Cancer nanotechnology:Enhancing tumor cell response to chemotherapy for hepatocellular carcinoma therapy
2020-01-06LiBiBinYuZhiqingYu
,Li Bi,Bin Yu,Zhiqing Yu,
aNational Engineering Research Center for solid preparation technology of Chinese Medicines,Jiangxi University of Traditional Chinese Medicines,Nanchang 330006,China
bSchool of Pharmaceutical Sciences,Southern Medical University,Guangzhou 510515,China
cIntegrated Hospital of Traditional Chinese Medicine,Southern Medical University,Guangzhou 510315,China
dDepartment of Chemical and Biomedical Engineering,West Virginia University,Morgantown 26506,USA
eSchool of Pharmaceutical Sciences,Zhengzhou University,Zhengzhou 450001,China
ABSTRACT Hepatocellular carcinoma(HCC)is one of the deadliest cancers due to its complexities,reoccurrence after surgical resection,metastasis and heterogeneity.In addition to sorafenib and lenvatinib for the treatment of HCC approved by FDA,various strategies including transarterial chemoembolization,radiotherapy,locoregional therapy and chemotherapy have been investigated in clinics.Recently,cancer nanotechnology has got great attention for the treatment of various cancers including HCC.Both passive and active targetings are progressing at a steady rate.Herein,we describe the lessons learned from pathogenesis of HCC and the understanding of targeted and non-targeted nanoparticles used for the delivery of small molecules,monoclonal antibodies,miRNAs and peptides.Exploring current efficacy is to enhance tumor cell response of chemotherapy.It highlights the opportunities and challenges faced by nanotechnologies in contemporary hepatocellular carcinoma therapy,where personalized medicine is increasingly becoming the mainstay.Overall objective of this review is to enhance our understanding in the design and development of nanotechnology for treatment of HCC.
Keywords:Hepatocellular carcinoma Cancer nanotechnology Cell response Chemotherapy
1.Introduction
Hepatocellular carcinoma(HCC)is the sixth most malignant human cancer and rank third in cancer-related deaths globally among cancer patients.The incidence also is geographically related,as is the mortality,with Eastern and South-Eastern Asia and Western Africa having a high incidence[1-3].HCC usually occurs in the established background of liver cirrhosisdue to the endopathic cause and exopathic cause often varies considerably across areas and populations.Normally,HCC can be treated curatively with surgical resection or liver transplantation if diagnosed early[4].However,since the majority of HCC patients are diagnosed at an advanced stage,their median survival times are generally less than 1 year,leading to a poor prognosis[5].One reason is surveillance programs are not widely implemented,despite being recommended in national guidelines[6].On the other hand,prevention of alcohol intake,vaccination against hepatitis B virus(HBV)infection,intake of vitamin D and calcium can prevent HCC in many cases[7].However,they could not be used to obliterate the disease.Furthermore,many investigations have shown that the occurrence of this multi-stage carcinogenesis evolves through dysregulation of multiple signaling pathways,genetic alterations with the initiation of inflammatory responses leading to the formation of a heterogeneous solid tumorous mass[8].Implementation of such programs has only resulted in an early diagnosis in some rich places[9].
To date,there are six treatments can extend life expectancy of patients with HCC:surgical resection,liver transplantation,radiofrequency ablation,transcatheter arterial chemoembolization(TACE),sorafenib and lenvatinib[10,11].One third of patients are eligible for potentially curative therapies,which include resection,transplantation or local ablation,and such curative treatment can extend median survival times beyond 60 months[12].Unfortunately,most patients are still diagnosed at advanced disease stages.In this setting,only treatment with sorafenib(the first FDA approved molecularly targeted drug for HCC treatment)is able to improve overall survival in the last few decades[13].In 2018,lenvatinibcapsules(Lenvima,Eisai Inc.)was approved by FDA for the first-line treatment of un-resectable hepatocellular carcinoma and showed better subordinate clinical endpoints when compared with sorafenib[14].Although the anti-cancer potency of sorafenib,lenvatinib and some other drugs have been proven,poor aqueous solubility,pharmacokinetics and undesirable side effects make the approach difficult to apply widely in HCC patients[15].The major side effects of these drugs treatment are elevated blood pressure,diarrhea,fatigue,hand-foot syndrome,skin rash/desquamation,and nausea[16].The poor aqueous solubility and side effects might be overcome by the use of nanotechnologies that can control and target the release of effective drugs to the specific tumor site[17].
With increasing numbers of nanotherapeutics and nanodiagnostics being commercialized or having reached clinical stage in recent years[18].Plenty of drug delivery vehicles have been developed from different biomaterials,such as synthetic polymers[19-24],natural polymers[25-28],microsphere liposomes[29-31],peptide[32-36],small molecules[37-41],and protein[42-46]et al.Specific cancer nanotechnologies have been proven to be efficacious with respect to better targeting and minimizing the non-specific cytotoxic effect to the surrounding organs[47-51].Targeting the receptors by drug delivery nanoparticles could significantly alleviate the adversities of chemotherapy[52,53].Significant achievements also have been witnessed in the field of HCC drug delivery systems[52,54,55].Understanding the biological processes of the distribution or/and retention of nanoparticles inside the HCC microenvironment is therefore essential to the development of personalized nanomedicine approaches.Herein,we examine the fundamentals behind targeting of nanoparticles to HCC and cancer cells.We will discuss the causes and recent treatment modalities of HCC,with an emphasis on cancer nanotechnology for enhancing HCC cell response to chemotherapy.With the perspective of developing new therapeutic nanotechnologies,we will also examine how the physicochemical parameters of the nanoparticles affect their localization,retention,cell binding,internalization,efficacy and toxicity.
2.Current treatment strategies for hepatocellular carcinoma
HCC has been well investigated as a complex multi-step process arising from combination of genetic and epigenetic alterations,somatic mutations,genomic instability and environmental factors[56].There are multiple factors responsible for the initiation and progression of HCC including virus-induced[56,57],alcohol-induced,fungi-induced obesity and type II diabetes[58,59].Common treatment strategies are composed of surgical(resection and liver transplantation)and nonsurgical approaches(transarterial chemoembolization,transartetial radiation,percutaneous local ablation,microwave ablation and systemic therapy).In nonsurgical drug therapy,not only small molecule drugs such as sorafenib and lenvatinib,but also gene therapy,immunotherapy and combination therapies have been used for HCC therapy[60-62].Among them,systemic therapies which using small molecule drugs to target various signaling pathways following surgical resection and liver transplantation have been applied particularly where locoregional therapy has been confirmed invalid[59,63].However,small molecular chemotherapy in HCC also suffers from the drawbacks of dose-limiting toxicities,development of multidrug resistance(MDR)and unfavorable side-effects like other cancers[64-67].Although various multikinase inhibitors have been used for systemic treatment of HCC,only few drugs such as sorafenib(orally active multikinase inhibitor)and lenvatinib(oral multi-targeted tyrosine kinase inhibitor)have been approved as deserves special mention drug for treatment of advanced HCC.Many investigations have indicated that it could prolong overall survival in patients and delay the time to progression of advanced HCC by using sorafenib or lenvatinib.However,fatigue,diarrhea,hypertension,skin toxicity,weight loss,hypophosphatemia are almost the frequent symptoms amongst patients with these two drugs’treatment.Further,various first line and second line therapies are currently under evaluation for the HCC treatment,however,potential randomized trial with another drugs such as sunitinib,tivantinib,brivanib and linifanib have been disappointing due to the adverse effects and not superior to sorafenib.Only lenvatinib capsules(Lenvima,Eisai Inc.)have been demonstrated that were noninferior than sorafenib for overall survival.In view of the failures of clinical trials for most of the chemotherapeutics drugs,elaborate and extensive information of the underlying molecular mechanisms undergoing in each patient’s tumor progression is critically necessary.Other small molecule targeted therapies such as regorafenib,doxorubicin,gefitinib,vatalanib,cediranib,bortezomib(proteasome inhibitor),rapamycin,sirolimus,and gemcitabine as single agent or in combination with mAb have been evaluated in clinical trials targeting pathways which are activated in HCC.Recently,combination therapies also have been widely studies and shown many positive results[65,68,69].
In addition,two other types of molecular-based therapies are currently under development for the treatment of HCC:epigenetic modifying therapies[70]and microRNAs(miRNAs)[71].Epigenetic modifications in certain genes have been proposed to drive progression of HCC.Therapies targeting epigenetic modifiers,such as DNA methyltransferases and histone deacetylases,are attractive approaches[72,73].A multicenter phase I/II trial of the histone deacetylase inhibitor belinostat for treatment of patients with HCC is currently being developed[74].Some other researches also showed that those with high immunoreactivity to the UV excision repair protein RAD23 homologue B(HR23B)to HDAC inhibitors had a higher rate of disease stabilization[75].Bitzer et al.have studied the resminostat plus sorafenib as the second line therapy and showed potential activity in patients with HCC[76].MiRNAs-based strategies that block the interaction of HDAC with its substrates have also been studies[77-79].The results showed that many kinds of miRNAs are important regulators of gene expression and associated with the development of HCC[80,81].
Otherwise,their value in clinical management has been investigated.Functional screens of miRNAs have identified key regulators of glypican-3,a surface proteoglycan that acts as a co-receptor,controls cell apoptosis,proliferation and is a potential target for treatment of HCC[78].In a word,evidence suggests that miRNA-based therapies are worthwhile approaches to cancer treatment.Shuai and his co-workers have investigated the folate-targeted theranosticsiRNA(Fa-PEG-gPEI-SPION/psiRNA-TBLR1)nanoparticles for enhanced HCC chemotherapy and obtained the positive results(Fig.4).
Recently,cancer immunotherapy rises rapidly and also plays a critical role in HCC treatment due to the liver as the mainimmune organ of the lymphatic system[79,82,83].Three kinds of hepatic cells named liver sinusoidal endothelial cells,Kupffer cells and dendritic cells(DCs)are main roles for the immune response in the HCC microenvironment[84].However,only few immunotherapy trials for HCC have been studied so far with contrasting results,suggesting that significant improvements are needed.It is beyond a doubt that such an inherent immune tumor environment needs to be taken into account and counterbalanced when immunotherapeutic approaches are designed and implemented in HCC.Therefore,combination therapy of monoclonal antibodies or chemotherapies along with transarterial chemoembolization is a promising approach in increasing the overall survival of HCC patients whose chances of relapse after surgery are evident[85,86].Previous studies have shown promising safety,efficacy and tumor targeting of the 131I-labeled metuximab and transcatheter arterial chemoembolization combination for unresectable HCC[87,88].Combination of low dose cyclophosphamide treatment with a vaccine(e.g.telomerase vaccine)in HCC has been evaluated in a single clinical trial[89,90].Unfortunately,the combination did not show antitumor efficacy in respect to tumor response and time to progression.Nearly 5 years,PD-1 and its ligand PD-L1 have been widely used as an immune regulating checkpoint with a well-established role in the development of HCC progression.A single clinical trial in HCC patients has been reported combining anti-PD-1 Ab and a GPC3 peptide vaccine.Results showed improved antitumor effects of a peptide vaccine correlating with the increased levels of vaccine-specific CTLs and reduced tumor-infiltrating T cells[88].Nivolumab is an immunotherapy that inhibits PD-1.It has been used as a second line systemic treatment in HCC patients who have been treated with or intolerant to sorafenib and has been granted approval by FDA in 2017.To increase responses to immunotherapy,combination of PD-1 or PDL1 and tyrosine kinase inhibitors are currently investigated significant improved clinical outcomes.Xu et al.generated and used SHR-1210(anti-PD-1 antibody)and apatinib(VEGFR2 inhibitor)to study combination therapy treatment with advanced HCC.It was found that combination therapy demonstrated manageable toxicity and encouraging clinical activityin patients[91].Markus Joerger et al.also provided data from this clinical case to support the potential of combination treatment of the oral multi-kinase inhibitor regorafenib with PD-1 or PDL1 targeted monoclonal antibodies to advanced HCC therapy[92].These discoveries can be used to promote the development of HCC immunotherapy.After the completion of genome-wide association studies(GWAS)and pharmacogenomics,personalized medicine has gradually become possible in HCC.Personalized medicine has been the mainstay for the treatment of HCC.Personal genetic analysis can hold the promise of identifying patients and family members,who would benefit from personalized medicine,modifying risk factors and so on[93,94].
3.Application of nanomedicine for hepatocellular carcinoma therapy
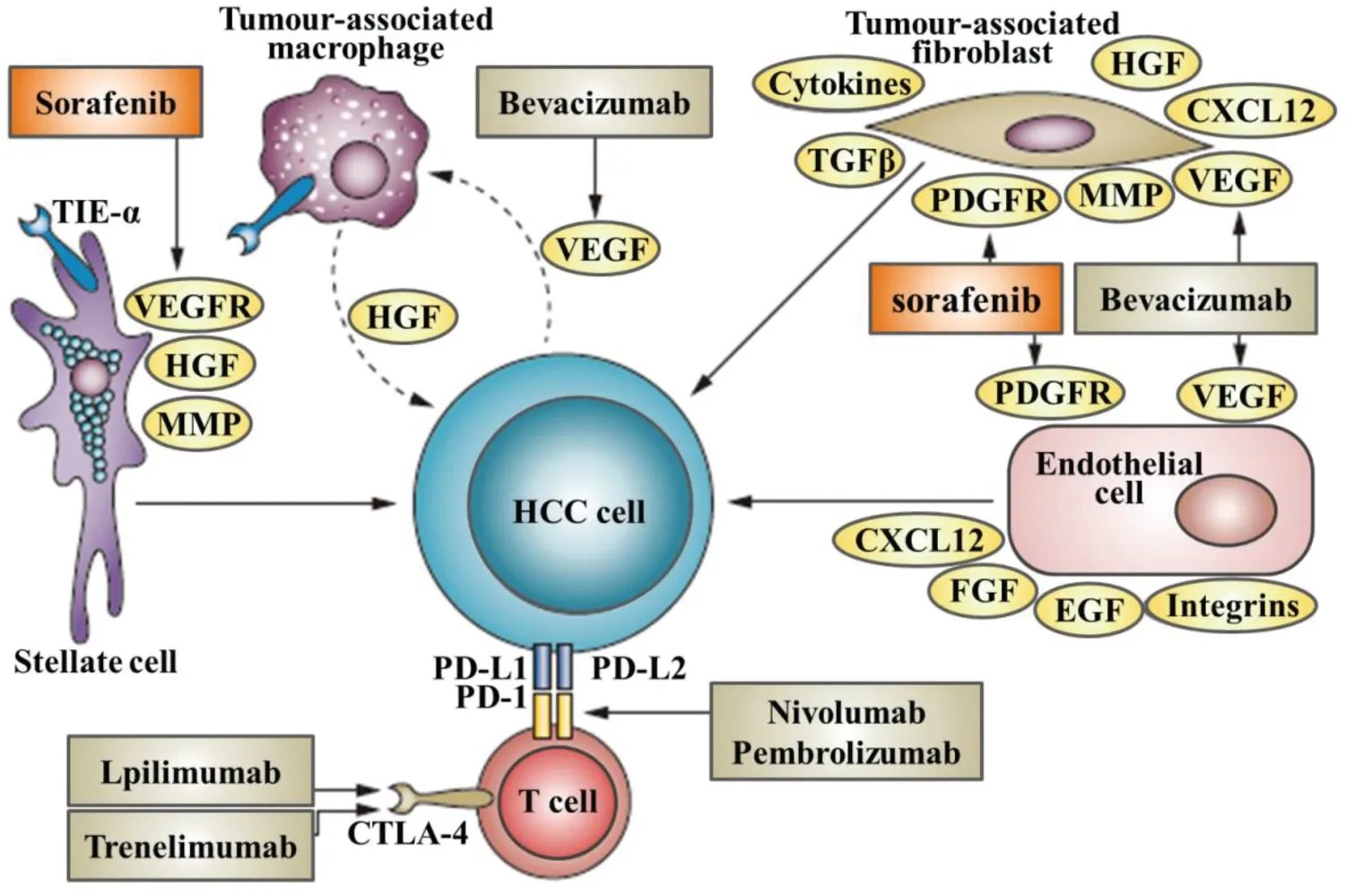
Fig.1-This diagram summarizes known therapies for treatment of HCC receptors,and the cellular locations of drug-target interactions.
Although the efficiency of these few chemotherapeutics molecules in the management of HCC,the drugs are not usually delivered at high concentrations into the malignant tissues.Thereby,hydrophobicity,toxicity and bioavailability of these small molecular drugs caused dose-limiting side effects are still the deliver challenge.Nanotechnology is a powerful tool for the delivery and targeting of therapeutics in HCC[95-97].Since HCC cells undergo genetic and phenotypic changes compared to other hepatic cells,targeting of HCC cells is an obvious avenue for treatment of HCC(Fig.1).Various targeting and delivery strategies have been explored for nanoparticles(NPs)[98-100],micelles[101,102],liposomes[103]both passively and receptor-mediated active targeting in HCC.Normally,nanotechnologies could overcome the unfavorable side-effects of systemic administration of chemotherapeutics by improving the pharmacokinetics,biodistribution,accumulating cytotoxic agents in tumor site and elevating the effectiveness of treatment through drug delivery nanosystems[104-107].Nanometer size range of nanosystems could help drugs reach the tumor cells through the leaky vasculature and enhance site-specific enhanced delivery[108].However,degradability,stability,circulation,metabolismas well as the balance between side effects and curative effect still have to be carefully considered for the design of efficient deliver nanosystems.Otherwise,HCC patients almost developed based on prolonged inflammatory processes,which emerged as a result of genetic and epigenetic alterations.Tumor suppressor genes,in particular p53 and retinoblastoma,are the frequently altered in HCC.Further,as a kind of highly vascular tissues,HCC progression is almost accompanied by abnormal angiogenesis at different stage and etiology.Thereby,angiogenesis related molecules such as VEGFR,RAF and EGFR are extremely useful targets in the development of selective therapeutics.Hence,the specific surface molecules of liver cells including ASGPR and endocytic cell surface receptors are highly expressed by HCC cells that are distinguished from other tissues.These specific related genotypic and phenotypic alterations have been utilized for HCC targeting diagnosis and therapy.Herein,some of the recent designs and findings in the field of nano-based medicines for passive and receptor-specific active targeting for the treatment of hepatocellular carcinogenesis would be discussed.
3.1.Passive targeting nanotechnology for hepatocellular carcinoma therapy
In case of passive targeting,nanomedicines can reach tumor via the leaky vasculature of the tumors by the enhanced permeability and retention(EPR)effect[109-111].Nanoparticles can be delivered to specific sites by passive targeting.It also has been reported that nanoparticles injected intravenously are taken up by the liver after only a few minutes due to the opsonization process.To date,various stimuli including pH,enzymatic,redox,light and heat have been used for passive targeting of nanoparticles carrying drugs in HCC[112,113].Recently,nanoassemblies based on supramolecular complexes of nonionic amphiphiliccyclodextrin and sorafenib as effective weapons to kill HCC cells have been developed(Fig.2)[114].Sorafenib robustly interacts with nonionic amphiphiliccyclodextrin,forming supramolecular complexes that behave as building passive targeting nanoassemblies.These nanoassemblies are highly stable in aqueous medium,retaining sorafenib up to 2 weeks.Interestingly,these passive targeting systems show very low hemolytic activity and a high efficiency to inhibit the growth of three different HCC cell lines,similarly to free sorafenib,which indicated the preferentialin vivoaccumulation of nanoassemblies could result in a superior therapeutic efficacy than the free drug.Antisense-miRNA-21 and gemcitabine(GEM)co-encapsulated PEGylated-PLGA NPs have been developed to overcome drug resistance and improve their therapeutic efficacyin vitro.For lenvatinib,Yuan et al.have developed aliposome-encapsulated contrast agent comprising lenvatinib-bound nanoparticles for screening of early-phase non-small cell lung cancer.However,nanotechnology has not been used for enhancing the HCC therapy of lenvatinib.
3.2.Active targeting nanotechnology for hepatocellular carcinoma therapy
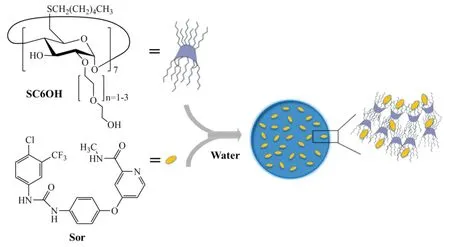
Fig.2-Sketched view of nanoassemblies formation in aqueous solution(Reproduced with permission from[67].Copyright 2017 Shenyang Pharmaceutical University).
Although nanosystems have gained tremendous success for delivering chemotherapeutic agents,their rapid clearance by the reticuloendothelial system(RES)after systemic administration restricts them from their delivery to hepatocytes which are the main site for HCC origination[115,116].As a result,the nanotechnological approaches of site specific and/or targeted drug delivery amenable to distinct hepatic cells and their receptors are being studied extensively by researchers[117-119].Further,active targeting was almost accompanied with passive targeting when nanosystems were designed[120-122].
Asialoglycoprotein receptor(ASGPR)has been identified as a commonly found lectin receptor which is profoundly expressed in mammalian liver cells[123,124].Using natural ligands such as asialofeutin as well as synthetic ligands could achieve specific liver ASGPR targeting[58].For example,nanoparticles and liposomes incorporating various lactosylated strategies have been evaluated for targeting efficacy to hepatic tumor cells.Polyethylene sebacate(PES)-Gantrez®AN 119 Dox NPs have been developed with ASGPR ligands by Sandhya et al.and showed the high efficacy coupled with greater safety as a promising nanocarrier for improved therapy of HCC[125].Meng and colleagues successfully demonstrated the galactose-installed photo-crosslinked pHsensitive degradable micelles(Gal-CLMs)for active targeting chemotherapy have a great potential for hepatocellular carcinoma chemotherapy(Fig.3)[126].In another study,cholesterol arabinogalactan anchored liposomes(CHOL-Al-AG)carrying DOX targeting ASGPR receptors on mammalian hepatocytes in HCC were studied where improved delivery of DOX was reported in terms of stability,loading efficiency,tumor regression bothin vitroandin vivocompared to unmodified liposomes[127].
Integrins are transmembrane receptors which are responsible for cell-cell and cell-extracellular matrix interactions[128].Hepatic stellate cells almost express integrins when it suffers fibrotic liver[129].For instance,the peptide sequence RGD acts as a targeting ligand for collagen VI and RGD-coupled liposomes has been extensively applied for targeting integrin receptors in HCC[130].To deliver the poor water soluble drug paclitaxel(PTX)effectively in HCC,Chen et al.have developed a kind of integrin-αvβ3 receptor targeted liposomal PTX[131].RGD-motif was successfully conjugated to DSPE-PEG to prepare RGD-modified liposomes(RGD-LP).Bothin vitroandin vivostudies demonstrated RGD-LP-PTX had enhanced anti-proliferative and tumor growth inhibitory effects activity against HepG2 cells and HepG2-bearing mice tumor respectively than RGD-LP or free PTX.Moreover,combination of DOX and sorafenib in iRGD decorated lipid-polymer hybrid core-shell structured NPs also indicated enhanced antitumor efficacy in HCCin vivomodels with improved bioavailability and longer circulation time[132].Recently,RGD modified lipid-coated NPs for the co-delivery of the hydrophobic drugs have been used by Wang et al.to against hepatocellular carcinoma.The combination of sorafenib and quercetin formulations was more effective than the single drug formulations in both NPs and solution groups.This RGD modified NPs achieved the most significant tumor growth inhibition effectin vitroandin vivo[133].
Vandetanib is an oral inhibitor of VEGFR,EGFR,RETtyrosine kinase and has been approved for medullary thyroid cancer[134].It has been explored for HCC as encapsulated NPs with targeting moiety with enhanced antitumor efficacy[135].
HCC cells overexpressed transferrin(Tf)receptors which have become useful targeting avenues for potential treatment[136].The ideal of sorafenib in albumin nano-shell was used and DOX was loaded in poly(vinyl alcohol)as nano-core,the interactions were simulated and then the nanomedicines were formed by a sequential freeze-thaw/coacervation method(Fig.5)[137].It indicated that rationally designed core-shell nanoparticles can effectively combine clinically relevant single-agent drugs for exerting synergistic activity against liver cancer.
CL4 RNA aptamer specific to EGFR and CD133 targeted aptamers was conjugated to PLGA nanoparticles was studied for salinomycin delivery to cancer stem cells in HCC treatment[138].
Like other cancers,folate receptors are significantly overexpressed in HCC and to target these receptors,its natural ligand,folic acid(FA)has been explored for nanoparticle drug delivery to the cancer cells[3,139].Xu et al.studied the antitumor potency of the folate receptor-targeted liposomes(FA-PEG-DSPE)co-delivery of antitumor agent docetaxel and iSur-pDNAin vitroandin vivo[140].
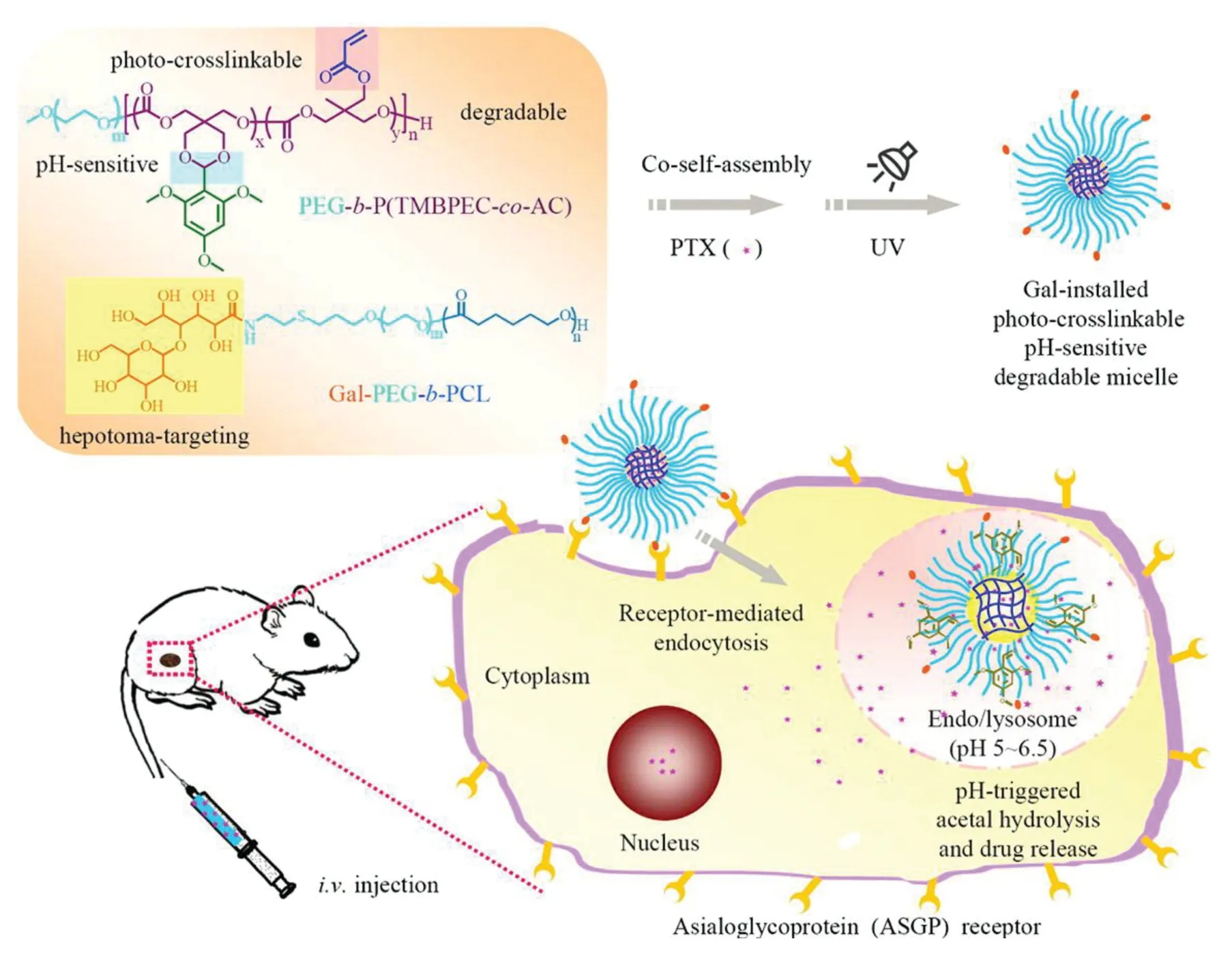
Fig.3-Illustration of pH-sensitive degradable micelles as an integrative nanovehicle for active targeting hepatocellular carcinoma chemotherapy(Reproduced with permission from[114].Copyright 2015 American Chemical Society).
VEGF receptors are found to be overexpressed in HCC due to the receptor activation via multiple signaling pathways[140,141].Recently,iRGD-decorated polymeric NPs for the efficient delivery of vandetanib to HCC have been investigated.Wang et al.studied the biodegradable PEG-PLA to nanoprecipitate this potent agent to form water-soluble NPs that are suitable for intravenous administration[142].Active targeting by aptamer-mediated delivery showed significant anti-tumor efficacy when tested bothin vitroandin vivo.The results also demonstrate that reformulating targeted therapeutic agents in NPs permits their systemic administration and thus significantly improves the potency of currently available,orally delivered agents.
Glycyrrhetinic acid(GA)receptor is overexpressed on the hepatocyte surface and has been extensively studied for drug targeting[143].Qi et al.reviewed the roles of this receptor and GA-mediated drug delivery in HCC[144].GA-modified nanoparticles formulations of DOX exhibited a much higher level of tumor accumulation than nontargeted NPs at 1 h after injection in hepatoma-bearing Balb/c mice.Zhang and colleagues studied poly(L-Histidine)(PHIS)mediated polymeric system(GA-PEG-PHIS-PLGA)with GA for targeted delivery of the anti-cancer agent and rographolide[145].
Passive and active targeting nanotechnologies have shown some potential for hepatocellular carcinoma therapies.However,tumor recurrence needs deeper or more thorough therapies,such as combination targeting,long circulation and so on.There are still many challenges for nanotechnology to fabricate more ideal delivery systems.
4.Influence of the architecture of nanomedicine for hepatocellular carcinoma therapy
The conjugation of ligands on the liver cells surface of nanosystems changes not only the properties of the targeting molecules but also the nanocarriers.From a chemical point of view,while ligands bind to the surface of NPs,it would lose the rotational and translational freedom bestowed to free molecules,otherwise,the new targeted entity achieves improved avidity due to the increased valency.Similarly,the size,shape,surface properties(charge,density and hydrophobicity),and composition of NPs can also be the influence factors[146,147].Fig.6 shows the various properties of NPs which could change the delivery behavior of drugs from NPs to HCC cells.As we know,targeted NPs have shown benefits that go beyond the simple delivery of anti HCC drugs in many cases.To fully understand the properties of targeted NPs,it is important to determine how the physicochemical properties of the NPs affect the interactions with their targets.Herein,we will discuss the main factors such as the ligand density,size and shape,surface and ligand charge and surface hydrophobicity of NPs to influence the behaviors of them.
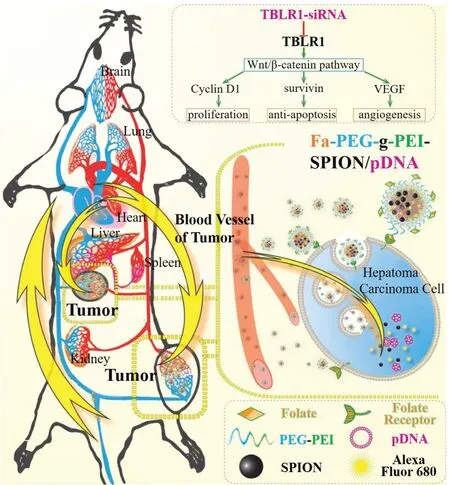
Fig.4-Illustration of the folate-targeted theranostic small interfering RNA(siRNA)nanomedicine Fa-PEG-gPEI-SPION/psiRNA-TBLR1 hepatocellular carcinoma chemotherapy.
4.1.The ligand density
For all delivery,the density of the targeting molecules on the surface of NPs impacts their affinity for the substrate due to increased cooperative effects.According to the thermodynamics,the more binding of a ligand to its substrate would promote the subsequent binding of its neighbors[148].Biologically,increasing the ligand density would aggrandize the interactions of the NPs with the cell membrane so that force the local concentration of receptors and lead to internalization of cell membranes.These synergistic effects impede the detachment of the NPs from the cell surface and result in increased avidity[149].
The increasing ligand density usually results in improved cellular uptakein vitro[150].However,targeting ligand density is critical to maximizing the efficiency of targeting NPs to cancer cells,and further increasing in ligand density will have deleterious effects on cell binding beyond the cooperative effect of the ligand get saturate.The reason of this effect might due to the improper orientation of the ligand,steric hindrance of neighboring molecules or competitive behaviors for the binding of the receptor[151].Similar negatively cooperative effect has been observed in ligand-clustered NPs where the ligands are arranged in patchy clusters by Hammond group[152].Valencia et al.have investigated and showed that the use of high densities of hydrophobic ligands can increase the macrophage uptake of the NPs without providing significant advantages in terms of receptor-mediated internalization[153].
Similar effects have been observedin vivowhere higher densities do not always result in improved efficacy.Many studies have shown that the alteration of the NPs surface to incorporate the ligands can modify the blood circulation and biodistribution profiles of the NPs.Gu et al.have developed the aptamer-targeted polymeric NPs and shown that increasing the ligand density above 5% would cause the increasing clearance of the NPs by the liver and the spleen and impeded the distribution to the tumor[154].
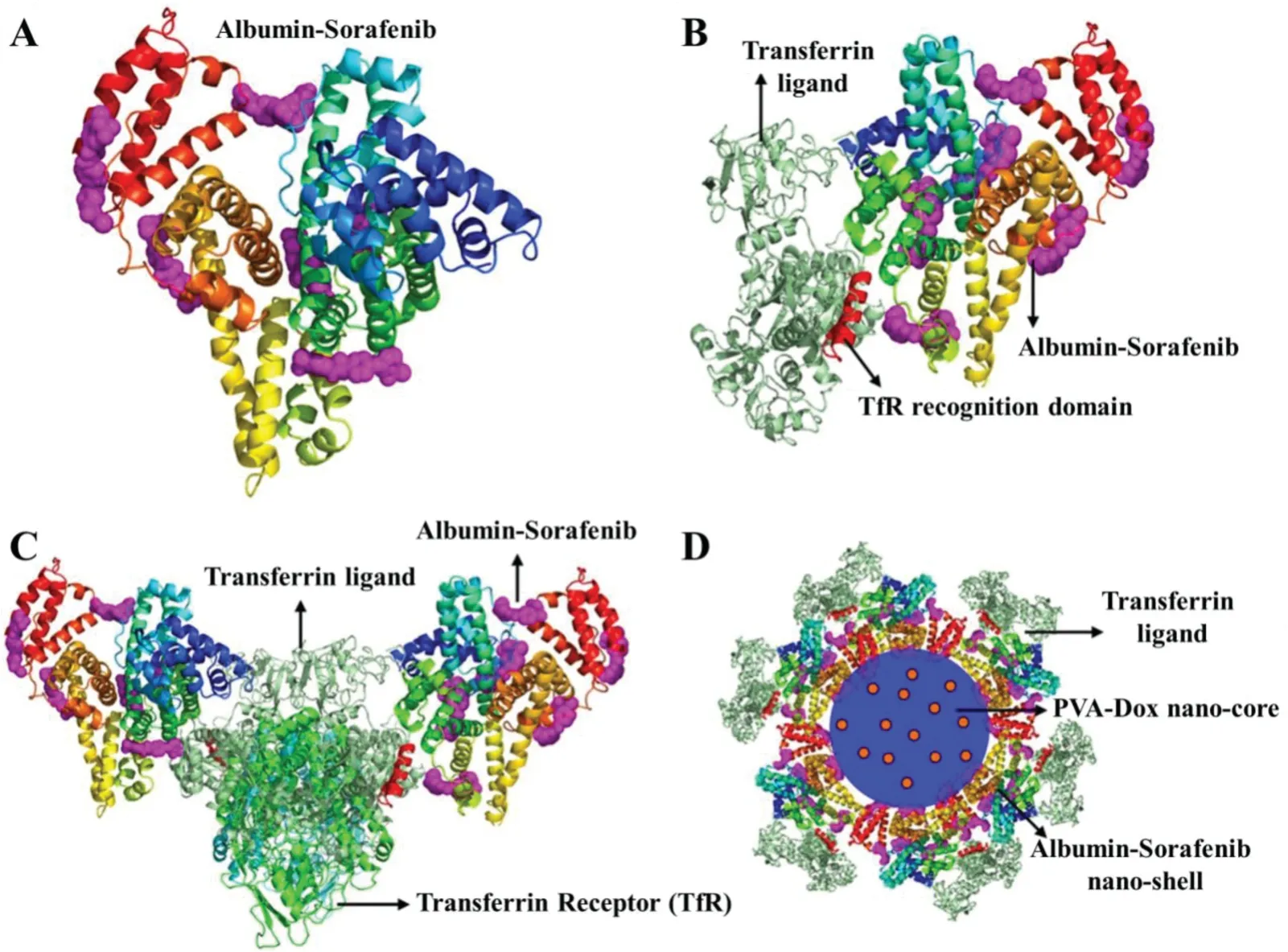
Fig.5-In silico docking simulation of core-shell nanomedicine(Reproduced with permission from[85].Copyright 2016 Lin et al.).
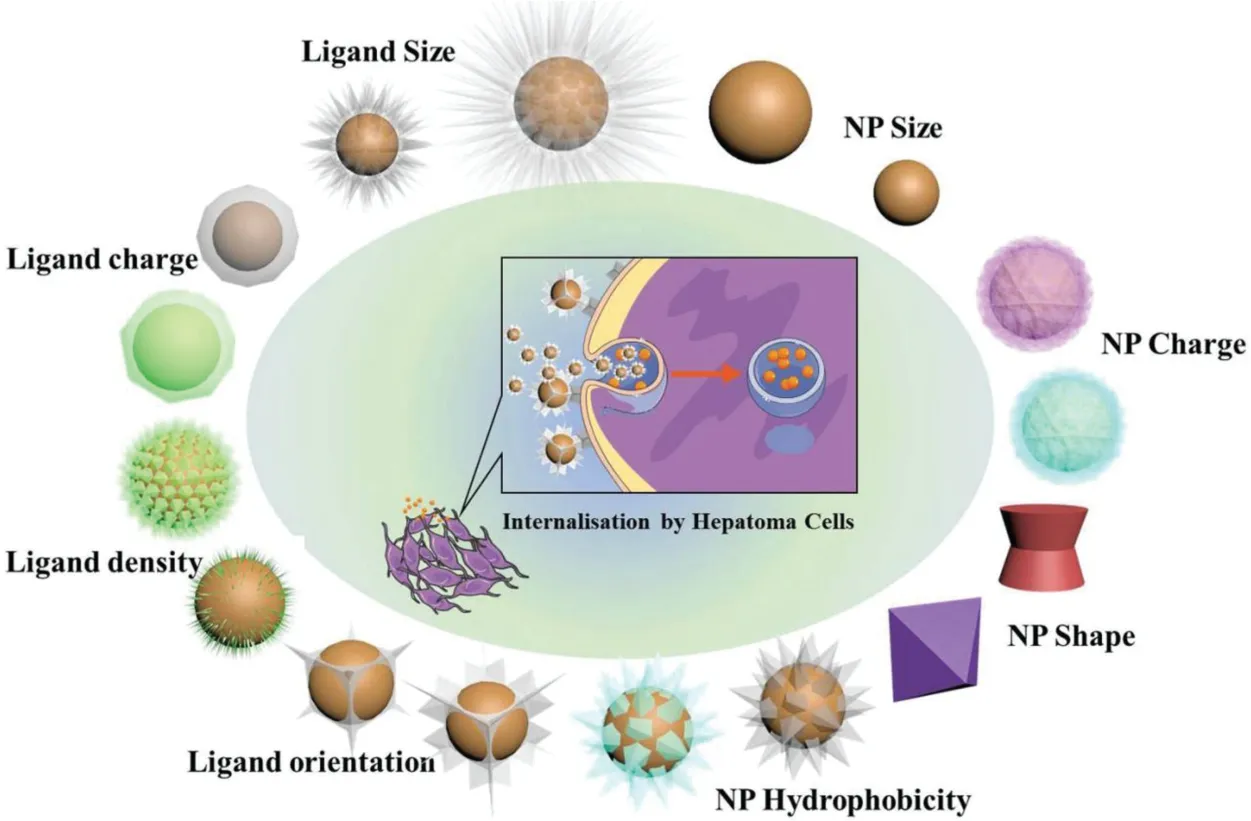
Fig.6-The physicochemical properties of the ligand and the NP affect their blood circulation profiles,their biodistribution and their ability to be internalized by cancer cells.
Finally,many studies indicated that tumor selective antibodies could suffer from a binding-site barrier preventing their in-depth diffusion in the tumor[155,156].This phenomenon is observed when high affinity molecules rapidly bind perivascular cells surrounding the cancer cells upon their extravasation to the tumor.The binding-site barrier also limits the in-depth diffusion of small molecular weight drugs with high affinity for HCC cells.This effect has been recently targeted to the receptors NPs which showed not enough HCC cells penetration compared to their non-targeted counterpart.Interestingly in that case,the binding-site barrier effect was not observed with larger NPs,presumably because of differences in the rates of HCC cells penetration and/or cell internalization.Therefore,it seems probable that adequately tuning the ligand density on the NP surface could mitigate the binding-site barrier effect and translate into adequate retention time and maximal cellular uptake throughout the HCC[157].
4.2.Size and shape
The size and shape of the nanomaterial must be taken into consideration early in the design of targeted NPs.For HCC,smaller sizes and spherical shape represent higher curvatures might promote hepatic targeting but can be problematic for post synthesis ligand functionalization.In addition,the tethering of high molecular weight ligands on the surface of NPs would increase their hydrodynamic radius[158].This increase in size must always be considered in light of the possible size restrictions involved in tumor accumulation.Besides the above mentioned effects,size can also affect cellular uptake.Although,the avidity of the particle increased with size range from 2 to 70 nm,the maximum uptake is a compromise between high avidity and optimal cell membrane wrapping around the NPs.Additionally,it is not clear how these conclusions can be expanded to other systems,as the interactions between NPs and cell highly-depend on the physicochemical properties of each material.In vivo,Lee et al.have reported that the size of actively targeted NPs could affect the intracellular deposition,not only in liver.In this case,although the intratumoral accumulation of smaller NPs was decreased due to shorter blood circulation times,the cytoplasmic and nuclear distribution of the 25-nm NPs was superior to that of the 60-nm colloids[158,159].Moreover,the shape of NPs might influence the cell uptake kinetics and internalization pathways through modulating the nanomaterial interact with the cell surface[160].
4.3.Surface and ligand charge
Chemically,the charge of unfunctionalized NPs and that of the ligand can affect the conjugation yield and the spatial display of the ligand on the surface.Repulsive or attractive forces between the surface of the NPs and the ligand can interfere with the conjugation or affect the final ligand structure and conformation.A chemical spacer with reasonable length,such as PEG,can be helpful to reduce the effect,but might simultaneously complicate synthesis and increase the final particle size[161,162].Previous study also showed that the final surface charge will affect the efficacy of the targeted NPs[163].For HCC,due to the interaction between cationic NPs and negatively charged cell membranes,positively-charged NPs show increased cellular binding and uptake,in a first pass effect and non-specific manner[164].Because most ligands are charged molecules,the NPs surface charge is determined by the combinations of ligand densities,materials,NP formulation strategies and so on.Although recent work was carried out to address how charge density affects interactions of actively targeted NPs with HCC cells and NPs charge can affect cellular uptake,it remains unclear what parameters offer the best hepatic targetingin vivo.
4.4.Surface hydrophobicity
Except surface charge and above factors,hydrophobicity can also affect the architecture of the ligand display.Further,this property can have serious effects since most polymeric NPs have hydrophobic cores.Previous studies have shown that during the self-assembly of polymeric hydrophobic particles,hydrophobic ligand such as folic acid could remain trapped in the particle core without being properly displayed on the surface.In some cases,the final surface hydrophobicity of NPs can also affect nonspecific interactions with HCC cells.Actively targeted NPs without steric stabilization seem to lose their substrate-binding capacity when proteins adsorb on their surface.Surface functionalization can delay adsorption of plasma proteins and has been demonstratedin vitroandin vivowhere the efficacy is dependent on both circulation times and ligand-substrate interactions.
Overall,although the aforementioned factors hold true for the majority of therapeutic NPs,no general strategies have been found suitable to address the limitation of the nanosystems.For HCC therapy,assessing the efficacy of a treatment still requires time and resources,and providing matching targeted delivery nano-systems remain therefore unlikely.However,most studies have demonstrated the targeting ligand might be key factor to decide thein vivoprofiles of nanoparticles.More and more targeted liposomes and polymeric NPs have been made to clinical development stages indicating that the decisive role of ligands.With the aim to transport in depth of tumor,multi-ligands or multi-functional ligands have been used to alternate the negative effects of mono-high ligand density.
5.Conclusion and future prospect
Since HCC is a complex multi-step process influenced by many factors for its initiation and progression,therapeutic interventions are challenging for this disease.With the rising incidence of HCC and high morbidity and mortality rates associated with this cancer,there is an urgent need to develop effective treatment and prevention strategies.We have reviewed the various targeted receptors and recent treatment modalities of HCC with emphasis on nanotechnology strategies in the field of HCC.While surgical resection and liver transplantation are the only strategies available for treating advanced stage patients,nanomedicines and immunotherapy might be promising approach for successful treatment of HCC in the future.Not only safety,toxicity,preclinical efficacy studies are required thoroughly before any clinical application of these delivery strategies are further explored.As GWAS and more comprehensive data sets become available,the clinical advantages of using nanomedicine for HCC treatment might be better understood.Based on the personalized medicine,combined nanomedicines with immunotherapy would be a fine choice for HCC therapy.Immunotherapy strategies have exhibited encouraging results for various cancer therapies.However,the dynamic nature of immune responses at the HCC sites and some other challenging such as complication of multi-immune and individual difference would lead to combination nanotechnology and immunotherapy an opportunity and challenge coexist in clinic.With the development of immunotherapy,all together these will forecast a bright future of nanomedicine for hepatocellular carcinoma therapy when it could control and regulate the immune response from exogenous immune stimulation.
Conflicts of interest
The authors confirm that this article content has no conflict of interest.The authors alone are responsible for the content and writing of this article.
Acknowledgments
This work is supported by the National Natural Science Foundation of China(Grants 81571799,81773193,81771929 and 81773642).
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.ajps.2019.04.005.
杂志排行
Asian Journal of Pharmacentical Sciences的其它文章
- The functions and applications of A7R in anti-angiogenic therapy,imaging and drug delivery systems
- Investigation of molecular aggregation mechanism of glipizide/cyclodextrin complexation by combined experimental and molecular modeling approaches
- Evaluation of the Mrp2-mediated flavonoid-drug interaction potential of quercetin in rats and in vitro models
- A novel oral prodrug-targeting transporter MCT 1:5-fluorouracil-dicarboxylate monoester conjugates
- Synthesis,characterization and in vivo evaluation of honokiol bisphosphate prodrugs protects against rats’brain ischemia-reperfusion injury
- Improved dissolution and oral absorption by co-grinding active drug probucol and ternary stabilizers mixtures with planetary beads-milling method
