Studies on chemical constituents of Ailanthus altissima (Mill.)Swingle and their antioxidant activity
2020-01-01WanyiShiYuxiWangZhiyangYanXiaoxiaoHuang
Wanyi Shi,Yuxi Wang,Zhiyang Yan,Xiaoxiao Huang
Key Laboratory of Computational Chemistry-Based Natural Antitumor Drug Research &Development,Liaoning Province,School of Traditional Chinese Materia Medica,Shenyang Pharmaceutical University,Shenyang 110016,China
Abstract Ailanthus altissima (Mill.) Swingle is a deciduous tree belonging to the Simaroubaceae family.The root and stem bark are traditional medicines for treating many diseases,such as diarrhea,dreaminess and intestinal tract bleeding.Phytochemical investigation of Ailanthus altissima (Mill.) Swingle led to the isolation of eight compounds.Chemical structures of these compounds were identified by analyzing their NMR spectroscopic data.Among them,compounds 1,4,5,6 and 8 were isolated from Ailanthus Desf.for the first time.The antioxidant activities of these compounds were determined by DPPH radical scavenging method.
Keywords: Ailanthus altissima (Mill.) Swingle; lignans; phenylpropanoids; antioxidant activity
1 Introduction
Ailanthus altissima(Mill.) Swingle is a deciduous tree belonging to the Simaroubaceae family.It is distributed in China’s north,east and southwest.Its stem and root bark have many bioactivities such as antitumor [1,2],antioxidant [3],anti-inflammatory [4]and antibacterial activities [5],thus widely used in the treatment of diarrhea,intestinal tract bleeding,hemorrhoids,pruritus and dreaminess in traditional Chinese Medicine.At present,indoles alkaloids [6],triterpenes [7],flavonoids [8],phenolic acids and other active components have been isolated fromA.altissima.Indoles alkaloids fromA.altissimainclude mainly canthin-6-one type alkaloids andβ-carboline type alkaloids,while triterpenoids consist mainly of bitter principles,dammarane type triterpenes and tirucallane type triterpenes [6-8].The present study extracted the root bark with 70% EtOH first,and then isolated 8 compounds from the EtOAc extract.These compounds were identified as 4 lignans and 4 phenylpropanoids by analyzing their physical and chemical properties and spectral data.
It is reported that reactive oxygen species(ROS) is the metabolic byproduct of aerobic respiration,responsible for maintaining redox homeostasis in cells [9].ROS includes oxygen radicals [superoxide (O2·-),hydroxyl (OH·),peroxyl(RO2·),and alkoxyl (RO·)]and certain nonradicals [10].ROS at physiological concentration is beneficial to human body,and is an essential substance for protecting the organism from microorganism attack [11].ROS at higher concentration may cause adverse effects.ROS produced by the respiratory cycle of oxidative phosphorylation may attack biological macromolecules (e.g.cellular DNA),giving rise to single-strand or double-strand breaks that may eventually lead to cell aging,cardiovascular diseases,mutagenic changes,and cancerous tumor growth [10].Some natural ingredients with antioxidant properties might have the potential to prevent or treat various diseases,possibly by inhibiting or delaying oxidative reactions [12].In order to quickly determine the antioxidant activity of these compounds,DPPH radical scavenging assay is commonly used [13].
2 Materials and methods
2.1 General material and instrument
NMR spectra (Bruker ARX-400,USA,TMS as an internal standard in CDCl3or DMSO-d6); Mass spectra (Bruker IFS-55 spectrometer,Germany);High performance liquid chromatograph (Shimadzu,SPD-20A,UV detector,LC-6 high pressure pump,Japan); Preparative column (YMC Pack ODS-A,5μm,150 mm × 4.6 mm,USA); AE electronic analytical balance (Mettler-Toledo,Shanghai,China); Silica gel (100-200 mesh,200-300 mesh,Qingdao Marine Chemical Co.,China); Silica gel GF254 (Qingdao Marine Chemical Co.,China);Reversedphase C18silica gel (60-80μm,Merck,Germany); Sephadex LH-20 (25-100μm,Green Herbs Science and Technology Development Co.,Ltd.China); HP20 macroporous resin (Mitsubishi Chemical Co.,Japan); Diphenylpicrylhydrazyl(DPPH) and trolox (Sigma-Aldrich,Steinheim,Germany).
2.2 Plant material
The root bark ofA.altissima(Mill.) Swingle was purchased in 2017 from Anguo medicine market,Hebei province,China,and authenticated by Prof.Jincai Lu,Department of Pharmacognosy,Shenyang Pharmaceutical University.
2.3 Extraction and isolation
The root bark ofA.altissima(60 kg) was extracted with 70% ethanol under reflux for 3 times,2 h for each,to obtain 1.5 kg crude extract,and then extracted with EtOAc to give 440 g EtOAc extract.The extract was subjected to the silica gel column chromatography and eluted with a CHCl3-CHOH gradient system (100:1→3:1) to give four fractions A (75 g),B (80 g),D (60 g) and E (120 g).Fraction A was separated by HP20 macroporous resin column chromatography using EtOH-H2O (50:50,90:10,95:5,v/v) and further separated by ODS column chromatography with EtOH-H2O (30:70,60:40,90:10,v/v) to obtain 3 subfractions C1 (8 g),C2(20 g) and C3 (18 g).Subfraction C2 was purified by Sephadex LH-20 CC,extracted with 95% EtOH,and eluted with two gradient systems (Petroleum ether-EtOAc (5:1,v/v) to CH2Cl2-MeOH (3:1,v/v))using silica gel column to yield 9 subfractions C2-1 to C2-9.These 9 subfractions were subjected to preparative HPLC with MeOH-H2O (53:47,v/v) to obtain compounds 1 (33.9 mg),2 (67.1 mg),3 (8.9 mg),and 4 (25.2 mg).Subfraction C3 was purified by Sephadex LH-20 CC,extracted with 95% EtOH,and eluted with two gradient systems (Petroleum ether-EtOAc (3:1,v/v) to CH2Cl2-MeOH (3:1,v/v))using silica gel column to obtain subfractions C3-1 to C3-14.These subfractions were subjected to preparative HPLC with MeOH-H2O (45:55,v/v)to obtain compounds 5 (89.8 mg),6 (4.4 mg),7 (184.7 mg),and 8 (4.1 mg).See Fig.1.
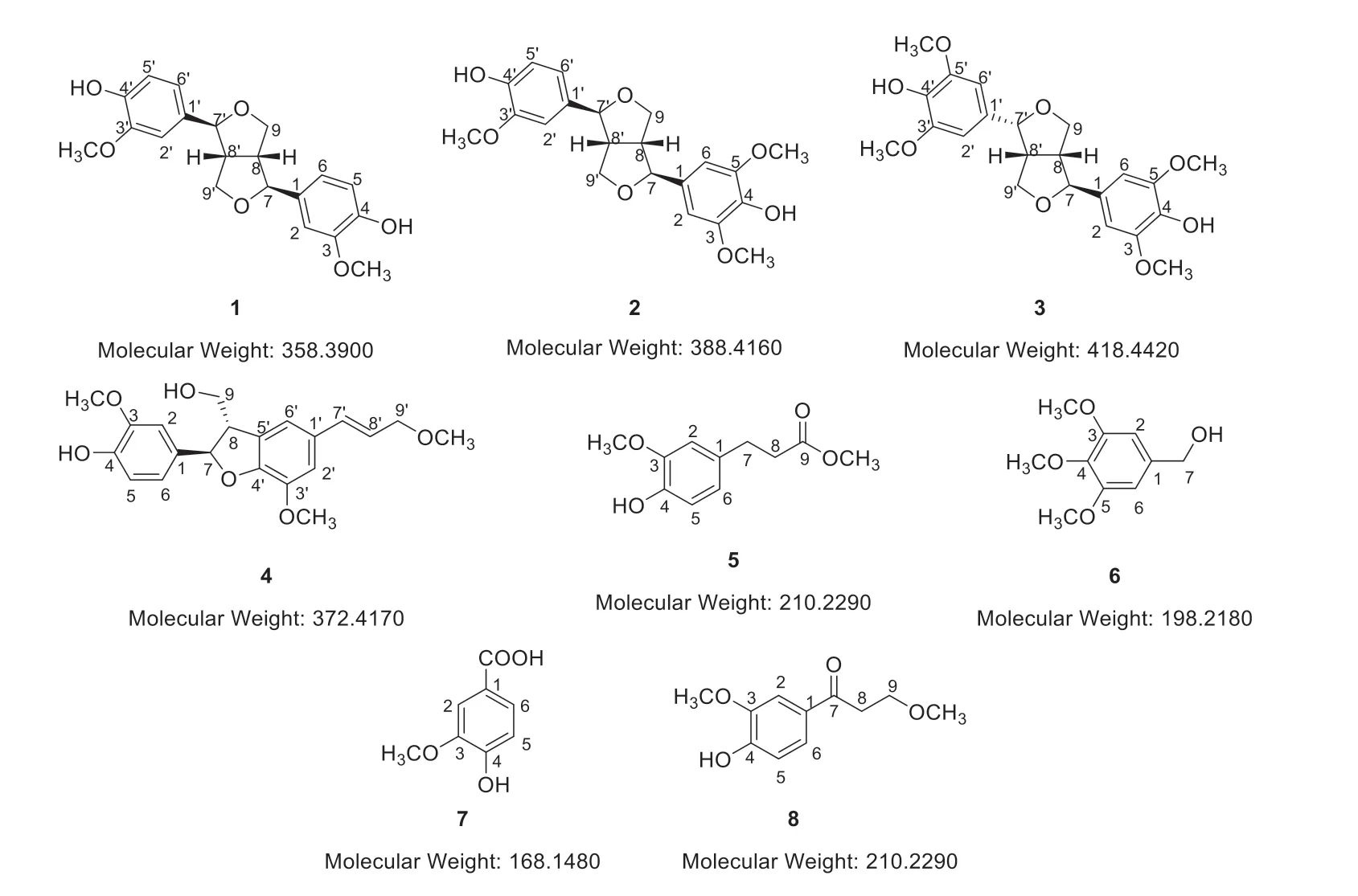
Fig.1 The structures of compounds 1-8
(+)-pinoresinol (1):yellowish oil, +35.2 (c0.1,MeOH),1H-NMR (400 MHz,CDCl3) see Table 1;13C-NMR (100 MHz,CDCl3) see Table 2; HRESIMS(m/z):[M+Na]+381.1374 (calcd.for C20H22O6Na,381.1309); Calculated molecular weight 358.390.
(+)-medioresinol (2):yellowish oil, +13.8(c0.1,MeOH),1H-NMR (400 MHz,CDCl3) see Table 1;13C-NMR (100 MHz,CDCl3) see Table 2;HRESIMS (m/z):[M+Na]+411.1491 (calcd.for C21H24O7Na,411.1420); Calculated molecular weight 388.416.
(-)-lirioresinol (3):yellow oil, -38.7 (c0.1,MeOH),1H-NMR (400 MHz,CDCl3) see Table 1;13C-NMR (100 MHz,CDCl3) see Table 2; HRESIMS(m/z):[M+Na]+441.1558 (calcd.for C22H26O8Na,441.1525); Calculated molecular weight 418.442.
(-)-jatroiinrelignan D (4):yellowish oil, -10.2(c0.1,MeOH),1H-NMR (400 MHz,CDCl3) see Table 1;13C-NMR (100 MHz,CDCl3) see Table 2;HRESIMS (m/z):[M+Na]+395.1580 (calcd.for C21H24O6Na,395.1471); Calculated molecular weight 372.417.
dihydroferulic acid methyl ester (5):yellow oil,1H-NMR (400MHz,CDCl3):δH6.82 (1H,d,J=8.0 Hz,H-5),6.70 (1H,d,J=2.0 Hz,H-2),6.67 (1H,dd,J=8.0,2.0 Hz,H-6),2.87 (2H,t,J=7.8 Hz,H-8),2.60 (2H,t,J=7.8 Hz,H-7),3.84(3H,s,3-OCH3),3.66 (3H,s,9-OCH3);13C-NMR(100 MHz,CDCl3):δC173.5 (C-9),146.5 (C-3),144.1 (C-4),132.4 (C-1),120.8 (C-6),114.5 (C-5),111.0 (C-2),55.9 (3-OCH3),36.1 (C-8),30.7 (C-7);Calculated molecular weight 210.229.
3,4,5-trimethoxybenzyl alcohol (6):brown oil,1H-NMR (400 MHz,CDCl3):δH6.58 (2H,br s,H-2,6),4.38 (2H,br s,H-7),3.90 (6H,s,3,5-OCH3),3.38 (3H,s,4-OCH3); Calculated molecular weight 198.218.
vanillic acid (7):yellow oil,1H-NMR(400 MHz,DMSO-d6):δH12.49 (1H,s,COOH),9.84(1H,s,4-OH),7.44 (2H,m,H-2,6),6.84 (1H,d,J=8.5 Hz,H-5),3.80 (3H,s); Calculated molecular weight 168.148.
1-(4-hydroxy-3-methoxyphenyl)-3-methoxy-1-propanone (8):yellow oil,1H-NMR (400 MHz,CDCl3):δH7.55 (1H,m,H-6),7.54 (1H,br s,H-2),6.95 (1H,d,J=8.5 Hz,H-5),3.81 (2H,t,J=6.5 Hz,H-8),3.20 (2H,t,J=6.5 Hz,H-9),3.95 (3H,s,3-OCH3),3.38 (3H,s,9-OCH3);13C-NMR(100 MHz,CDCl3):197.0 (C-7),150.6 (C-3),146.7(C-4),130.2 (C-1),123.7 (C-6),114.0 (C-5),110.0(C-2),68.3 (C-9),59.1 (C-3),56.2 (9-OCH3),38.3(C-8); Calculated molecular weight 210.229.
2.4 Antioxidant assay
1,1-diphenyl-2-picrylhydrazyl (DPPH) is a free radical stable at room temperature,which acts as an acceptor for electrons or hydrogen radicals [13].Alcohol solution of DPPH is purple in color.After capturing single electron,the DPPH solution turns the color yellow,whose absorbance value is 517 nm.Prior to the start of the assay,a series of 20,50,100,and 200μg/mL ethanol solutions were prepared for compounds 1-8,and a DPPH ethanol solution with an appropriate absorbance of 517 nm was prepared.
The method of DPPH scavenging assay was a modified method [14].A series of different samples in ethanol (0.1 mL) were added to a 96-well plate and DPPH was added in ethanol(0.1 mL).The mixture plate was shaken vigorously and then incubated immediately in the dark for 30 min.The absorbance of the reaction solution was determined as 517 nm using a microplate reader (Bio-Tek,Winooski,VT).The DPPH radical-scavenging activity was calculated as follows:
DPPH scavenging rate=[1-(AS-ASB)/(AC-ACB)]× 100%
S:Add sample (0.1 mL) and DPPH (0.1 mL);SB:Add sample (0.1 mL) and ethanol (0.1 mL);C:Add ethanaol (0.1 mL) and DPPH (0.1 mL);CB:Add ethanol (0.2 mL)
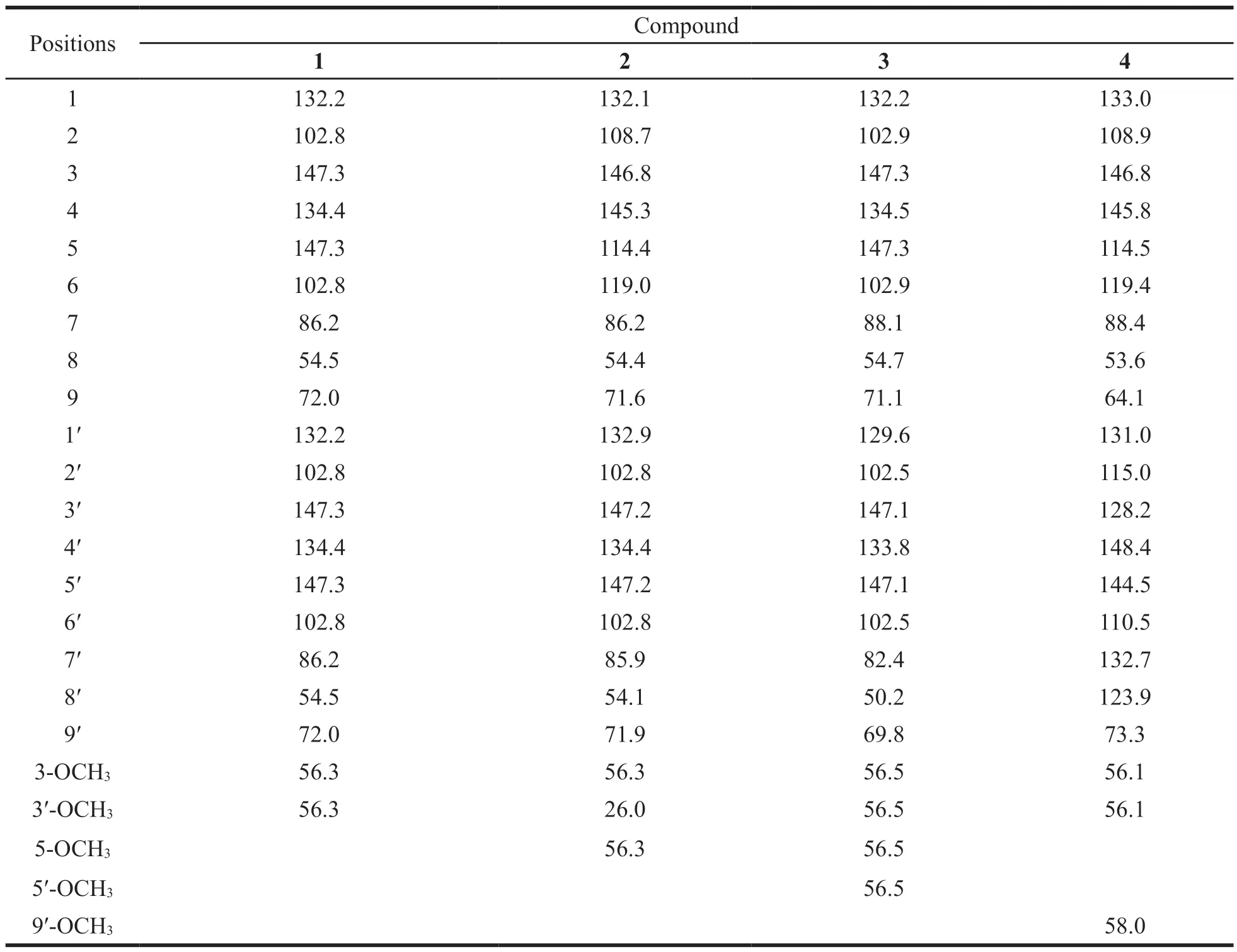
Table 2 13C-NMR data for compounds 1-4 (100 MHz,CDCl3)
3 Results and discussion
3.1 Structure elucidation
The molecular formula of compound 1 was determined as C20H22O6.The1H-NMR spectrum of compound 1 showed the presence of two 1,3,4-trisubstituted benzene ring signals at 6.88 (2H,d,J=8.2 Hz,H-5,5′),6.88 (2H,d,J=1.8 Hz,H-2,2′),6.82 (2H,dd,J=8.2,1.8 Hz,H-6,6′).δH4.74(2H,d,J=4.1 Hz,H-7,7′) indicated that compound 1 was a furofuran lignan.The relative configuration of 1 was assigned as thetrans-relationship based on1H-NMR data,δH4.25 (2H,m,H-9a,9′a),3.89 (2H,m,H-9b,9′b),3.09 (2H,m,H-8,8′),4.74 (2H,d,J=4.1 Hz,H-7,7′),because the coupling constant between H-7 and H-7′ was 4.1 Hz,and 0.30 < ΔδH-9=ΔδH-9′=0.36 < 0.4 [15].This compound was an optically pure compound,having single peaks with a variety of chiral splitting columns.By comparing the spectrum data with the reported literature [16],compound 1 was identified as (+)-pinoresinol.
Compound 2 was found to have a molecular formula of C21H24O7with 10 degrees of unsaturation.The1H-NMR and13C-NMR data of 2 were similar to that of 1,except for the slight discrepancy caused by methoxy (5-OCH3).Combined analysis of1H-NMR data showed compound 1 and 2 have the same skeleton.δH4.74 (1H,d,J=4.3 Hz,H-7′),4.72 (1H,d,J=4.4 Hz,H-7),4.26 (2H,m,H-9a,9′a),3.90(2H,m,H-9b,9′b),3.10 (2H,m,H-8,8′) indicated that the coupling constant between H-7 and H-7′ was 4.3 and 4.4 Hz,and 0.30 < ΔδH-9=ΔδH-9′=0.36 <0.4 [15].Therefore,the relative configuration of 2 was assigned astrans-relationship.By comparing the spectral data of compound 2 and (+)-medioresinol in literature [17],compound 2 was identified as(+)-medioresinol.
Comparison of1H-NMR data of 3 and 2 indicated that these two compounds have similar proton signals,except for the difference caused by 5′-OCH3.It also indicated that two compounds have the same skeleton.Analysis of13C-NMR data and HRESIMS data showed that the number of methoxy groups increased to 4.δH4.14 (1H,m,H-9a),3.86(1H,m,H-9′a),3.86 (1H,m,H-9b),3.33 (1H,m,H-9′b),3.33 (1H,m,H-8),2.90 (1H,m,H-8′),4.85(1H,d,J=5.2 Hz,H-7),and 4.42 (1H,d,J=7.1 Hz,H-7′) illustrated the coupling constant between H-7 and H-7′ was 5.2 and 7.1 Hz,and ΔδH-9=0.28 <0.4,ΔδH-9′=0.53 > 0.5.It implied the configuration of H-7 and H-8 wastrans-relationship,and that of H-7′ and H-8′ wascis-relationship [15].Compared with the spectral data of the known compound(-)-lirioresinol reported in literature [18],compound 3 was identified as (-)-lirioresinol.
Compound 4 has a molecular formula of C21H24O6,as determined by the peak atm/z395.1580[M+Na]+(calcd.for C21H24O6Na,395.1471) in HRESIMS.According to the analysis of the1H-NMR and13C-NMR data,δH5.56 (1H,d,J=7.1 Hz,H-7),3.60 (1H,m,H-8),3.91 (1H,m,H-9a),3.86(1H,m,H-9b) showed that compound 4 possesses the structural fragment of benzo-dihydrofuran.The coupling constant of 7.4 Hz between H-7 and H-8 indicated the configuration of H-7 and H-8 wastrans-relationship.Compared with the spectral data of (-)-jatroiinrelignan D in literature [19],compound 4 was identified as (-)-jatroinrelignan D.
The1H-NMR data of compound 5 illustrated the existence of one 1,2,4-trisubstituented benzene ring at δH6.82 (1H,d,J=8.0 Hz,H-5),6.70 (1H,d,J=2.0 Hz,H-2),6.67 (1H,dd,J=8.0,2.0 Hz,H-6),two methoxy groups at 3.84 (3H,s,3-OCH3),3.66(3H,s,9-OCH3),and a group of coupling methylene at 2.87 (2H,t,J=7.8 Hz,H-8),2.60 (2H,t,J=7.8 Hz,H-7).5 possesses a carboxyl at δC173.5 (C-9).Compared with the spectral data of dihydroferulic acid methyl ester in literature [20],compound 5 was identified as dihydroferulic acid methyl ester.
The molecular formula of compound 6 was determined as C10H14O4.δH6.58 (2H,br s,H-2,6)was the specific signal of 1,3,4,5-tetrasubstituented benzene ring,and δH3.90 (6H,s,3,5-OCH3),3.38 (3H,s,4-OCH3) illustrated the existence of three methoxy groups.Compared with the spectral data of 3,4,5-trimethoxybenzyl alcohol in literature [21],compound 6 was identified as 3,4,5-trimethoxybenzyl alcohol.
Compound 7 was found to have the molecular formula C8H8O4.δH12.49 (1H,s,4-OH) was the signal of carboxyl,and δH7.44 (2H,m,H-2,6),6.84 (1H,d,J=8.5 Hz,H-5) was the signal of 1,3,4-trisubstituented benzene ring.Compared with the spectral data of vanillic acid in literature [22],compound 7 was identified as vanillic acid.
Compound 8 has a molecular formula of C11H14O4,with 5 degrees of unsaturation.1H-NMR of 8 showed the existence of one 1,2,4-trisubstituented benzene ring at δH7.55 (1H,m,H-6),7.54 (1H,br s,H-2),6.95 (1H,d,J=8.5 Hz,H-5),two methoxy groups at 3.95 (3H,s,3-OCH3),3.38 (3H,s,9-OCH3)and a group of coupling methylene at 3.81 (2H,t,J=6.5 Hz,H-8),3.20 (2H,t,J=6.5 Hz,H-9).δC197.0 (C-9) was the carbonyl signal and it indicated that carboxyl was linked to benzene ring.Compared with the spectral data of 1-(4-hydroxy-3-methoxyphenyl)-3-methoxy-1-propanone in literature [23],compound 8 was identified as 1-(4-hydroxy-3-methoxyphenyl)-3-methoxy-1-propanone.
3.2 Antioxidant activity
All compounds were tested for their antioxidant activity by DPPH radical scavenging assays.The experimental results were calculated and analyzed to obtain the EC50for all compounds.The values of EC50calculated by SPSS 16.0 are shown in Table 3.Compared the standard Trolox,all compounds except compound 7 had lower value of EC50.Among them,compounds 3,5 and 6 exhibited more prominent antioxidant effects.Compound 7,an aromatic compound,contains three substituents of phenolic hydroxyl,methoxy,and carboxyl and has low antioxidant activity.The possible reason is that it is hard for carboxyl group to accept protons.
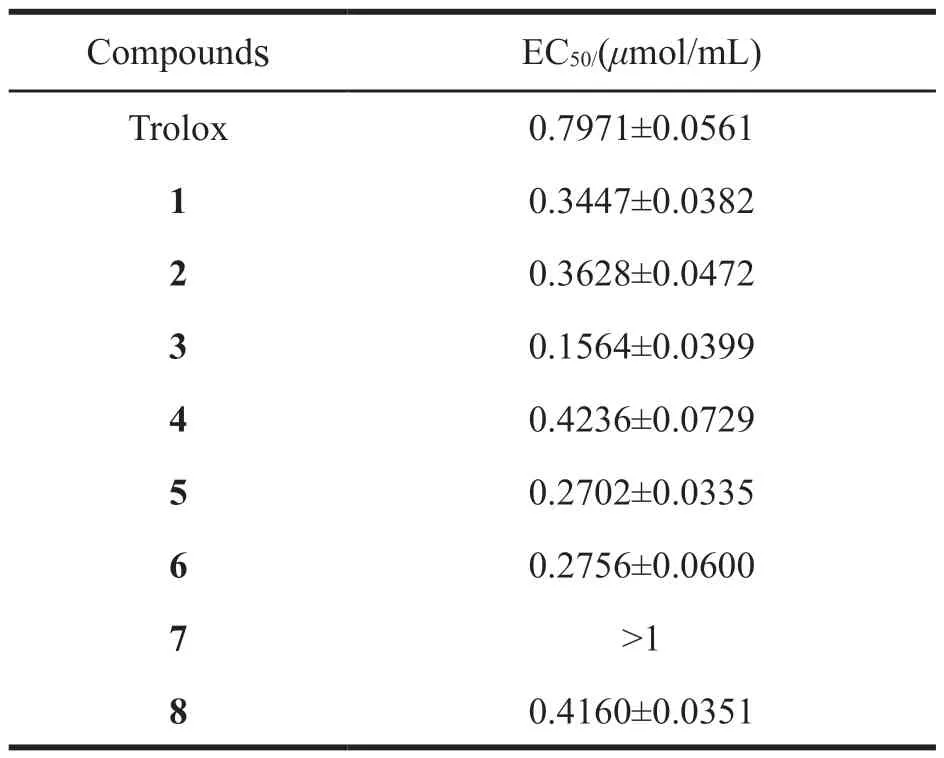
Table 3 EC50 of compounds 1-8 about DPPH scavenging assay
4 Conclusion
Five compounds were isolated fromAlanthusDesf.for the first time,identified as (+)-pinoresinol(1),(-)-jatroinrelignan D (4),dihydroferulic acid methyl ester (5),3,4,5-trimethoxybenzyl alcohol (6)and 1-(4-hydroxy-3-methoxyphenyl)-3-methoxy-1-propanone (8).All of them have potent antioxidant activity,and compounds 3,5 and 6 have significant scavenging effect on DPPH radical.
杂志排行
Asian Journal of Traditional Medicines的其它文章
- Contribution Regulations for Asian Journal of Traditional Medicines
- Research progress on chemical constituents and pharmacological activities of Viburnum tinus L.
- Studies on the chemical components and biological activities of the genus of Juncus
- Research progress on chemical constituents in Periploca forrestii and their pharmacological activities
- Uncovering the potential targets of Viburnum odoratissimum for the treatment of related diseases
- Physicochemical properties and antioxidant activities of polysaccharides extracted from Panax ginseng C.A.Meyer with graded percipitation method
