ML,et al. Non-canonical NLRP3 inflammasome activation and IL-1β signaling are necessary to L. amazonensis control mediated by P2X7 receptor and leukotriene B4 [J]. PLoS Pathog,2019,15(6):e1007887.
[8] Martínez-García JJ,Martínez-Banaclocha H,Angosto-Bazarra D,et al. P2X7 receptor induces mitochondrial failure in monocytes and compromises NLRP3 inflammasome activation during sepsis [J]. Nat Commun,2019,10(1):2711.
[9] Dumont A,de Rosny C,Kieu TL,et al. Docosahexaenoic acid inhibits both NLRP3 inflammasome assembly and JNK-mediated mature IL-1β secretion in 5-fluorouracil-treated MDSC:implication in cancer treatment [J]. Cell Death Dis,2019,10(7):485.
[10] Plazyo O,Romero R,Unkel R,et al. HMGB1 Induces an Inflammatory Response in the Chorioamniotic Membranes That Is Partially Mediated by the Inflammasome [J]. Biol Reprod,2016,95(6):130.
[11] 熊炜,赵倩.胎膜早破及绒毛膜羊膜炎孕母孕期不同时间点的血清IL-6及IL-18的表达水平及意义[J].广东医学,2018,39(6):900-903.
[12] 周静,陈萍,郑雅萍,等.感染性早产产妇血清MCP-1与sTNFR-Ⅰ和HMGB1及TNF-α的表达[J].中华医院感染学杂志,2019,29(3):436-439.
[13] 陈丹丹,卢丹.蛋白酶/抗蛋白酶系统与胎膜早破合并绒毛膜羊膜炎的关系[J].中国实用医刊,2017,44(2):122-124.
[14] 翁廷松,何美玲,刘非,等.未足月胎膜早破合并绒毛膜羊膜炎孕妇血清中NE,Ox-AT及IL-8水平变化及临床意义[J].现代生物医学进展,2017,17(1):133-136.
[15] Yuasa M,Saito M,Molina C,et al. Unexpected timely fracture union in matrix metalloproteinase 9 deficient mice [J]. PLoS One,2018,13(5):e0198088.
[16] Muhammad S,Planz O,Schwaninger M. Increased Plasma Matrix Metalloproteinase-9 Levels Contribute to Intracerebral Hemorrhage during Thrombolysis after Concomitant Stroke and Influenza Infection [J]. Cerebrovasc Dis Extra,2016,6(2):50-59.
[17] Oh KJ,Romero R,Park JY,et al. The earlier the gestational age,the greater the intensity of the intra-amniotic inflammatory response in women with preterm premature rupture of membranes and amniotic fluid infection by Ureaplasma species [J]. J Perinat Med,2019,47(5):516-527.
[18] Oh KJ,Romero R,Park JY,et al. A high concentration of fetal fibronectin in cervical secretions increases the risk of intra-amniotic infection and inflammation in patients with preterm labor and intact membranes [J]. J Perinat Med,2019,47(3):288-303.
[19] Holmstr?觟m E,Myntti T,Sorsa T,et al. Cervical and Amniotic Fluid Matrix Metalloproteinase-8 and Interleukin-6 Concentrations in Preterm Pregnancies with or without Preterm Premature Rupture of Membranes [J]. Fetal Diagn Ther,2019,46(2):103-110.
[20] Zuo G,Dong JX,Zhao FF,et al. Expression of matrix metalloproteinase-9 and its substrate level in patients with premature rupture of membranes [J]. J Obstet Gynaecol,2017,37(4):441-445.
(收稿日期:2019-09-23 本文編辑:封 华)
