A cabazitaxel liposome for increased solubility,enhanced antitumor effect and reduced systemic toxicity
2020-01-06JianYou
,Jian You,
aCollege of Pharmacy,Shenyang Pharmaceutical University,Shenyang 110016,China
b College of Pharmaceutical Sciences,Zhejiang University,Hangzhou 310058,China
ABSTRACT The potential side effects of cabazitaxel(CBZ)in the field of cancer treatment have become a great limitation to its further clinical application.Liposomal delivery is a well-established approach to increase the therapeutic index of hydrophobic drugs.In this study,a PEGmodified liposome was developed for efficiently encapsulating CBZ,thus enhancing its specific tumor inhibition effect and reducing the systemic toxicity.It was found that the loading efficiency of CBZ into the liposome could be improved with the increase of lipophilic materials,as it could be over 80% under the weight ratio of 20:1(total lipid:CBZ).The diameter of CBZ loaded liposome(CBZ@Lipo)was~100 nm.And the liposome suspending in aqueous medium was stable at 4 °C for at least one month,according to the change of its size distribution.The killing ability of CBZ@Lipo to cancer cells was significantly lower comparing to that of CBZ solution,which could be attributed to the slow release of CBZ from the liposomes.However,CBZ@Lipo could induce an obvious apoptosis of the cancer cells at low concentration.Furthermore,CBZ@Lipo exhibited an expressively enhanced tumor growth inhibition effect comparing to CBZ solution.More importantly,CBZ@Lipo showed an obviously higher biosafety proved by lower hemolysis probability,stable body weight of mice during the whole experiment and no obvious lesion in histology analysis.Our work provided a useful reference of the formulation of CBZ,which had potential for greater clinical application.
Keywords:Cabazitaxel Liposome Stability Apoptosis Biosafety
1.Introduction
Chemotherapy is still the reliant treatment strategy for most of cancers in the mid or late stage.At present,most of the chemotherapy drugs are administrated as intravenous injection,which requires a good water solubility of the drugs.However,most drugs are poorly water-soluble.Thus,tween 80,ethanol and other organic reagents are usually used to improve the solubility of the drugs[1].Depressingly,such preparations have shown common adverse reactions in clinical applications[2].Researchers have explored varieties of drug carriers to avoid the severe side effects caused by solubilizer[3-5].Liposomes with good biocompatibility,cytocompatibility and biodegradability have drawn considerable attention in cancer therapy due to its notable ability to prolong blood circulation,keep continuous tumor accumulation as well as increase the solubility of hydrophobic drugs[6,7].
Cabazitaxel(CBZ),a second-line drug for prostate cancer is a small molecule compound which belongs to chemical semisynthetic taxane class[8].CBZ is one of tubulin inhibitors,whose characteristics and acting mechanism are similar to paclitaxel and docetaxel[9].It can bind to tubulin,promote self-assembly so as to form microtubules and further prevent the microtubules from disintegrating thereby inhibiting cell mitosis[10,11].Jevtana®,a commercial product of CBZ,is often used together with prednisone for the treatment of hormone-refractory metastatic prostate cancer[12].Unfortunately,Jevtana®is almost insoluble even in the mixed solvent of water and organic reagent which causes many adverse reactions in clinical treatment,such as neutropenia,hyper sensitivity reactions,gastrointestinal disturbances and renal failure[13,14].Therefore,it is of great significance to find an appropriate drug delivery system for improving the water solubility as well as reducing the side effect of CBZ.
Here,we designed a CBZ encapsulated liposome(CBZ@Lipo)which could efficiently improve the poor solubility and biosafety of CBZ.By optimizing various liposomes,a final formulation of CBZ@Lipo with high drug encapsulation efficiency,small and uniform particle size as well as excellent stability was achieved.Comparing with CBZ solution,CBZ@Lipo displayed a better therapeutic effect and lower toxicity bothin vivoandin vitrostudy,thus possessing great potential for future clinical application.
2.Materials and methods
2.1.Materials
Distearoyl-sn-glycerol-3-phosphoethanolamine-N-[maleimide(polyethyleneglycol)-2000](DSPE-PEG2000),egg phosphatidyl lipid-80(E80),and cholesterol were purchased from Lipoid GmbH(Ludwigshafen,Germany).CBZ was purchased from Taxus Co,Ltd.(Jiangsu,China).6-coumarin,3-(4,5-dimethy lthiazol-2-yl)-2,5-diphenyltetrazolium bromide(MTT reagent),and DAPI were purchased from Sigma(St Louis,USA).EthD-1 and Calcein AM(Live/Dead Viability/Cytotoxicity Kit)were acquired from Life Technologies(Carlsbad,CA).FITCAnnexin V/PI apoptosis kit was provided by MultiSciences Biotech(Zhejiang,China).RPMI 1640 medium(RPMI),Dulbecco’s modified Eagle’s medium,fetal bovine serum(FBS),and penicillin/streptomycin(100 U/ml)were purchased from JiNuo Biotechnology Co.,Ltd.(Zhejiang,China).Dimethyl sulfoxide(DMSO),methanol,and trichloromethane(CHCl3)were obtained from Sinopharm Chemical Reagent Co.All other chemicals were of analytical grade and used without further purification.The deionized water used in all experiments was prepared using a Milli-Q system(Millipore,Boston).
2.2.Cell culture and animals
CT-26(mouse colon cancer)and 4T1(mouse breast cancer)cells were obtained from the Shanghai Cell Bank,Chinese Academy of Sciences(CAS).CT-26 cells were cultured in RPMI 1640 medium and 4T1 cells were cultivated in Dulbecco’s modified Eagle’s medium(DMEM)-containing 10%fetal bovine serum(FBS),1%penicillin and 1%streptomycin sulfate(Invitrogen,USA).All cells were kept at 37°C in a humidified atmosphere containing 5%CO2.
All animal experiments were performed in accordance with the regulations of the Institutional Animal Care and Use Committee(IACUC)of Zhejiang University.Balb/c mice(4-5 weeks old,18±2 g)were raised under aseptic condition in animal isolators with free access to food and water and accepted a circulation of 12 h light/dark.Tumor models were established by subcutaneously injecting 1.0×106CT-26 cells into Balb/c mice or 1.0×1064T1 cells into the mammary fatty pad of BALB/c mice.During the experiment,animals were observed daily for any clinically relevant abnormalities.Tumor over-size(>3000 mm3),severe loss of body weight(>20%)and appearance of large ulceration were considered as poor life quality.As a result,any mice showed the above behaviors was sacrificed by CO2inhalation.
2.3.Preparation of CBZ loaded liposome(CBZ@Lipo)
In order to obtain an optimal formulation,CBZ loaded liposomes with four different weight ratios of phospholipid and cholesterol(2:1,4:1,6:1,8:1)or different drug content were prepared by film dispersion method.Briefly,CBZ,E80,cholesterol and DSPE-PEG2000 were dissolved in chloroform.Then chloroform was evaporated under vacuum at 30°C for 60 min to form a thin film on the walls of the flask.A certain volume of PBS solution(pH 7.4)was then added into the film followed by a stirring at 30°C water bath to obtain a crude dispersion of liposomes.After sonicating for 10 min,homogeneous liposomes were obtained and were further purified by size-exclusion chromatography using a Sephadex G-50column eluted with PBS.CBZ solution was also prepared by dissolving the CBZ in Tween-80 and ethyl alcohol(1:1,v/v)as control.Three formulations of CBZ@Lipo were prepared in the same way to determine the suitable weight ratios of CBZ to total lipid.
2.4.Characterization of CBZ@Lipo
The average size of CBZ@Lipo was measured by a light scattering method using a Malvern Zeta sizer(Malvern,UK)at 25±1°C after appropriate dilution with distilled water.The UV-visible spectrum was measured by a spectrophotometer(Agilent Cary 60 UV-Vis,Santa Clara)in the wavelength range of 200-800 nm.To investigate the morphology of CBZ@Lipo,samples were dried on copper grid and negatively stained with phosphotungstic acid(1%),and then observed by transmission electron microscopy(TEM;JEOL JEM-1230,JEOL,Japan)at an accelerating voltage of 120 kV.The encapsulation efficiency(EE)of CBZ within the liposomes was calculated according to the formula Fi/Ft×100%,where Fi is the amount of CBZ loaded in the liposomes and Ft is the initially feeding amount of CBZ.
2.5.Stability study
Stability studies were carried out by investigating the size change of CBZ@Lipo during long-time storage.CBZ@lipo(0.5 mg CBZ/ml)was suspended in PBS(pH7.4)and stored at 4 °C for 30 d Samples were collected at definite time intervals and the particle size of the liposomes was measured by Malvern Zeta sizer.
2.6.In vitro release study
The release of CBZ from the liposomes was investigated by the dialysis method.Briefly,CBZ@Lipo(0.5 mg CBZ/ml)was placed in a dialysis bag with a molecular cut-off of 3.5 kDa,and then the bag was put into PBS buffer(pH7.4)containing 0.25% SDS(w/w)with a mild stirring at 37 °C.Samples were collected at 0.5,1,2,6,12 and 24 h,and concentration of CBZ in the supernatant was measured using HPLC method.The chromatographic conditions were set as follows.C18reverse-phase column(200 mm×4.6 mm,5 μm)was used under a temperature of 30°C.The mobile phase was acetonitrile-ultrapure water(60:40,v/v),and the detection wavelength was 230 nm.To give some meaningful information towards the clinical potential,we investigated the effect of plasma proteins on drug release[15].1 ml CBZ@Lipo or CBZ solution(1 mg/ml)was mixed with plasma which was proposed from rat blood(1:1,v/v)to make the final concentration of CBZ@Lipo or CBZ solution to 0.5 mg/ml,then the mixture was placed in dialysis bags with a molecular cut-off of 3.5 kDa,and then the bag was put into PBS buffer(pH7.4)containing 0.25%SDS(w/w)with a mild stirring at 37°C.The same method was used to investigate the release of CBZ with plasma proteinin vitro.
2.7.Cellular uptake
In order to investigate the internalization of CBZ@Lipo into tumor cells,coumarin-6 labeled CBZ@Lipo was obtained according to the method of preparing CBZ@Lipo.4T1 or CT-26 cells were seeded in a 24-well culture plate(Corning,NY,USA)at the density of 5×104cells per well and were incubated with coumarin-6 labeled CBZ@Lipo for different time in darkness.Then,the cell nuclei were stained with DAPI solution(20 μg/ml).The cells were repeatedly rinsed with PBS,fixed with 4%paraformaldehyde and observed using a Fluorescence Inversion Microscope System(F900,Edinburgh Instruments Ltd.,UK).
2.8.Cytotoxicity assay in vitro
CT-26 or 4T1 cells were seeded into 96-well plate at a density of 5×103per well,and were treated with CBZ@Lipo or CBZ solution under various CBZ concentrations for 48 h.Cells without any treatment were used as control.Cytotoxicity was measured using an MTT assay according to the manufacturer’s suggested procedures.The cell viability was calculated as the percentage of surviving cells compared with the control group and was reported as the mean values of six wells.The apoptotic response of the cells was analyzed using flow cytometry.Briefly,cells were collected after different treatments,re-suspended in PBS and stained with Annexin V-FITC(5 μl)and propidium iodide(5 μl)according to the manufacturer’s suggested procedures(Keygen Apoptosis Detection Kit),followed by a flow cytometry analysis.
Furthermore,4T1 cells were seeded in a 96-well cell culture plate and treated with CBZ@Lipo(0.5 mg CBZ/ml)for 24 h.The cells were stained with Calcein-AM at 37 °C,followed by the observation using a fluorescence microscope.
2.9.Hemolysis test
Healthy rabbit blood was diluted with normal saline(4:5 ratio by volume).CBZ@Lipo or CBZ solution was diluted with normal saline to obtain the samples with various CBZ concentrations.Then diluted blood was added into these samples and the mixtures were incubated for 3,6,24 and 48 h at 37 °C.Normal saline solution was used as a blank control with deionized water as a positive control.After that,all the mixtures were centrifuged for 5 min at 3000 rpm and the supernatant was carefully collected for spectroscopic analysis at 545 nm.The hemolysis was calculated using an spectrophotometer(UNIC-7200,China)following the formula(ODtest-ODnegativecontrol)/(ODpositivecontrole-ODnegativecontrol)×100%,where OD stands for optical density,ODteststands for the optical density of test group,ODnegativecontrolstands for that of normal saline control group,and ODpositivecontrolstands for that of deionized water group.
2.10.Biodistribution of CBZ@Lipo
DiR labeled CBZ@Lipo was obtained according to the method of preparing CBZ@Lipo.Heterotopic CT-26 tumor model was obtained by subcutaneously injecting a suspension of CT-26 cells(1×106cells)into BALB/c mice.Orthotopic breast cancer model was established by inoculating a suspension of 4T1 cells(1×106cells)into the mammary fatty pad of BALB/c mice.When the average volume of tumors was about 200 mm3,the mice were intravenously injected with DiR labeled CBZ@Lipo(150 μg DiR/kg and 5 mg CBZ/kg).Then the fluorescence images of the mice were taken at 2,6,12,24 and 48 h postinjection byin vivoimaging system(CRI Maestro,USA).In order to investigate the distribution of CBZ@Lipo in various tissues,all mice were sacrificed at 48 h and major organs including heart,liver,spleen,lung,kidney and tumor were collected forex vivofluorescent imaging and quantitative analysis.
2.11.In vivo anti-tumor efficacy of CBZ@Lipo
CT-26 xenograft tumor models were acquired by subcutaneously injecting of 1×105CT-26 cells into BALB/c mice.When the average size of tumor reached to about 200 mm3,the tumor-bearing mice were randomly divided into three groups(6 mice per group).The mice in group 1 to 3 were intravenously injected with 0.2 ml saline,CBZ solution(5 mg CBZ/kg)and CBZ@Lipo(5 mg CBZ/kg)respectively every other day until day 14.The weight of mice was measured by an electronic balance and the tumor size was recorded using a vernier caliper,and the volume was calculated as(length×width×height×π)/6,the mice were sacrificed at the end of the experiments.The heart,liver,spleen,lung,kidney and tumor of each mouse were excised,fixed in 4%formalin,embedded in paraffin and finally sectioned for hematoxylin and eosin(H&E)staining.The proliferation and apoptosis of tumors were also detected by Ki67 and TUNEL staining,respectively.All the slices were observed by an inverted fluorescence microscope.
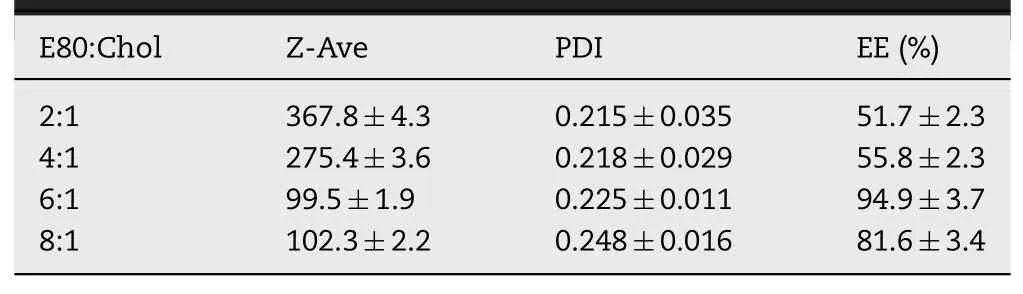
Table 1-Particle size distribution and entrapment efficiency of CBZ@Lipo with different weight ratio of E80 to cholesterol(n=3).
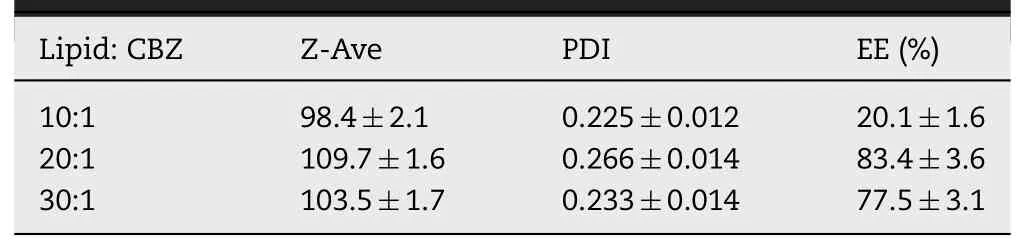
Table 2-Particle size distribution and entrapment efficiency of CBZ@Lipo with different weight ratio of total lipid to CBZ(n=3).
2.12.Statistics
2-sample statistical comparisons were conducted using Student’s test and multigroup comparisons were carried out by one-way analysis of variance(ANOVA)test with post hoc contrasts by Student-Newman Keuls test.∗P<0.05 was considered to be statistically significant difference.
3.Results and discussion
3.1.The preparation of CBZ@Lipo
CBZ loaded liposomes were prepared with various weight ratios of phospholipids to cholesterol(2:1,4:1,6:1,8:1,m/m)under a fixed CBZ concentration of 0.2 mg/ml.The liposomes with phospholipids to cholesterol ratio of 6:1 or 8:1 presented an obviously smaller diameter,narrower size distribution and higher EE of CBZ compared to ones of other ratios(Table 1)and their suspension was transparent and blue-opalescent(Fig.1A).Furthermore,increasing the ratio from 1:20 to 1:10(CBZ:total lipid)induced a significant decrease of CBZ encapsulation(Table 2).
The representative size distribution of CBZ@Lipo as an optimized ratio of 6:1(phospholipids:cholesterol)and 1:20(CBZ:total lipid)was shown in Fig.1B,with average diameter of about 100 nm.The morphology of CBZ@Lipo was observed by TEM,exhibiting a spherical structure with a particle size of about 100 nm(Fig.1C).
The liposome suspension was kept in a sealed container and the size distribution was measured at predetermined time intervals.It was found that there was no significant change of the average diameter of CBZ@Lipo during the storage,suggesting a high stability of the liposomes for at least one month(Fig.1D).
Fig.1E showed a slow CBZ release from the liposomes,with only 50% accumulative release after 24 h.As control,nearly 80% of CBZ solution could quickly leak from the dialysis bag after 2 h.When CBZ@Lipo was mixed with plasma(1:1,v/v),the speed of drug release was slow,with only 30%CBZ released after 24 h.
3.2.Cellular uptake
The uptake behavior of CBZ@Lipo into 4T1 or CT-26 cells was investigated by observing the fluorescence of Coumarin-6.As exhibited in Fig.2A and B,the fluorescence intensity in both cell lines increased gradually over time,indicating that CBZ@Lipo could efficiently internalize into tumor cells and accumulate in the cytoplasm.Semi-quantitative analysis based on Fig.2A and B also showed a progressive cellular uptake of CBZ@Lipo with a saturation tendency after 12 h(Fig.2C).
3.3.Cell cytotoxicity in vitro
The cytotoxicity was detected by MTT assay.Both CBZ@Lipo and CBZ solution showed a dose-dependent killing ability to both 4T1 and CT-26 cells(Fig.3A and B).CBZ solution presented an obviously stronger killing effect to cancer cells than CBZ@Lipo.When the incubation concentration of CBZ solution was 100 μg/ml,only about 10%cells were alive,while over 80%cells was alive with the same concentration of CBZ@Lipo.The significantly decreased antitumor activity could be attributed to the slow release of CBZ from the liposomes.Interestingly,both CBZ@Lipo and CBZ solution induced a remarkable cell apoptosis under a lower CBZ concentration(e.g.10 and 20 μg/ml),as determined with Annexin V/PI staining followed by a cytometer analysis(Fig.3C).
The 4T1 cancer cell-killing effect by CBZ@Lipo under different CBZ concentrations was also tested with Calcein AM staining to indicate the living cells(green,Fig.3D).The results showed that CBZ@Lipo presented a dose dependent cell killing effect.
3.4.Hemolysis test
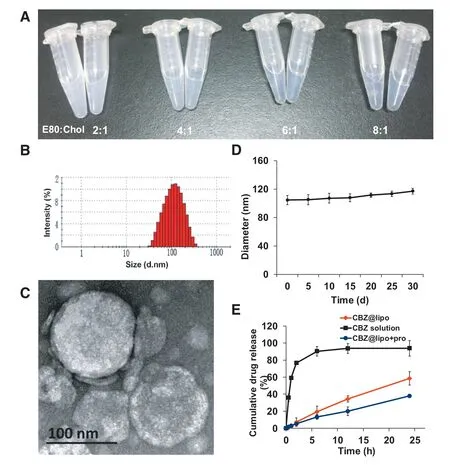
Fig.1-Formulation screening and characterization.(A)Photographs of the liposomes with different formulations according to various weight ratios of E80 to cholesterol.(B)Particle size distribution of CBZ@Lipo.(C)TEM image of CBZ@Lipo.Scale bars,100 nm.(D)Particle size change of CBZ@Lipo within 30 d after preparation(n=3).(E)Cumulative CBZ release from CBZ solution or CBZ@Lipo at 37°C(n=3).(For interpretation of the references to color in this figure,the reader is referred to the web version of this article.)
For hemolysis test,the red blood cell suspensions(2%)were incubated with CBZ solution or CBZ@Lipo with different CBZ concentrations(0.09,0.03 and 0.01 mg/ml)for 48 h,with normal saline solution as the negative control and deionized water as the positive control.It was found that CBZ solution exhibited an obvious hemolysis within the whole concentration range,and the degree of hemolysis is dose dependent.However,even at the highest tested concentration,there was almost no hemolysis caused by CBZ@Lipo after 48 h incubation,just like the negative control did(Fig.4A).The supernatant was collected at 24 h for the determination of spectrophotometer(Fig.4B).Conspicuously,the hemolysis degree of CBZ solution was much more serious than that of CBZ@Lipo,positively related to drug concentration(Fig.4C).The results suggested that,compared to CBZ solution,CBZ@Lipo presented a significant enhanced biosafety and biocompatibility.
3.5.In vivo biodistribution
The mice bearing CT-26 tumors were intravenously injected with DiR labeled CBZ@Lipo,followed by the fluorescence observation at different time points.CBZ@Lipo could efficiently rapidly accumulate to tumor site at 2 h post-injection,which probably thanks to its small particle size(less than 150 nm),and the accumulation could be further increased over time(Fig.5A).Various organs including heart,liver,spleen,lung,kidney and tumor were stripped for fluorescent imaging at 48 h post-injection.The results indicated that the CBZ@Lipo could efficiently accumulate into the tumors after an intravenous injection.
Orthotopic tumor models are considered to be more clinically relevant and can be used as better predictive models of drug efficacy than subcutaneous models.The mice bearing orthotopic 4T1 tumors were obtained and intravenously injected with DiR labeled CBZ@Lipo.As presented in Fig.5B,CBZ@Lipo could gradually accumulate into the tumor site in 2-48 h.It was noticeable that the accumulative amount of CBZ@Lipo in 4T1 tumors was obvious more than that in CT-26 tumors(Fig.5C),suggesting that the liposomes could accumulate into tumor region of different models,especially into orthotopic tumors.
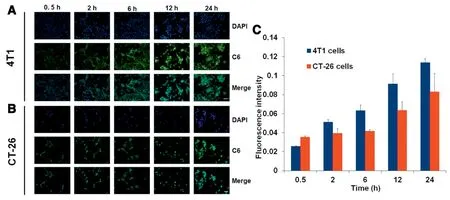
Fig.2-Cellular uptake of CBZ@Lipo in different cells lines.Cell uptake of C6labeled CBZ@Lipo(green fluorescence)by 4T1 cells(A)or CT-26 cells(B).The cell nucleus was stained with DAPI(blue).Scale bars,50 μm.(C)The quantitative analysis based on the images in(A)and(B)by“Image J”software(n=3).(For interpretation of the references to color in this figure legend,the reader is referred to the web version of this article.)
3.6.In vivo anti-tumor efficacy
CT-26 tumor model was employed to investigate the tumor growth inhibition effect of CBZ solution and CBZ@Lipo.Mice were injected with CBZ solution or CBZ@Lipo(5 mg CBZ/kg per injection,one injection per two days)(Fig.6A).Mice in CBZ solution group had a gradually increased loss of body weight during the treatment,and were sacrificed at day 6 due to over 20%body weight loss.Thus,only 6 days’tumor growth curves were shown in the three groups of CT-26 tumors(Fig.6B).It was obvious that CBZ@Lipo had a stronger inhibition effect compared to CBZ solution.In order to investigate tolerance of the mice to CBZ@Lipo and the biosafety of the liposomes,mice in saline and CBZ@Lipo groups was continued to be dosed until day14.It was found that the average body weight of mice in both saline and liposome groups remained constant during the whole experiment(Fig.6C),and the mice was still energetic after a total CBZ dose of 35 mg/Kg.These results indicated the obviously decreased systemic toxicity of CBZ@Lipo compared to CBZ solution,and suggested that CBZ@Lipo might have a high biosafety,making it fit for clinical application.
Furthermore,in order to evaluate the anti-tumor efficacy of CBZ@lipo,main organs including heart,liver,spleen,lung and kidney and tumor were also collected for histology analysis at the end of experiment.Results showed that CBZ solution caused some cardiotoxicity,manifesting as cardiac muscle cell swelling(Fig.6D).H&E imaging showed a health heart in CBZ@Lipo group like saline group,further demonstrating a low systemic toxicity for the CBZ loaded liposomes.Tumor proliferation and apoptosis analysis were also examined(Fig.6E).The tumor treated with CBZ@Lipo showed obviously lower Ki67 positive rate but higher TUNEL positive rate compared to the CBZ solution group.As a conclusion,CBZ@Lipo could inhibit CT-26 tumor growth more effectively and have a higher biosafety than CBZ solution.
3.7.Discussion
Over the past decades,continuous evolution related to cancer research and treatment has been performed.Many chemotherapeutic drugs have been developed and further improved,which could achieve good therapeutic effects.
Paclitaxel(PTX)is a broad-spectrum anti-tumor medicine,which has a wide clinical application for various cancer treatments.Due to its poor solubility in water,emulsifier EL(polyoxyethylated castor oil)is usually used in PTX formulation to increase the solubility.However,emulsifier EL can also cause various adverse reactions,such as allergies,toxic kidney damage,neurotoxicity and cardiovascular toxicity[16].In order to avoid the occurrence of adverse reactions,patients are arranged to orally take dexamethasone,diphenhydramine,intramuscularly dexamethasone or intravenously inject cimetidine before paclitaxel treatment,which unfortunately still cannot completely and effectively prevent the occurrence of allergic reactions[17].
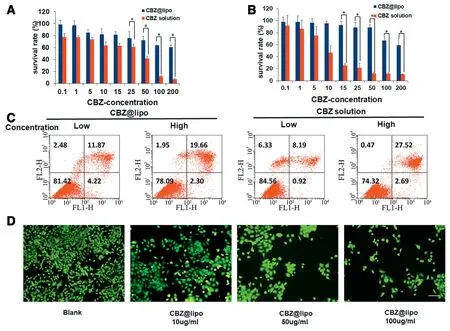
Fig.3-Cell cytotoxicity in vitro.(A)Survival rate of 4T1(A)or CT-26(B)cells treated with CBZ@Lipo containing various concentration for 48 h(n=6).(C)The apoptosis of cells preprocessed by CBZ@Lipo or CBZ solution by flow cytometry.Flow cytometry analysis of the cell apoptosis after treated with CBZ@Lipo or CBZ solution in low(10 μg/ml,CBZ)or high(20 μg/ml,CBZ)concentration.(D)The live cell(green)assay with exposure of 4T1 cells to blank group and CBZ@Lipo containing various concentration of CBZ.The live cells stained with Calcein AM after incubated with CBZ@Lipo containing various concentration of CBZ.Scale bars,100 μm.∗P<0.05.(For interpretation of the references to color in this figure legend,the reader is referred to the web version of this article.)
CBZ is an anticancer drug belongs to chemical semisynthetic taxane class.As a new generation of PTX,CBZ is usually used for the treatment of multidrug-resistant tumors.It is also employed to solve the problem of ineffective prostate cancer therapy or the low tolerance after treated with other paclitaxel drugs.Compared with paclitaxel,CBZ has obvious advantages.The effectiveness of paclitaxel is limited by the high substrate affinity of P-glycoprotein(P-gp).It has been shown that cancer cells that express P-gp become resistant to taxanes[18,19].Cabazitaxel is a novel tubulin-binding taxane that differs from docetaxel because of its poor affinity for P-glycoprotein(P-gp).So cabazitaxel can retain its activity in docetaxel-resistant tumors.At the same time,cabazitaxel is superior to paclitaxel in penetration of the blood-brain barrier in preclinical models[20].However,like PTX,the main defect of CBZ is its poor solubility.Thus,CBZ formulation for clinical application was designed to contain large amount of surfactant(Polysorbate 80)and organic solvent(Ethanol),which can also cause various adverse reactions(for example neutropenia,anemia,leukopenia,thrombocytopenia,hyper sensitivity reactions,gastrointestinal disturbances and renal failure).Therefore,a new dosage form of CBZ with a good therapeutic outcome and high biosafety is urgent to be explored.Preparing CBZ into liposomes can effectively solve this problem.In addition,the unique phospholipid bilayer structure of liposomes determines its targeting,low toxicity and long-term efficacy.And as a medium of anticancer drugs,liposomes have the advantages of simple manufacture,non-toxicity to the body,significant reduction of drug side effects and tumor targeting in the clinic[21,22].
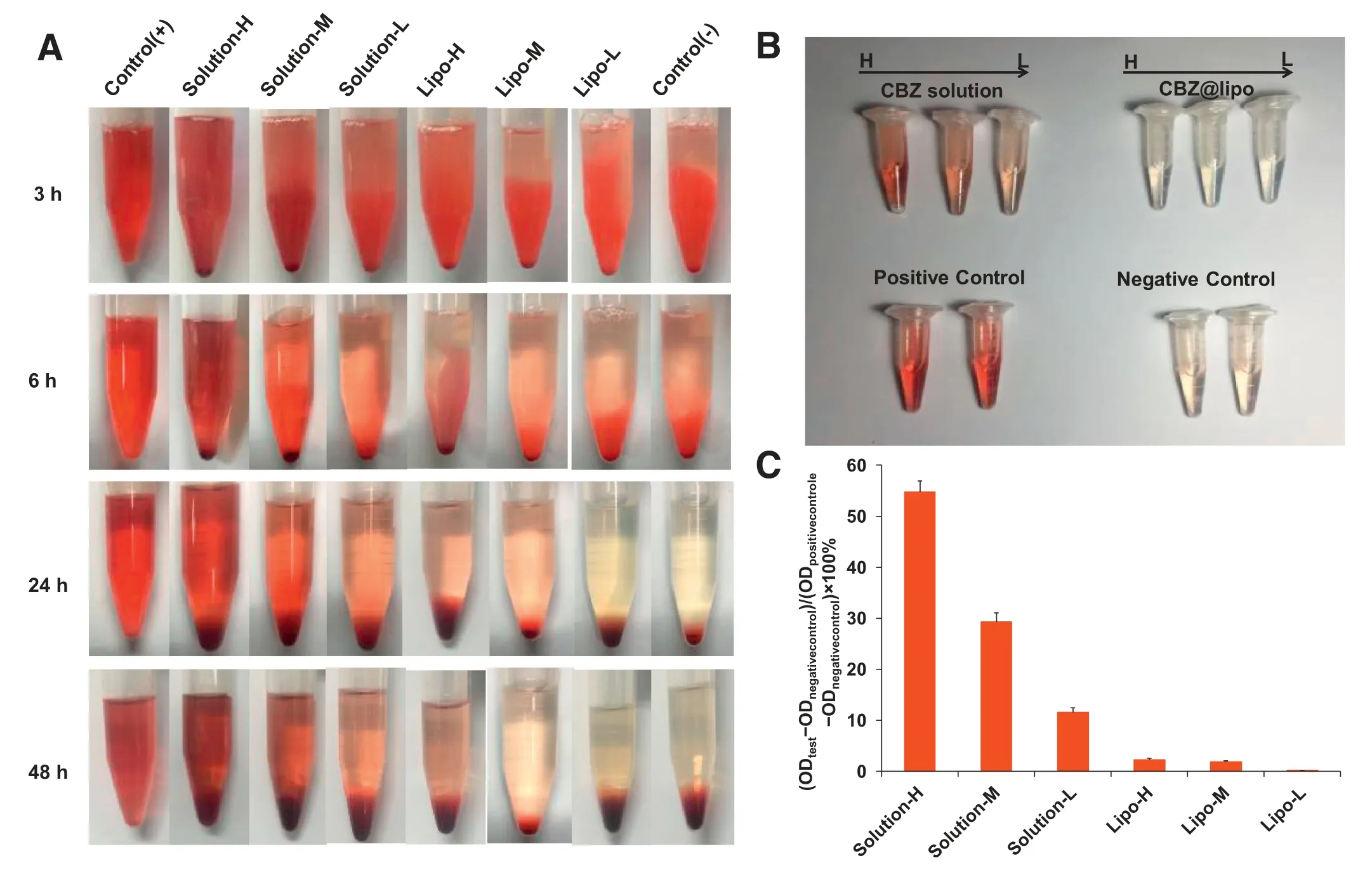
Fig.4-Red blood cell hemolysis test.(A)Photographs of defibrinated blood mixed with CBZ solution or CBZ@Lipo in different concentration(high:0.9 mg/ml,CBZ;middle:0.3 mg/ml,CBZ;low:0.1 mg/ml,CBZ)for 3,6,24,48 h.(B)Photographs of the supernatant collected from each group at 24 h.(C)The UV absorbance of the supernatant collected from each group at 24 h(n=3).
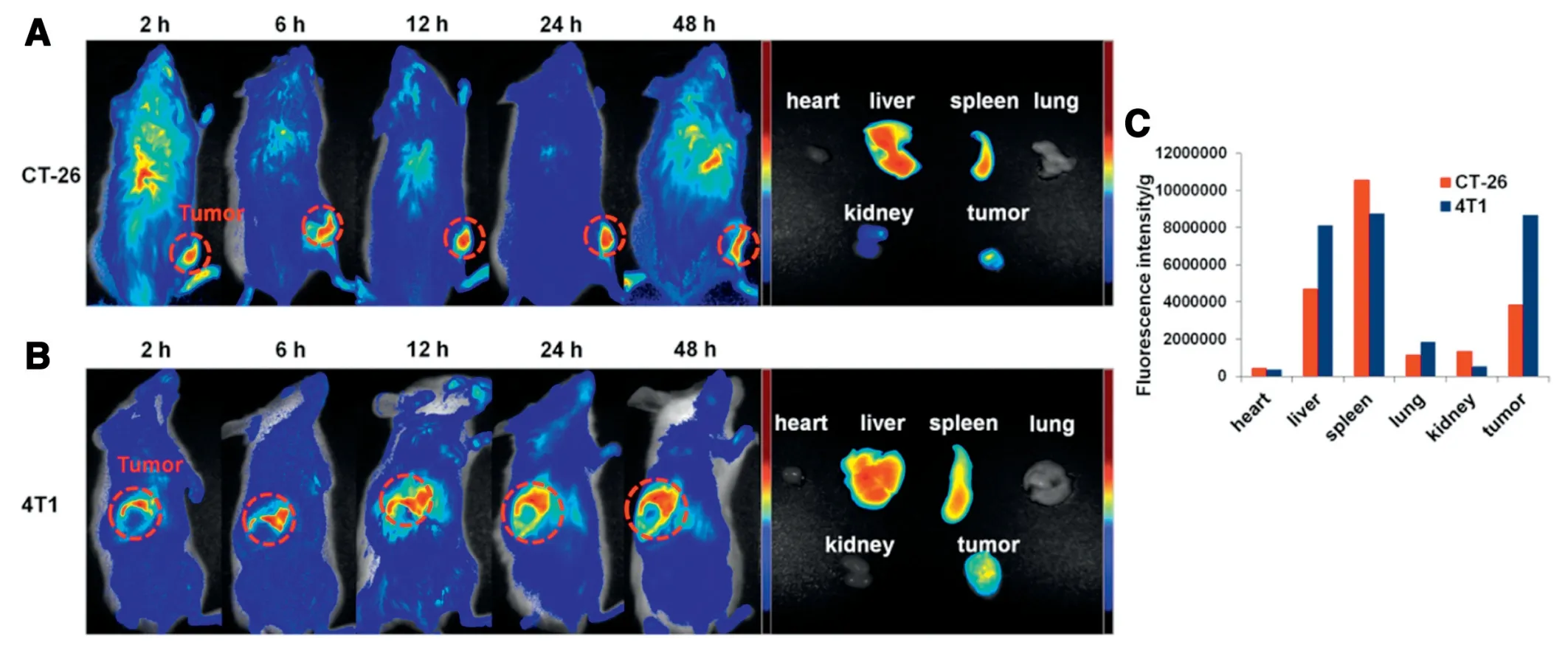
Fig.5-Biodistribution of CBZ@Lipo.In vivo fluorescence imaging of 4T1(A)or CT-26(B)bearing BALB/c mice post intravenously injection with CBZ@Lipo(left panel).The ex vivo fluorescent images of various tissues including heart,liver,spleen,lung,kidneys and tumor at 48 h post-injection(right panel)were showed.(C)based on the CBZ@Lipo in various tissues was calculated as the average fluorescent intensity,representing the amount of the liposomes.Fluorescence semi-quantification of the biodistribution in tumor and major organs 48 h post-injection with CBZ@Lipo,which was expressed as fluorescence intensity per gram.
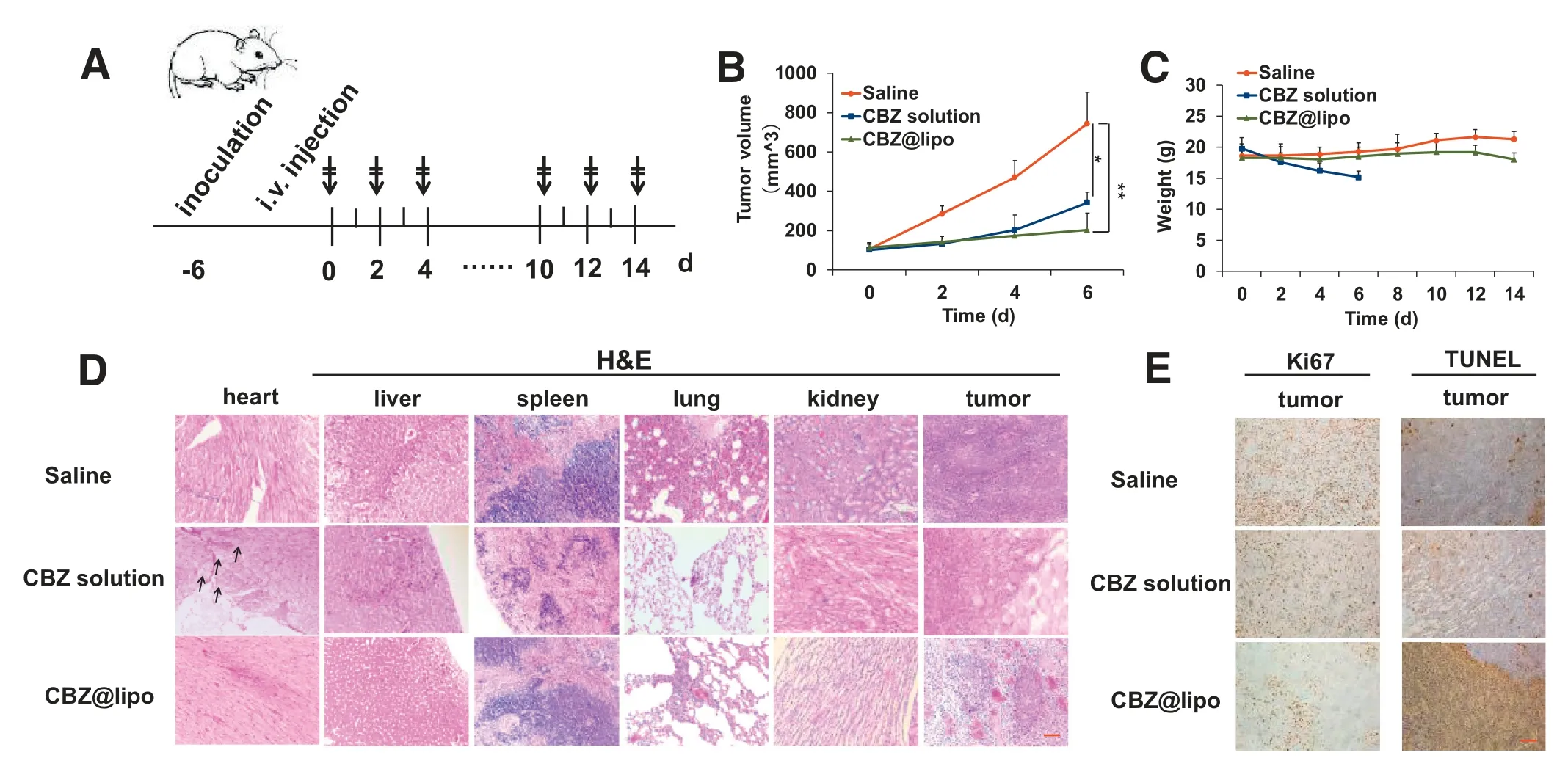
Fig.6-In vivo anti-tumor efficacy of CBZ solution or CBZ@Lipo(n=6).(A)Illustration of the drug administration scheme.(B)Tumor growth curves for mice bearing CT-26 tumors treated with saline,CBZ solution,or CBZ@Lipo.(C)Mice body weight change within 14 d(D)Histological observation of various tissues including heart,liver,spleen,lung,kidney and tumor after hematoxylin and eosin(H&E)staining.(Arrows,cardiomyocytes swell.Scale bars,50 μm).(E)Ki67 and TUNEL staining of tumors in each group.Scale bars,50 μm.
In our study,an optimized liposome formulation containing CBZ was screened(Table 1),which had a uniform particle diameter of about 100 nm and a narrow size distribution(Fig.1B).As for thein vitrorelease of CBZ from CBZ@lipo or CBZ solution,it is obvious that CBZ@lipo showed a significantly sustained release effect with only 50% accumulative release after 24 h comparing to the quick leak of drugs from CBZ solution.However,when plasma was added into CBZ@lipo,the release of CBZ was obviously lower(Fig.1E).We speculated that this was due to the combination of drug and plasma proteins,resulting in a lower release of free CBZ.As for the release of CBZ from CBZ solution with equal volume of plasma,a large amount of sediment was produced once the plasma was added into the CBZ solution,so the data was not presented.The liposome formulation,CBZ@Lipo,could rapidly internalize into tumor cells(Fig.2A and B).Although CBZ solution showed higher cancer cell killing ability than CBZ@Lipo,the cancer cell apoptosis could be efficiently induced by the treatment of CBZ@Lipo(Fig.3).Importantly,CBZ@Lipo presented remarkably lower hemolysis activity than CBZ solution(Fig.4),which could benefit from the slow release of the drug from liposomes.Also,CBZ@Lipo could efficiently accumulate into different type of tumors after an intravenous injection(Fig.5).As a result,CBZ@Lipo exhibited a good anti-tumor activity,which is better than that of CBZ solution.More importantly,the mice showed an obviously higher tolerance to CBZ after the treatment of CBZ@Lipo,which suggested a higher biosafety for the liposome formulation(Fig.6C).In conclusion,in this study,we designed a liposome that could effectively encapsulate CBZ.The CBZ@Lipo with a small and uniform particle size and a good biosecurity could reduce the side effects caused by organic solvent.Compared with CBZ solution,CBZ@Lipo had significant advantages in biosafety and efficacy,indicating that it has great potential to be a promising anti-tumor drug in clinical practice.
4.Conclusion
Herein,we reported a PEG-modified liposome to efficiently encapsulate CBZ,enhancing its tumor inhibition effect as well as reducing the systemic toxicity.Loading efficiency of CBZ into the liposomes could be improved with the increase of lipophilic materials.The average diameter of CBZ@Lipo was about 100 nm.The liposome suspending in aqueous medium was stable at 4 °C for at least one month.The significantly lower killing ability of CBZ@Lipo to cancer cells was presented compared to that of CBZ solution,which could be attributed to the slow release of CBZ from the liposomes.However,CBZ@Lipo could induce an obvious apoptosis of the cancer cells at low concentration.Furthermore,CBZ@Lipo exhibited a significantly enhanced tumor growth inhibition effect compared to CBZ solution.More importantly,obviously higher biosafety was shown of CBZ@Lipo,as determined by hemolysis potential,body weight and histology analysis.Our work provided a useful reference of the formulation containing CBZ,which had potential for the furture clinical application.
Conflicts of interest
The authors report no conflicts of interest.The authors alone are responsible for the content and writing of this article.
Acknowledgement
This work was supported by the National Key R&D Program of China(No.2017YFE0102200),the Natural Science Foundation of China(81373348 and 81573365)and Basic Public Welfare Research Project of Zhejiang Province,China(LGF18H300004).
杂志排行
Asian Journal of Pharmacentical Sciences的其它文章
- Cancer nanotechnology:Enhancing tumor cell response to chemotherapy for hepatocellular carcinoma therapy
- The functions and applications of A7R in anti-angiogenic therapy,imaging and drug delivery systems
- Investigation of molecular aggregation mechanism of glipizide/cyclodextrin complexation by combined experimental and molecular modeling approaches
- Evaluation of the Mrp2-mediated flavonoid-drug interaction potential of quercetin in rats and in vitro models
- A novel oral prodrug-targeting transporter MCT 1:5-fluorouracil-dicarboxylate monoester conjugates
- Synthesis,characterization and in vivo evaluation of honokiol bisphosphate prodrugs protects against rats’brain ischemia-reperfusion injury
