Improved dissolution and oral absorption by co-grinding active drug probucol and ternary stabilizers mixtures with planetary beads-milling method
2020-01-06
aSchool of Pharmacy,Shenyang Pharmaceutical University,Benxi 117004,China
bShenyang Medical College,Shenyang 110031,China
cSchool of Pharmaceutical Engineering,Shenyang Pharmaceutical University,Benxi 117004,China
dSchool of Traditional Chinese Medicine,Shenyang Pharmaceutical University,Benxi 117004,China
ABSTRACT The objective of this work is to construct a nanosuspension drug delivery system of probucol,a BCS II drug,in order to improve its dissolution and oral bioavailability.The wet milling procedure using planetary beads-milling equipment was utilized to grind the raw probucol to ultrafine nanoparticle/nanocrystal aqueous suspension that was further solidified by freeze-drying process.Cellulose derivatives of different substitution groups and molecular weights,including HPMC,HPC,and MC,were evaluated as the primary stabilizer of probucol nanosuspension.Ternary stabilizers system composed of a primary stabilizer(cellulose derivative,i.e.HPC),a nonionic surfactant(Pluronic® F68),and an anionic surfactant(SDS)was employed to obtain probucol nanosuspension of finer particle size and enhanced dissolution in aqueous media.The probucol nanosuspension with good physical stability showed no obvious change of particle size even after storing over 7 d at 4°C or 25°C.The solidified probucol nanosuspension with trehalose as the cryoprotectant showed the highest dissolution rate(>60% at 2 h)compared to other cryoprotectant.The in vivo pharmacokinetic evaluation indicated about 15-folds higher AUC value of the probucol nanosuspension compared to that of coarse probucol suspension after oral administration to rats.The probucol nanosuspension prepared by wet-milling and ternary stabilizers system may find wide applications for improving the dissolution and oral absorption of water-insoluble drugs.
Keywords:Probucol Nanosuspension Ternary stabilizers systems Planetary beads-milling Bioavailability
1.Introduction
Probucol,4,4′-[(1-methylethylidene)bis(thio)]bis[2,6-bis(1,1-dimethylethyl)]phenol,is a hypocholesterolemic,antiatherosclerotic,and potent anti-oxidant drug.It has been used for the treatment and prevention of atherosclerotic lesions,diabetes mellitus and cardiovascular diseases in clinical in the past few decades[1,2].In recent years,the application of probucol in anti-percutaneous transluminal coronary angioplasty(PTCA)restenosis has received great interests[3].In clinical practice,a large daily dose of one gram of probucol is normally recommended due to its poor water solubility(about 5 ng/ml at 25°C)and thus very low oral bioavailability(up to 6%)[4,5].As a typical BCS II(biopharmaceutics classification system,type II)drug,it is expected that the oral bioavailability of probucol can be enhanced by improving its apparent solubility and dissolution rate[6,7].A variety of solubilization techniques have been designed to solve this problem[8].The self-emulsifying drug-delivery systems(SEDDS)and solid dispersions prepared by co-precipitation,including spray-drying,freeze-drying and hot melt extrusion,has been investigated to enhance the dissolution of various water insoluble drugs[9-11],and water-soluble excipients have also been introduced to form complex with certain candidate drugs[12].In the past decade,nanonization has become a practical technology to improve the oral bioavailability of water-insoluble drugs[13-16].
Nanosuspensions are liquid dispersion consisting of solid drug nanoparticles,which can greatly improve thein vitroandin vivoperformance of pharmaceutical preparations,including increased saturation solubility and dissolution rate,oral absorption,etc[14,15,17].Two main approaches are involved in production of nanosuspensions,i.e.top down and bottom up technologies[18].The bottom up method,such as precipitation method that involves using organic solvent and low drug content,is generally difficult to scalable.The top down technologies include media milling,high pressure homogenization,and supercritical fluid process[19].Among these methods,media milling is one of the most efficient ways to obtain sub-micron drug particles aiming to improve oral bioavailability[20].Wet-milling process is one of the well developed media milling processes,by which the drug(s)is milled in liquid normally containing hydrophilic polymer and/or surfactant stabilizer(s).In addition to reducing the drug particle size,wet-milling process is found to be able to solve the problems related to the aggregation of any micronized drug[21-23].
The particle size of drug particles/crystals usually decreases to a constant value after grinding,which depends on the processing parameters.The polymeric stabilizers or surfactant stabilizers may also perform dual roles:assisting to reduce particle size,and inhibiting crystal growth[14].The wetting of the surface of hydrophobic drug particles by the surfactant stabilizers in the nanosuspension formulations most likely to obtain further enhancement of the dissolution rates compared to micronized suspensions[16,24].The oral nanocrystal products using wet milling technology have been successfully launched into the market[15,25],for instance,the felodipine micro powder prepared using a wet media milling technique was investigated and showed significant enhancement ofin vitrodissolution andin vivooral bioavailability[26].
Although various nano-formulations have been extensively studied or marketed,there are still many difficulties remained to be solved,such as low drug loading,poor physical stability,etc.[5,27].In this study,probucol nanosuspensions were prepared by co-grinding of probucol and aqueous solution of stabilizer(s)using planetary beads-milling technique.The ternary stabilizers system was used to obtain probucol nanosuspensions with improved dissolution and stability.The physicochemical properties of the suspensions and its freeze-dried powders were characterized by particle size analysis,X-ray powder diffraction(X-RPD),differential scanning calorimetry(DSC)analysis and scanning electron microscope(SEM).In vitrodissolution andin vivopharmacokinetic studies in rats were carried out to evaluate the effect of such ternary stabilizers system on improving thein vitro/in vivoperformance of the probucol nanosuspension.
2.Materials and methods
2.1.Materials
Probucol was purchased from Wuyicihang Pharmaceutical Co.(Hebei,China).Hydroxypropyl methylcellulose(HPMC-603,HPMC-606 and HPMC-615),methylcellulose(MC,Metolose SM-4),Hydroxypropyl cellulose(HPC-SL)were generously provided by ShinEtsu(Tokyo,Japan).poloxamer 188(Pluronic®F68,abbreviated as F68)and PVP K30 were kindly gifted by BASF(Berlin,Germany).Sodium dodecylsulfate(SDS)was purchased from Bodi Chemical Industry Co.,Ltd(Tianjin,China).Microcrystalline cellulose(MCC)and Avicel RC-591 were kindly donated by FMC(USA).Methanol and acetonitrile of HPLC grade were purchased from Hanbang Chemical Co.,Ltd(Jiangsu,China).All other chemicals used were of analytical grades.
2.2.Preparation and solidification of probucol nanosuspensions
Probucol nanosuspensions were prepared using wet milling procedure.In brief,coarse suspensions of 5 g of raw probucol in 25 ml aqueous solution containing stabilizer(s)were placed in a 100 ml mixing bowl.Here,the cellulose derivative was used as polymeric stabilizer,F68 and SDS as conjunct stabilizers.Subsequently,150 g of zirconia beads(0.5 mm in diameter)were introduced as the milling media.The mixtures were grinded using a PM 400 MA Planetary Mill(Shunchi Technology Co.,Ltd,China)at 1000 r/min for 3 h.Subsequently,the nanosuspension was separated from the milling media by decanting the suspension,followed by the washing of the beads with water.
The probucol nanosuspensions were lyophilized using FDU-1100 Freeze Dryer(Eyela,Japan),20%(w/w)cryoprotectant powders(trehalose or lactose)were added to probucol nanosuspensions.Samples were pre-frozen in the refrigerator(-40°C)for 12 h and subsequently lyophilized in freeze dryer at-25°C for 12 h,and then followed by secondary drying phase of 3 h at elevated temperature(20°C).
2.3.Nanosuspensions characterization
2.3.1.Particle size analysis
Particle size and zeta potential were measured using a Nicomp®380/ZLS Photon Correlation Spectroscopy(PSS,Santa Barbara,CA,USA).The sizes of the dispersed particles were calculated using intensity distribution from Gaussian distribution.The polydispersity index(PI)calculated from Gaussian distribution indicated the width of particle size distribution was not more than 0.3 of all the tested samples.Nanosuspensions were stored in glass bottle and sealed with polypropylene caps at 4°C and 25°C for one week.The changes to the particle sizes were determined at various time intervals to assess the physical stability of the nanosuspension.
2.3.2.X-ray powder diffraction(X-PRD)
X-PRD(X Ray Powder diffraction)measurement was performed using a LabX XRD-6000 X-ray Diffratometer(Shimadzu,Japan)using Cu Kαradiation(graphite monochromator)generated at 40 mA and 40 kV.Powders of bulk probucol,mixture of polymeric stabilizer,F68,and SDS,lyophilized probucol nanosuspensions without cryoprotectant,and physical mixture of polymeric stabilizer,F68 and probucol were measured,respectively.The obtained data were typically collected with a step width of 0.04° and a count time of 2 s.
2.3.3.Differential scanning calorimetry(DSC)
DSC analysis was performed using a DSC-60 instrument or Thermal Analyzer-60 WS(Shimadzu,Japan).For the DSC measurement,the same samples used for X-PRD measurement were weighed into aluminum pan,and then sealed with a pinhole-pierced cover.The thermal curves were recorded at a scan rate of 10°C/min from 30°C to 200°C.
2.3.4.Scanning electron microscope(SEM)
The morphology and particle size of the dispersed probucol and probucol bulk drug were observed using S-3400 SEM(Scanning electron microscope)(Hitachi,Japan)at 10.0 KV electron acceleration voltage,or using SU-70 SEM(Scanning electron microscope)(Hitachi,Japan)at 5.0 KV electron acceleration voltage.One drop of the nanosuspension sample was placed on a piece of mica,dried in nitrogen stream,and sputtered with gold before the SEM observations.
2.4.In vitro dissolution experiments
Thein vitrorelease test was performed using the paddle method as described inChinese Pharmacopoeia(2015)using a ZRS-8G Dissolution Apparatus(Tianda Tianfa Technology Co.Ltd.,China).In this study,900 ml SDS(2%w/w)solutions(sink condition)or purified water(non-sink condition)at 37.0±0.5°C were used as the dissolution media respectively,and the rotation speed was set at 50 r/min.The samples equivalent to 15 mg or 3 mg probucol were added to the dissolution medium respectively.At pre-determined time intervals,the dissolution sample(5.0 ml)was withdrawn from the beakers and replaced with the equal volume of fresh medium.The dissolution samples were filtered through a membrane filter of 0.1 μm pore size(Millipore,USA)and the content of probucol was then assayed by Hitachi L-7110 HPLC System(Hitachi Ltd.,Tokyo,Japan)equipped with a Kromasil C18(5 μm,150 mm×4.6 mm)column(Agilent,USA).The mobile phase consisted of methanol:water(97:3,v/v)was delivered at a flow rate of 1.0 ml/min,and the detection wavelength was set at 242 nm.
2.5.In vivo study
Male wistar rats weighing 210-250 g was used.The rats were fasted overnight prior to dosing and remained fasting until 4 h after the dose administration.The lyophilized samples and the reference coarse suspension at a dose of 250 mg probucol/kg body weight were administered by oral gavages to rats.The lyophilized samples were reconstituted in purified water before administration.The reference coarse suspension was obtained by grinding the commercially available probucol tablets to fine powders and then dispersing in water.Blood samples were collected from rats orbit vein at 0 h(pre-dose),2 h,4 h,6 h,8 h,10 h,12 h,and 24 h after dose administration,and placed into tubes treated with heparin.Plasma samples were obtained by centrifugation of the blood samples and stored at-20°C until further analysis.All animal experiments were carried out in accordance with The Guidelines for Animal Use issued by the Life Science Research Center of Shenyang Pharmaceutical University.
Plasma probucol concentration was determined by HPLC method using amiodarone as the internal standard[28]as described below.100 μl of internal standard solution(1.0 μg/ml in methanol)was added to 200 μl of plasma samples,mixed well using a vortex mixer(VORTEX 3,IKA)and stood for 30 s.1.25 ml n-hexane was added and mixed.The mixture was centrifuged at 5000 r/min for 10 min,1 ml of the supernatant was transferred into another tube and the organic solvent was evaporated under nitrogen stream.The residue was reconstituted with 100 μl of methanol,centrifuged at 10 000 r/min for 10 min.20 μl supernatant was injected into the HPLC system.Samples were analyzed on a Hitachi L-7110 HPLC System(Hitachi Ltd.,Tokyo,Japan)using a Kromasil C18(5 μm,150 mm×4.6 mm)column(Agilent,USA).The mobile phase consisted of methanol:water(93:7,v/v)was delivered at a flow rate of 1.4 ml/min.The linear range of this method was 0.10~6.0 μg/ml with anR2(correlation coefficient)value of not less than 0.99.Precision and accuracy results of three different concentrations within calibration range demonstrated good precision and accuracy(RSD<15%).The recoveries were in the range of 80%-90% for three different control samples and coefficient of variation was found to be less than 10%.
The plasma probucol concentrations were determined using a linear regression equation.Maximum concentration(Cmax)and maximum times(Tmax)were obtained directly from the concentration-time data.The other pharmacokinetic parameters(AUC0-24 handT1/2)were analyzed by DAS 2.1.1 software(supplied by Chinese Pharmacological Society).
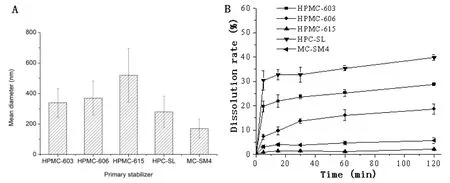
Fig.1-The mean diameters(A),and dissolution curves(B)under non-sink condition of probucol nanosuspensions prepared using different cellulose derivatives(HPMC-603,HPMC-606,HPMC-615,HPC-SL and MC-SM4)as the primary stabilizers.The formulations contained 2%cellulose derivative,1%F68 and 0.1%SDS.
3.Results and discussion
3.1.Formulation of probucol nanosuspensions
3.1.1.Effect of the primary stabilizer on the preparation of probucol nanosuspensions
Selection of proper steric and electrostatic stabilizers and their optimum contents play key roles in formulating any drug nanosuspension[29].Appropriately selected types and contents of stabilizer(s)are the important variables for physically stable drug nanosuspension.For the preparation of probucol nanosuspensions,hydrophilic cellulose derivative was investigated as the primary steric stabilizer,which was combined together with nonionic surfactant and anionic surfactant to form ternary stabilizer systems.
A series of low viscosity HPMC-603(3 mPa·s),HPMC-606(6 mPa∗s),HPMC-615(15 mPa∗s),HPC-HL and MC-SM4 were selected as the primary stabilizer at the same concentration(2%,w/v).When combined with F68(1%,w/v)and SDS(0.1%,w/v),both HPMC,HPC and MC were effective in reducing particle sizes(Fig.1A)compared to the bulk probucol(SEM observation indicated large size and wide size distribution of probucol between several to decades microns,as shown in Fig.3A)and improving short-term physical stability of the nanosuspensions,whereas three cellulose derivatives showed different effects on the dissolution of the obtained nanosuspensions.As shown in Fig.1B,HPC and HPMC-603 were effective in increasing the dissolution rate of the prepared probucol nanosuspensions,whereas MC was not so effective although it displayed to be a good stabilizer to decrease particle size.
With the increase of the viscosity(molecular weights)of HPMC,the drug particle sizes increased and the dissolution rates of the resulted nanosuspensions decreased,which can be attributed to the reduction of power as a result of decreased kinetic energy between different milling medias,and this result was in agreement with that of van Eerdenbrugh et al.[21].Further analysis from the structure,compared to HPMC,MC molecule contains a number of hydrophobic groups(methoxyl),and absence of hydrophilic hydroxypropyl group,thus relatively weak affinity to water molecules,and consequently lower dissolution rate of obtained probucol nanosuspension.HPMC-603 showed more effective in improving the dissolution rate,which may be attributed to its abundant hydroxypropyl group.
Although the results indicated that both HPMC and HPC were good stabilizers for obtaining ultrafine dispersion of probucol,HPC that contains more hydroxypropyl group showed higher affinity to water molecule and lead to higher dissolution rate of probucol.HPC was chosen as the co-milling primary steric stabilizer in further studies.
It is generally known that the dissolution behavior of nanosuspension was greatly dependent on the nature of the polymeric stabilizers used.It can adsorb onto freshly formed drug crystal surface during milling process.Such kind of adsorption is related to either hydrogen bonding between two hydroxyl group exist on probucol molecules and cellulose derivatives(HPMC and MC),or affected by the hydrophobic interactions between the cellulose derivatives and the hydrophobic groups present on the surface of probucol particle[30].As the viscosities and surface tension values of these cellulose derivatives used are almost the same,it is reasonable to postulate that the different results on particle size and dissolution rates were caused by the characteristics of the polymeric chain itself due to the different contents of methoxyl and hydroxypropyl groups.
3.1.2.Effect of nonionic and anionic surfactants on the preparation of probucol nanosuspensions
The binary and ternary stabilizer(s)systems composed of primary stabilizer(cellulose derivative),nonionic surfactant(F68)or/and anionic surfactant(SDS)were investigated to prepare nanosuspensions,the typical formulations and mean particle sizes were listed in Table 1.
The results showed that within certain concentration range of F68(0%,0.5%,and 1.0%),the particle size of probu-col crystals decreased with the increase of the content of F68,while extra amount of F68(2.0%)did not further facilitate the size reduction.Addition of 0.1%and 1.0%SDS into the grinding mixtures significantly decreased the mean particle sizes from 323.7 nm of the binary stabilizers system(B2)to 279.6 nm(T1)and 192.4 nm(T2),respectively.The anionic surfactant in the ternary stabilizers system can also significantly improve drug dissolution of the resulted nanosuspensions.The dissolution rates(sink condition)at 2 h of the two ternary formulations containing 0.1% and 1.0% SDS were approximately 40% and 67%,respectively.
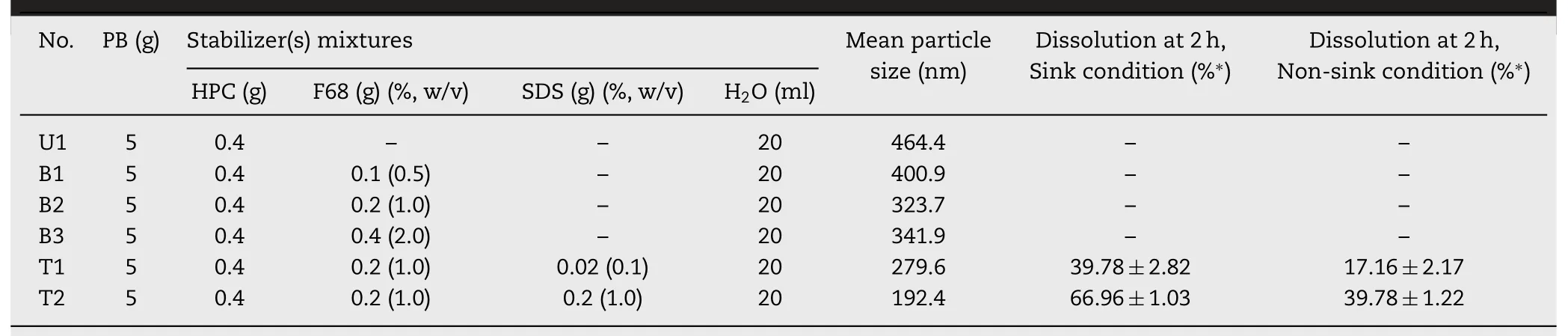
Table 1-Mean particle sizes of probucol suspensions grinded using unary,binary,and ternary stabilizer(s)systems.
Using the ternary stabilizers systems and planetary media milling method,relatively stable probucol nanosuspensions with improved dissolution was successfully prepared.A typical probucol nanosuspension prepared using ternary stabilizers system(2% HPC,1% F68,and 0.1% SDS)was diluted and kept at 4°C and 25°C,and the particle sizes were monitored.During the 7 d storage period,the particle sizes showed ignorable changes.These results indicated that the primary stabilizer,the nonionic surfactant and anionic surfactant,all contribute to size reduction and enhanced dissolution of probucol.
Both nonionic and anionic surfactants are widely incorporated into the grinding systems to improve the wettability and size reduction of the insoluble active ingredients,or to increase the physical stability of the resulted nanosuspensions[8].Anionic surfactant(SDS)may play versatile functions to the drug nanosuspensions,including micelle solubilization,electrostatic repulsion between the nanoparticles,and increased hydrophilicity of the nanoparticles,consequently improving the physical stability and dissolution rate of probucol nanosuspension.The nonionic surfactants,Pluronic®F68(generic name:poloxamer 188),i.e.,poly(oxyethylene)-poly(oxypropylene)-poly(oxyethylene)triblock copolymer,is a synthetic amphiphilic macromolecule with a number average molecular weight of 8500 Da.Both the surface activity and the steric stabilization effect of F68 can help to improve the grinding process.The role of surfactant in the nanosuspension is to prevent a rapid,local drug domain aggregation,which allow the water-insoluble drug diffuse to bulk solution to inhibit further crystallization[31].
3.2.Solidification of probucol nanosuspension
In order to obtain preparations of probucol nanosuspension with good long-term stability,various diluents or cryoprotectants were added into the grinded nanosuspension solutions,which were subsequently solidified using wet-granulation,spray-drying,or freeze-drying methods.
Some typical formulations and their dissolution rates at 1 h under non-sink condition were shown in Table 2.The results clearly indicated that the drying processes of both wet-granulation and spray-drying significantly decreased thein vitrodissolution rates compared to the pristine nanosuspension.The powders obtained by freeze-drying method using cryoprotectants including lactose,glucose and sucrose showed relatively higher dissolution rates at 1 h of about 30%-37%that was only about half of the dissolution rate of the pristine nanosuspension(about 67%at 1 h).The solidified powder using trehalose as the cryoprotectant showed the highest dissolution rate(64.9%)compared to other freeze-dried formulations,which could be attributed to that trehalose was more effective in preventing the aggregation of nanoparticles during the drying process.
Fig.2 showed the representative dissolution patterns of the probucol nanosuspension prepared by the ternary stabilizers system and its lyophilized powder under sink or non-sink dissolution conditions.The nanosuspension indicated faster and higher drug dissolution,while the dissolution of the physical mixture was not detected under both sink condition and nonsink condition.The solidified powder using trehalose as the cryoprotectant showed comparable dissolution rates to that of the pristine nanosuspension at both sink and non-sink dissolution conditions.And an interesting observation was that its dissolution rate at non-sink condition did not incline with time,which was different to that of the pristine nanosuspension tested at the same condition.A possible explanation to this phenomenon is that the sugar dissolved in the dissolution media may inhibit the aggregation or growth of probucol nanoparticles.
3.3.Characterizations of probucol nanosuspension
The nanosuspension of typical formulation had a zeta potential of-36.63±2.7 mV,as measured by photon correlation spectroscopy(PCS)method.The high value of zeta potential can be attributed to the introduction of SDS into the grinding mixtures.Fig.3 showed the SEM images of bulk probucol and probucol nanocrystals.The bulk probucol particles had irregular shape and wide size distribution,while the particle size of probucol nanocrystals was reduced markedly.
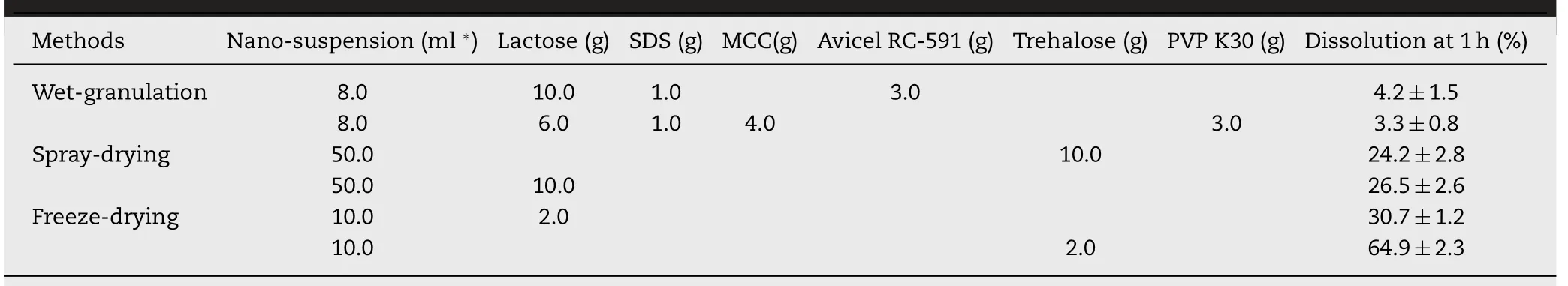
Table 2-Dissolution rates of the solidified probucol nanosuspensions obtained by wet granulation method or spray drying method under non-sink condition,respectively.
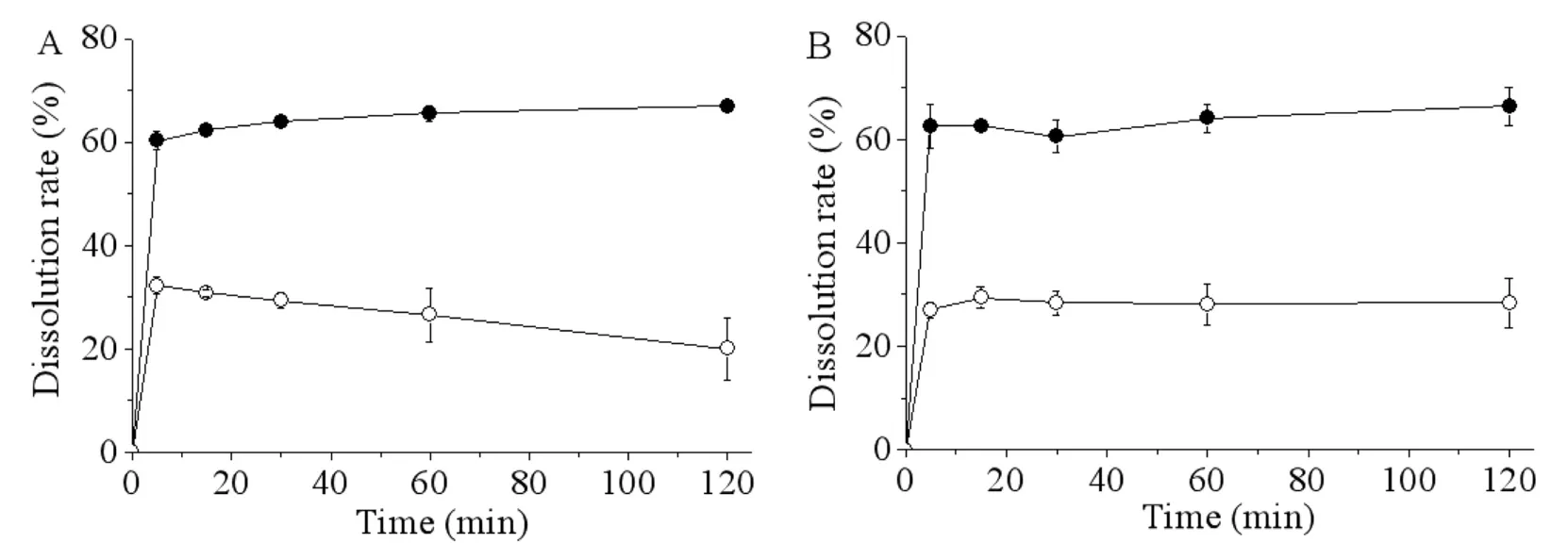
Fig.2-Dissolution profiles of probucol nanosuspension(A)and lyophilized powder of probucol nanosuspension using trehalose as the cryoprotectant(B)at sink dissolution conditions(●)or non-sink dissolution conditions(○)(n=3).
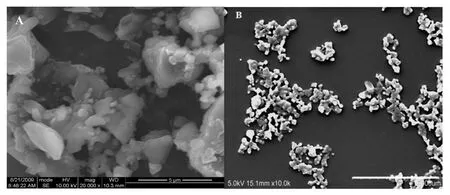
Fig.3-The SEM images of bulk probucol(A)and probucol nanocrystals obtained by wet milling(B).Scale bar:5 μm.
Probucol has been reported having at least two polymorphs with onset melting points of 116°C(Form II)and 125°C(Form I),respectively,Form I polymorph is the thermodynamically stable form[32].The X-PRD patterns and DSC thermograms of SDS,F68,HPC,probucol,their physical mixture,and the lyophilized powder of the nanosuspension are shown in Fig.4.Probucol,the physical mixture,and lyophilized nanosuspension showed the same X-ray diffractograms of probucol(Fig.4A).DSC analysis indicated that the melting endothermic peak of bulk probucol recorded was 127°C.Poloxamer 188 exhibited a sharp endotherm of melting point at 52°C.The melting point of lyophilized nanosuspension and the physical mixture was 124°C,which is 3°C lower than raw probucol.In addition,the width of endothermic peaks also changed(Fig.4B).Although the drug particles were still at crystalline state after intensive grinding treatment,the loweredTmindicated different crystalline state,or the presence of residual F68 and SDS that associate to the nanoparticle surface and probably existed interaction between drug and surfactant.
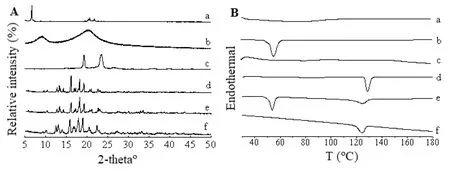
Fig.4-X-RPD patterns(A)and DSC thermograms(B)of SDS(a),F68(b),HPC-SL(c),probucol(d),their physical mixture(e),and the lyophilized powder of probucol nanosuspension(f).
Crystalline state is one of the key factors that influence the dissolution behavior of water insoluble active substances.The high energy input during the production of nanosuspension may induce changes in the crystalline state,for example,increasing the amorphous fraction or even creating completely amorphous particles,which can lead to higher dissolution rate and oral bioavailability[33].It may be due to experimental conditions such as presence of moisture content,sample weight and its preparation and polarity of poloxamer 188[34].The formation of a different crystalline state is likely suggesting that the shift in theTmis the consequence of a small amount of F68 and SDS bounded to probucol nanoparticle[35].Both X-RPD and DSC studies demonstrated all kinds of energy input during milling did not cause obvious formation of amorphous probucol.
As the physical mixture had the same compositions to the nanosuspension,a conclusion can be drawn that the enhanced dissolution of probucol nanosuspension was due to particle size reduction instead of the incorporation of stabilizers or surfactants in the formulation.It also should be noted that the dissolution rate inclined within the test period under non-sink condition,which may be attributed to the formation of large particles due to the crystal precipitation or particle aggregation at high drug concentration.
3.4.In vivo bioavailability
The bioavailability of the prepared probucol nanosuspension was preliminarily estimated after oral administration to rats.In order to understand the dependence ofin vivoabsorption andin vitrodissolution rate,the oral bioavailability of freeze-dried powders of probucol nanosuspension with or without cryoprotectant(thus different dissolution rates)were compared with the coarse probucol suspension.The plasma probucol concentrationversustime curves for all the formulations were shown in Fig.5.
It can be seen from the results that the lyophilized probucol nanosuspension with trehalose as the cryoprotectants had the highestCmax(6.48±1.08 mg/l),and the highestAUC(29.87±1.91 mg/l•h),which were significantly higher than those of coarse probucol suspension(Cmax:0.42±0.16 mg/l/AUC(0-24h):2.48±0.15 mg/l•h)and other two freeze-dried formulations.TheAUCandCmaxvalues of the lyophilized probucol nanosuspension with trehalose as cryoprotectants were remarkably increased by 12.04 folds and 15.43 folds compared to the coarse suspension.
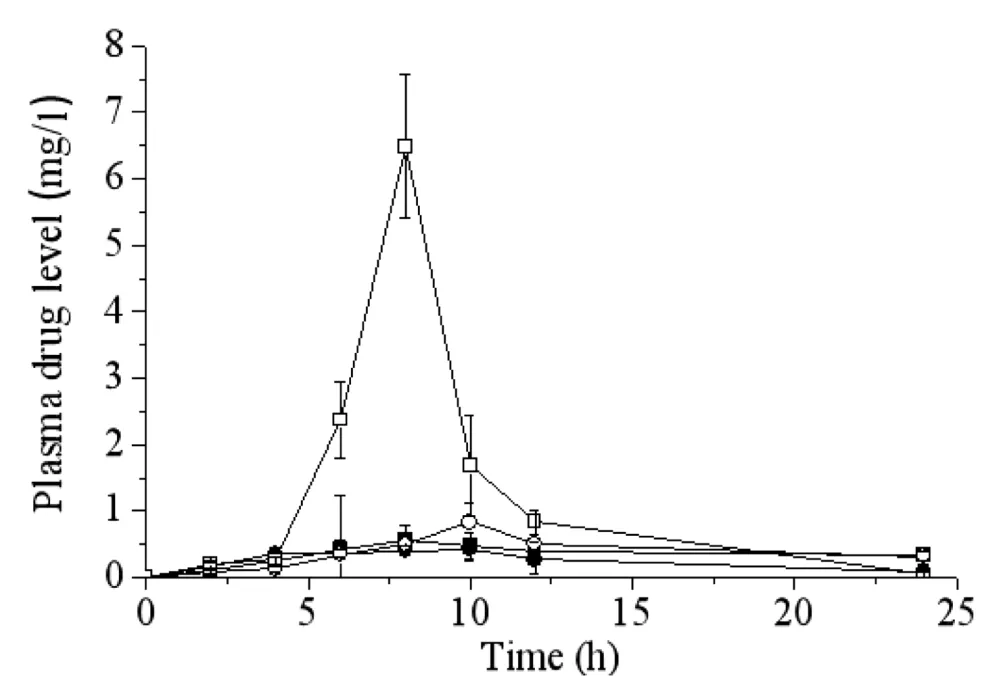
Fig.5-Plasma drug concentration versus time curves after oral administration of lyophilized probucol nanosuspension with trehalose as cryoprotectants(□),lyophilized probucol nanosuspension with lactose as cryoprotectants(○),lyophilized probucol nanosuspension without cryoprotectants(■),and coarse probucol suspension(●)to rats(n=6).
The AUC values of these four formulations followed the order of lyophilized probucol nanosuspensions with trehalose,lactose,and without cryoprotectant,and coarse suspension.The results also suggested dissolution dependentAUCvalues of various formulations.Although the ternary stabilizers system was effective in reduction of drug particle size and enhancement ofin vitrodissolution of probucol and stability,the selection of drying methods,optimized formulation and processing parameters were still necessary for the solidification of probucol nanosuspension to prevent nanoparticles aggregation and consequently decreased dissolution and oral bioavailability.
4.Conclusion
Poor water solubility of probucol was the major obstacle to its oral absorption,and thus extremely low bioavailability was normally observed in patients.In our present study,probucol nanosuspensions were successfully prepared by using media wet-milling technique with a planetary beads-mill.The ternary stabilizers system composed of a primary stabilizer(water soluble cellulose derivative,e.g.HPC),a nonionic surfactant(Pluronic®F68),and an anionic surfactant(SDS)was effective in reduction of particle size,improving dissolution rate and physical stability.The three components in the ternary stabilizers system exert the dissolution enhancement effect via various mechanisms.The hydrophilic hydroxypropyl group in the cellulose derivative molecule is believed to be the important factor for facilitating probucol dissolution.The probucol nanosuspension obtained using ternary stabilizers system was solidified by freeze-drying method,the dried powders showed differentin vitrodissolution rates andin vivooral absorption dependent on the cryoprotectant used.Nanosuspension using trehalose as the cryoprotectant displayed the highest plasma drug level and AUC value when compared to other formulations,which showed about 15-folds higherAUCvalue than that of coarse probucol suspension.The nanosuspension prepared with ternary stabilizers system may find wide application of this old drug due to its remarkably improved dissolution and oral absorption.
Conflicts of interest
The authors report no conflicts of interest in this work.
Acknowledgment
The authors wish to express their gratitude for financial support from the National Basic Research Program of China(973 Program,No.2009CB930300).
杂志排行
Asian Journal of Pharmacentical Sciences的其它文章
- Cancer nanotechnology:Enhancing tumor cell response to chemotherapy for hepatocellular carcinoma therapy
- The functions and applications of A7R in anti-angiogenic therapy,imaging and drug delivery systems
- Investigation of molecular aggregation mechanism of glipizide/cyclodextrin complexation by combined experimental and molecular modeling approaches
- Evaluation of the Mrp2-mediated flavonoid-drug interaction potential of quercetin in rats and in vitro models
- A novel oral prodrug-targeting transporter MCT 1:5-fluorouracil-dicarboxylate monoester conjugates
- Synthesis,characterization and in vivo evaluation of honokiol bisphosphate prodrugs protects against rats’brain ischemia-reperfusion injury
