Monitoring maturation of neural stem cell grafts within a host microenvironment
2019-11-28OlgaKopach
Olga Kopach
Olga Kopach,Department of Clinical and Experimental Epilepsy,Queen Square Institute of Neurology,University College London,London WC1 N3BG,United Kingdom
Abstract
Key words: Neural stem cells; Embryonic progenitors; Neurogenesis; Maturation of neurophysiological properties; Integration into network; Neural stem cell therapy;Neurodegeneration; Ischemic injury
INTRODUCTION
Stem cell therapy has emerged to become a universal “rescue” tool for a broad range of neurological disorders which are as yet incurable with canonical treatment approaches.Since the discovery of stem cells,this avenue of research has become mainstream,not least due to the enigmatic nature of stem cells,but mostly because of the immense therapeutic potential of stem cells,unveiled either by experimental studies or clinical trials.Undoubtedly,the prominent therapeutic effects produced by neural stem cell (NSC) use in different types of neuropathology give credit to NSC as a multipurpose tool for curing neurodegeneration in a variety of diseases of the central nervous system[1-4].Among all the advanced features of this cell type,the multi-lineage potential of NSC is probably the most attractive in terms of a cell-based therapy for the treatment of neurodegenerative disorders which are accompanied by extensive neuronal cell death that require a replacement of the pool of nonrecoverable cells.These include such disorders as stroke,epilepsy,different forms of dementia,including Alzheimer’s and Parkinson’s diseases,among others[5,6].The high capacity of NSC - either of fetal or adult brain origin - to differentiate between the lineages of neuronal or glial cell types,provides the damaged brain with a newly developed pool of cells consisting of a mixture of the entirely different phenotypes:e.g.,cholinergic,serotonergic,GABAergic,other neuronal subtypes,mixed with oligodendrocytes,astrocytes,reparative microglial subtypes,etc.This innate peculiarity of NSC to differentiate into diverse phenotypes,confirmed by several lines of evidence as a result of monitoring cell grafts in the post-stroke brain[7-11],can underlie the versatile beneficial effects when employed as cell-based therapy[12].Consequently,such a therapy could lead to the “self-repair” of the damaged tissue by amplifying the remodelling of the injured brain through the rebuilding of damaged neuronal ensembles,neurite remodelling and the rewiring of the whole circuitry using one therapeutic approach.Together,this offers a potential advantage of NSC use in terms of boosting neurorestorative effects and amplifying the recovery of neurological function.In addition,employing NSC as a therapeutic approach has proved to be safe,owing to the restricted proliferation of neural precursors – unlike stem cells - that implies a lower risk of malignant transformation that could subsequently develop in the brain[9,11,13].The latter is an essential requirement that cellbased therapies should meet.
NEURAL STEM CELL FOR MULTI-LINEAGE DIFFERENTIATION WITHIN A HOST TISSUE
Among two stem cell therapy approaches applicable at present – the transplantation of already differentiated cell phenotypes from induced pluripotent stem cells (iPSC)and the engraftment of neural progenitors into the injured brain - the use of NSC has emerged as one that opens a door for “self-repair” of the damaged tissue.The rationale for this lies with the multi-lineage differentiation of NSC regulated by a host(endogenous) microenvironment.This implies that after engraftment into the injured tissue,NSC differentiation occurs in a way that is pertinent to impairments taking place within the damaged area.In the light of the microenvironment characteristics featuring the post-ischemic brain tissue,a high level of excitotoxicity which originates from overwhelming glutamate,necrotic,pro-apoptotic factors being released following massive ischemic cell death,is a critical determinant that dramatically lowers the cell viability of already differentiated iPSC-derived neurons after transplantation.Similarly,the overactivation of pro-inflammatory signalling pathways pertinent to the post-ischemic impairments would suppress the survival of vulnerable neurons transplanted into the post-stroke brain.In support of this,there has been a routine low survival rate of iPSC-derived neurons after transplantation into the post-ischemic brain - the process greatly exacerbated by the proximity of cell grafts to the stroke lesion[14-17].Furthermore,the lowered viability of engineered iPSC-derived cell grafts following transplantation has been a general problem for gene therapy applications in clinical trials for Alzheimer’s and Parkinson’s diseases[18-20].Establishing how the transplanted neurons can be protected over time within a pathological milieu is a key prerequisite for achieving the optimal outcome of cellbased therapy.This is where the advantage of the high intrinsic plasticity of NSC over the differentiated neuronal phenotypes in terms of gaining cell viability while within a detrimental host microenvironment,has appeared.
Emerging data from functional studies of the NSC-derived neurons indicate that neuronal differentiation and maturation occur at a much faster rate within a host brain tissue than inin vitrocell cultures.For instance,the maturation of electrophysiological properties of the NSC-derived neurons in organotypic hippocampal tissue has been completed for up to 3 weeks after engraftment[21,22].By contrast,the maturation of biophysical properties of stem cell-derived neurons in dissociated cell cultures normally requires months to achieve a similar result.Electrophysiological studies collectively suggest that it is often necessary to use enriched media (a “cocktail”) composed of a mixture of transcriptional factors and master regulators to force the maturation of neurophysiological properties of iPSC-derived neurons[22,23].In this context,the accelerated neuronal maturation of NSC grafts within an endogenous microenvironment is highly advantageous,since only mature neurons will contribute to neurological function and lead to tissue remodelling for functional recovery.Although the precise mechanisms that underlie the accelerated neuronal maturation of NSC within a host brain tissue remain largely unknown,the potential of NSC for achieving fast therapeutic outcomes argues the case for pursuing further research to explore this in detail.
Among the benefits of NSC use as a promising therapeutic approach,one may assume engaging other mechanism(s),which remain as yet enigmatic.It can include,in particular,triggering the pool of resident NSC to cause it to become activated.The resident NSC - the population of adult stem cells available across the mature brain at the subventricular and subgranular dentate gyrus zones of the hippocampus,cerebellum,forebrain,olfactory bulbs - revealed the innate therapeutic potential with regard to the regeneration of the impaired brain tissue[24-26].Growing interest within this newly exploring research area piles up further arguments for the high intrinsic plasticity of NSC and the control of the NSC fate by a host endogenous environment.
MONITORING NEURONAL MATURATION - CHALLENGES AND IMPORTANCE
Despite the lengthy period of time since the therapeutic effects of stem cell applications in brain injuries were first documented,to date neurophysiological mechanisms mediating these effects are still beyond our comprehensive understanding.For decades,in innumerable attempts to assess how far competent NSC-derived cells become over time within the adult brain,most data across the field have generally illustrated many antigens/markers that differentiated cells can express.While a combination of immunocytochemical (histochemical) profiles firmly documents the cell lineage[10,27,28],along with an ample expression of various receptors,proteins,etc.,across the pool of differentiated cells,it provides,however,no rigorous evidence for the functional properties of these cells.Cells displaying a clear immunoreactivity might yet possess neither functional receptors nor signalling pathways constituted to ensure appropriate neurophysiological activity.Given that the appropriate level of neurophysiological activity - of individual cells and integrated neuronal network activity - determines the function,the anticipated beneficial effects of cell-based therapy would ultimately rely on the timing maturation of the neurophysiological properties of differentiated neurons,followed by their functional integration into the host circuits.Eventually,this dictates the overall outcome of the therapy being applied.
Notwithstanding their importance,the functional studies investigating how far the stem cell-derived neurons are physiologically credible following neurogenesis,have been scarce.There have only been a few studies,with some exceptional examples as follows[7,11,22,29,30],which have performed meticulous investigations of the biophysical properties and the neurophysiological activity of stem cell-derived neurons.The depth to which the majority of works tested the maturation of stem cell-derived neurons consisted of basic patterns of firing and synaptic activity recorded from differentiated neurons,typically at the very late time-points after transplantation (a few months in the post-stroke brain).Certainly,major challenges lay in selecting the difficult electrophysiological technique.Among technical difficulties,the methodology of conventional whole-cell recordings carrying onin vivodemands the termination of an acute experiment; therefore,the assessment of neurological function through behavioural testing commonly precedes studies at the neuronal level.Consequently,a huge leap exists for the time window between cell engraftment to when the neurophysiological properties of stem cell-differentiated neurons have been tested.Therefore,a number of important questions remain to be answered.First of all,the time window that stem cell-derived neurons require to set up their neurophysiological properties to match the level of functional activity displayed by endogenous neurons.Second,what is the time scale for differentiated neurons to become functionally integrated into the host circuits? Third,is there a difference in the timing of neuronal maturation between different milieus (i.e.,varied pathological microenvironments)? This knowledge is essential when it comes to making decisions with regard to a scheme for stem cell transplantation (timely initiation of the treatment) and the assessment of anticipated benefits,along with the potential risks associated with the therapy application,depending on the severity of the tissue damage[31].
Evidence-based advances of theex vivobrain tissue preparations have attracted attention to this experimental approach as an alternative toin vivostudies.Brain slices fulfil expectations for functional studies at the sub-cellular,cellular and neuronal network levels due to the preserved tissue layer architecture consisting of innate cell assemblies.Over time,organotypic brain slices have been effectively used to discover important insights into the cellular and molecular mechanisms of neurodegeneration -first of all,because of feasibility for the long-term maintenance of viable tissue,with much fewer costs as compared to animal model use,and because varied combinations of advanced techniques and analytical tools become applicable to brain tissue at either immature or mature developmental stages[32-34].One of the other problems in studies of neurodegenerative disorders is that the generated animal models do not replicate the neuropathological changes obtained from post-mortem studies of the brain neurodegeneration,for instance,in stroke (cerebral ischemia),Alzheimer’s and Parkinson’s diseases,other forms of dementia[35-38].Whilst the use of animal models remains in a constant debate in terms of whether or not they are relevant to human neurodegenerative disorders associated with the clear clinicopathological profile of memory loss and cognitive decline (debatable in animal species),mechanistic studies require model systems for exploring the mechanisms of neurodegeneration and treatment strategies.In this context,once again organotypic brain slices perfectly fit these aims.
Taking all the above into account,monitoring the time-dependent maturation of NSC grafts within a host hippocampal tissue has recently been employed.Functional studies have been carried out in organotypic hippocampal slices,aimed at answering the questions as highlighted earlier.The experimental data from electrophysiological recordings,combined with electron microscopy and immunohistological approaches,have revealed that NSC-derived hippocampal neurons have matured electrophysiological properties,and have functionally integrated into the host circuits within 3 weeks of engraftment[21].Moreover,the neurophysiological maturation of NSC-derived neurons achieved a similar level of activity as that exhibited by endogenous CA1 pyramidal neurons (varied electrophysiological parameters were quantitatively compared between the groups).Next,a morphological comparison has been performed with regard to the synapses which NSC-derived neurons constituted with endogenous cells.The visualised structures,either presynaptic terminals containing numerous vesicles or postsynaptic structures,revealed the typical morphology,confirmed by synaptic function (i.e.,recordings of the postsynaptic currents) detected as early as the first two weeks after engraftment[21].Extrapolating from the experimental data from this and other studies[22],the maturation of neuronal excitability and synaptogenesis within a host tissue can be envisaged to last up to a few weeks - a time scale much faster than established in dissociated cell cultures across a vast literature (NSC-derivedvsiPSC-derived neurons[23]).Consequently,the therapeutic outcome from NSC-based therapy could,therefore,be anticipated to emerge shortly after initiating the treatment - within only a few weeks.In the light of such a time range,accelerated NSC maturation can provide a mechanistic basis of the speedy therapeutic effects in a recovery of neurological function observed one week after stem cell transplantation into the stroke-damaged brain[11,39].Collectively,the outlinedin vivoandin vitrodata suggest that NSC-based therapy is advantageous in promoting the remodeling of brain tissue to amplify a recovery of neurological function,given that no effective therapy currently exists.
GLIAL LINEAGE AT WORK
A substantial bias in the neurogenesis of NSC grafts to glial lineage has been found while monitoring NSC neurogenesis within the ischemic-injured brain tissue[21].In the post-ischemic environment (organotypic hippocampal slices subjected to ischemic conditions - oxygen-glucose deprivation[40]),NSC grafts have been largely differentiating into glia,with a prompt rise in NSC-derived oligodendrocytes,followed by astrocytes.Notably,NSC-derived oligodendrocytes have already been identified at week 1,and astrocytes - by two weeks.In the meantime,NSC-derived neurons matured in terms of their electrophysiological properties with a dramatically slower rate within the post-ischemic milieu than in a physiological environment[21].Based on the experimental data from a direct comparison between electrophysiological parameters,the promoted glial lineage has been a hallmark of NSC neurogenesis within the post-ischemic tissue (approximately 70% of grafted NSC differentiated into glia),opposing the reduced neuronal lineage (a drop from approximately 70% to approximately 30% in the proportion of NSC-derived neurons;Figure 1).Similar effects with regard to both the differentiation and the maturation of fetal NSC grafts in the post-stroke brain were observedin vivo[7].The rationale for such a strong influence of the post-ischemic environment on NSC neurogenesis rests in how far the post-ischemic milieu is overburdened with extracellular glutamate[41],potassium,mediators of inflammation[42],pro-apoptotic factors,enzymes,and other compounds[43,44]that produce long-lasting excitotoxic actions,resulting in delayed neuronal cell death[40,45].
Oligodendrocytes and astrocytes are thought to have diverse roles in brain physiology and neuropathology,and both can actively communicate with neurons and other cell types[46-49].Therefore,the peculiarity of NSC neurogenesis within postischemic tissue may mirror the numerous roles that these glial cell types would play there.Promoted glial lineage implies the glia-mediated neuroprotective and neurotrophic supports of the oxygen-glucose-deprived endogenous neurons as the first steps of defence against the ischemic impairments.Owing to the neuroprotective role of oligodendrocytes,protecting,in particular,the survival of CA1 hippocampal neurons,the NSC-derived oligodendrocytes may constitute endogenously-driven neuroprotection by providing a metabolic supply (paracrine signaling action),for instance,viathe production of lactate,oligodendrocyte-derived trophic factors,GDNF[46,47].In addition to this mechanism,the revealed impact of oligodendrocytes on astroglial development[39,48]may explain that NSC-derived oligodendrocytes precede the derivation of astrocytic phenotype[21].As the most abundant cell type in the mammalian brain[49],astrocytes are highly secretory cells,able to produce large amounts of proteins in order to provide trophic support.The astrocytic-mediated surveillance of neurotoxic inflammation[50],together with a high capability of taking up glutamate and potassium[51]are essential to lower excitotoxicity within the postischemic tissue.The stem cell-derived astrocytes have been shown to replicate the functional properties of astroglia,including the uptake of glutamate and promoting synaptogenesis[48,52].All the aforementioned lines of evidence support the possibility that NSC-derived oligodendrocytes and astrocytes provide the post-ischemic tissue clearance off debris,lower down the high level of excitotoxicity,and eventually improve the survival of oxygen-glucose-deprived endogenous neurons in postischemic conditions.These together favor the maturation of NSC-derived neurons within the endogenous post-ischemic environment as the subsequent step of NSC-based therapy to advance the remodelling of the ischemic-injured tissue and to facilitate its functional recovery.
SUMMARY AND SOME REMARKS
The great ability of NSC grafts to differentiate into neurons,astrocytes or oligodendrocytes within damaged brain tissue marks these cells as a versatile tool for neural replacement strategies in neurodegenerative disorders of various origins.The potential of NSC-based therapy with regard to brain neurodegeneration treatment is,therefore,mediated by multiple mechanisms to effectively amplify the therapeutic outcome[31,53,54].While ethics restrict the use of embryonic NSC,the reprogramming of somatic cells can offer an alternative source for generating the progeny-restricted neural progenitors applicable for cell-based therapies.Given that iPSC feature a patient-specific phenotype,this will ultimately meet any safety concerns effectively.The phenotypic specificity appears particularly useful in generatingin vitrohuman models of neurological disorders linked to genetic mutations,and iPSC have become widely exploited in this avenue of research.The iPSC capability of recapitulating both genetic and phenotypic profiles over the developmental stagesin vitroas in the adult human brain has enabled functional studies in human cells directly for exploring the pathogenesis of genetically-triggered neurodegenerative disorders.Many protocols for the manufacture of nerve cell phenotypes are being actively developed and made available,and the most recent advances in the technology of genome editing,including the CRISPR/Cas9-based correction of gene mutations,constantly refine stem cell clones to facilitate functional studies of brain neurodegeneration.This research direction has marked a new milestone in up-to-date strategies and therapeutic approaches tailored to amplify the remodeling of the injured brain tissue and boost the recovery of neurological function.
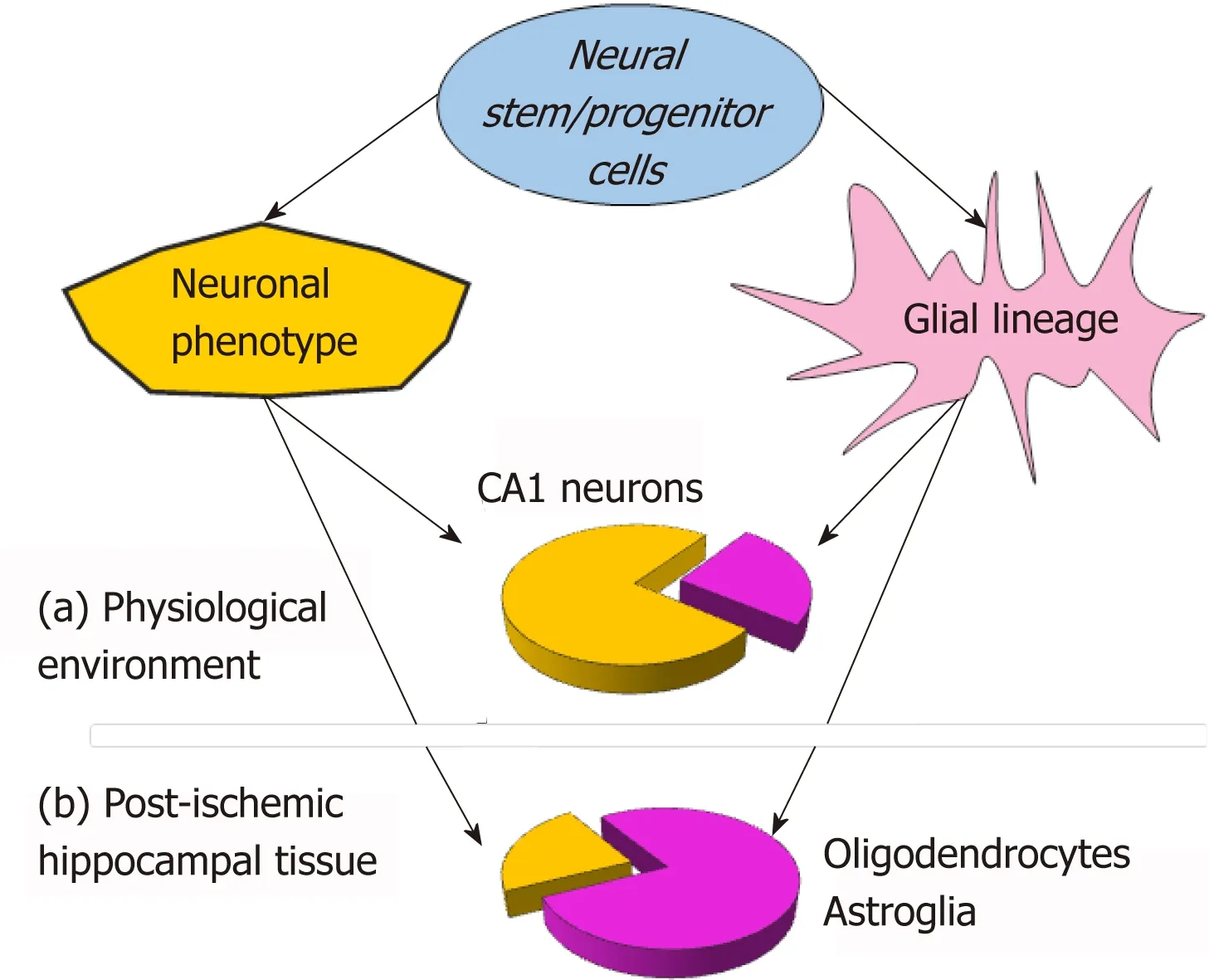
Figure 1 Schematic illustration for neurogenesis of neural stem cell (NSC) grafts within a host hippocampal tissue,showing the difference in relative proportions for neuronal (yellow) vs glial (purple) NSC lineage between physiological environment (a) and the post-ischemic milieu (tissue subjected to oxygen-glucose deprivation) (b) at an earlier time-window – first 2 weeks after engraftment into organotypic hippocampal tissue.
杂志排行
World Journal of Stem Cells的其它文章
- Unexpected encounter of the parasitic kind
- Colon cancer stemness as a reversible epigenetic state: Implications for anticancer therapies
- CRISPR/Cas system: An emerging technology in stem cell research
- Cytokine interplay among the diseased retina,inflammatory cells and mesenchymal stem cells - a clue to stem cell-based therapy
- Developments in cell culture systems for human pluripotent stem cells
- Ameliorating liver fibrosis in an animal model using the secretome released from miR-122-transfected adipose-derived stem cells
