Cytokine interplay among the diseased retina,inflammatory cells and mesenchymal stem cells - a clue to stem cell-based therapy
2019-11-28VladimirHolanBarboraHermankovaMagdalenaKrulovaAlenaZajicova
Vladimir Holan,Barbora Hermankova,Magdalena Krulova,Alena Zajicova
Vladimir Holan,Barbora Hermankova,Magdalena Krulova,Alena Zajicova,Department of Transplantation Immunology,Institute of Experimental Medicine of the Czech Academy of Sciences,Prague 14220,Czech Republic
Vladimir Holan,Barbora Hermankova,Magdalena Krulova,Department of Cell Biology,Faculty of Science,Charles University,Prague 12843,Czech Republic
Abstract
Key words: Retina; Degenerative diseases; Stem cell therapy; Mesenchymal stem cells;Cytokines; Growth factors
RETINAL DEGENERATIVE DISORDERS
The retina is a highly specialized tissue.This structure is composed of several layers of functionally different cell types that are inter-connected.Disease or a damage to any particular cell or layer has secondary effects on the surrounding cell types,and the progression of retinal damage results in retinal degenerative disorders.Inherited and age-related retinal degenerative disorders represent the most common cause of reduced vision and blindness.Among the most common retinal degenerative diseases are age-related macular degeneration,diabetic retinopathy,retinitis pigmentosa and glaucoma.These disorders have different etiology,various causes and starting mechanisms,and distinct retinal cell types are affected.However,they are all associated with chronic inflammation,immune cell infiltration and enhanced cytokine secretion.
Because all retinal degenerative diseases in the advanced stages are associated with a loss or a function damage of specialized retinal cells,their replacement or a support of the surviving cells would be the only effective approach to stop spreading of the disease or even return the visual function of the retina.Currently,a direct transplantation of healthy retinal explants is strongly limited.Therefore,the transfer of stem cells that can support survival and functioning of the remaining retinal cells,or even replace missing cells,offers a perspective approach to stop and treat retinal degenerative diseases.Recent experimental and preclinical data suggest a great potential of such cell therapies for the treatment of so far incurable ophthalmological diseases.
TYPES OF STEM CELLS FOR RETINA REGENERATION
Stem cells are characterized by their permanent growthin vitroand by their ability to differentiate or even transdifferentiate into other cell types.According to their origin,stem cells can be divided into embryonic stem cells and adult stem cells.The third type of stem cells is artificially prepared from any somatic cell by reprogramming its properties to a pluripotent state.For this intervention,genes associated with stemness are introduced into somatic cells,which gain some characteristics of stem cells.These cells,called induced pluripotent stem cells,have attracted a lot of attention as a possible source of autologous stem cells that could avoid immune rejection.However,these genetically modified cells have proved to be immunogenic even in an autologous host[1],and their often uncontrolled growth and the formation of teratomas limit their clinical potential.Similarly,the use of embryonic stem cells,which have a high differentiation potential and can be relatively easily differentiated into numerous different cell types,have ethical limitations associated with their origin.They are always used as allogeneic cells,and they often suffer from uncontrolled growth.In comparison with embryonic stem cells or induced pluripotent stem cells,adult stem cells have a lesser differentiation potential but can be obtained as autologous(patient’s own) cells,do not form teratomas or cancers and often fulfil the demands for use in regenerative medicine.Among the numerous types of adult stem cells,the highest potential have been proposed from mesenchymal stem/stromal cells (MSCs),which can be obtained relatively easily from the patient,propagatedin vitro,if needed differentiatedex vivoand finally used as autologous therapeutic cells.
Numerous experimental studies have shown the beneficial effects of MSCs in the treatment of ophthalmological diseases.Intravitreal or subretinal transplantation of MSCs significantly delayed retinal degeneration and supported normal retinal functions[2-4].In addition,in other types of eye diseases,such as experimental autoimmune uveitis[5,6],dry eye syndrome[7]or corneal epithelium damage[8,9],the therapeutic effects of MSCs on tissue regeneration have been demonstrated.The ability of MSCs to support interaction between retinal cells with neurons of optic nerve has been also documented.For example,Meadet al[10]showed that dental pulp stem cells promoted neuroprotection and axon regeneration after optic nerve injury.In other models,MSCs transplanted to the damaged area of the retina differentiated into retinal nerve cells[11]and promoted regeneration in a rat optic tract model[12].
MSCs
MSCs represent a heterogenous population of non-hematopoietic cells with multilineage differentiation potential.Originally,these cells were described as spindle shaped cells derived from bone marrow that adhere to plastic and form fibrocyte-like colonies[13].For therapeutic purposes,MSCs are isolated mainly from the bone marrow or adipose tissue,but they can be obtained from nearly all tissues of the body.According to the International Society for Cellular Therapy,human MSCs are characterized by their ability to adhere to plastic in standard culture conditions,by their potential to differentiate into adipocytes,chondroblasts and osteoblasts,and by being positive for the surface markers CD105,CD73 and CD90 and negative for CD45,CD34,CD14,CD19 and CD11b[14].MSCs from different sources (including bone marrow,adipose tissue,umbilical cord blood,etc) possess similar properties[15,16].Under appropriate conditions,MSCs can be differentiated or even transdifferentiated into different cell types.It has been demonstrated that in the presence of selective chemicals or retinal cells,MSCs can differentiate into cells expressing retinal cell markers and characteristics[17-19].We have shown that highly purified mouse bone marrow-derived cells fulfilling all the criteria proposed for MSCs and cultured in the presence of retinal cell extract and supernatant from activated T cells (to mimic the inflammatory environment of diseased retina) differentiated into cells expressing rhodopsin,S-antigen,recoverin,retinaldehyde binding protein,calbindin and retinal pigment epithelium (RPE) 65,which are the markers of specialized retinal cells[20].The ability of MSCs to extensively proliferate,to be expandedin vitroand to differentiate into various cell types makes them attractive targets for regenerative and reparative medical applications.However,before clinical application these cells have to be precisely characterized and their preparation standardized,as it has been recently proposed[21].
The therapeutic effects of MSCs are mediated by multiple mechanisms,as demonstrated in Figure 1.Among them,the immunomodulatory and secretory properties appear to be the most important.It has been shown that MSCs inhibit T and B cell functions,attenuate production of cytokines,decrease activity of cytotoxic T and NK cells and suppress transplantation,anti-cancer and inflammatory reactions[22,23].On the other hand,MSCs are potent producers of numerous cytokines and growth factors[24,25].It has been shown that mouse MSCs spontaneously produce transforming growth factor (TGF-β) and inducible interleukin-6 (IL-6),which are the basic cytokines regulating the development of anti-inflammatory regulatory T cells(Tregs) and pro-inflammatory Th17 cells[25].The immunomodulatory properties make MSCs a promising tool for the treatment of harmful inflammatory reactions that accompany inherited retinal degenerative diseases and injuries.However,the spectrum and concentrations of immunoregulatory molecules produced by MSCs strongly depend on the cytokine environment[26,27].This has to be taken into account when MSCs are delivered into the inflammatory environment of the diseased retina.
PRODUCTION OF CYTOKINES BY RETINAL CELLS
The retina is composed of a few layers of functionally different cell types that ensure visual acuity and internal homeostasis.The cells of the retina produce numerous cytokines and growth factors that support,in a paracrine mode,the survival of other retinal cells and contribute to the immune privilege of the eye[28].However,from the very beginning of retinal disease or damage,the spectrum of produced cytokines is significantly changed,and the retina starts to produce elevated levels of molecules that are not produced or only minimally secreted in a steady-state.
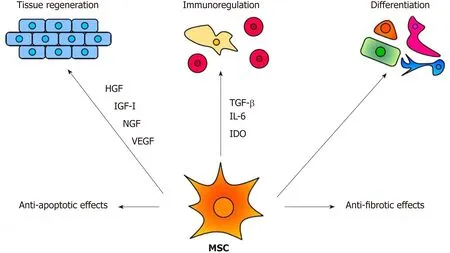
Figure 1 The main mechanisms of the therapeutic effect of MSCs.
It has been shown that increased levels of pro-inflammatory molecules,such as tumor necrosis factor (TNF-α),IL-6 or inducible nitric oxide synthase (iNOS),are found in the retina or aqueous humor of patients with degenerative retinal diseases or in animal models of retinal disorders[29-32].All of these molecules have a wider spectrum of immunoregulatory activities but generally contribute to the development of a local inflammatory reaction.On the contrary,IL-10,IL-11 or TGF-β,which are also produced by retinal cells[32,33],have anti-inflammatory effects.Therefore,the balance between the production of pro- and anti-inflammatory molecules influences the extent of the inflammation and damage in the diseased retina.
The complexity of the action of individual cytokines is supported by the observation that another pro-inflammatory cytokine,IL-6,that is produced by several retinal cell types,increases the survival of retinal ganglion cells[34].Eastlakeet al[35]have shown that Müller glia cells produce numerous factors,such as granulocytegrowth factor,monocyte chemoattractant protein-1,platelet-derived growth factor-BB,vascular endothelial growth factor (VEGF) or TGF-β2 and that their production is increased in the gliotic retina.Recently,IL-33 produced by Müller cells of the retina was identified as a key regulator of inflammation and photoreceptor degeneration after retinal stress injury[36].Thus,retinal microglia and RPE cells were proposed as the main sources of the majority of factors with immunomodulatory effects that play a pivotal role in the initiation and propagation of the neurodegenerative processes[37-39].In addition to their high secretory potential,Müller glia were recently shown to be able to directly restore vision afterde novoinduction of genesis of rod photoreceptors in mammalian retinas[40].It has been shown that cells of individual parts of the eye,such as the cornea,ciliary body or retina are able to inhibit the intraocular immune response and to contribute to the immune privilege of the eye[41].In this respect,we observed that the explants of the mouse retina or the supernatants from the cultures of retinal explants inhibited the production of pro-inflammatory cytokines by activated spleen cells (unpublished results).
In addition to the production of immunoregulatory cytokines,such as TGF-β or IL-6,cells of the retina produce a number of molecules that function in the paracrine mode as growth and trophic factors.Brain-derived neurotrophic factor,ciliary neurotrophic factor,glial cell line-derived neurotrophic factor,nerve growth factor,neurotrophin-3 and basic fibroblast growth factor released by microglia and other retinal cell types have been shown to protect retinal cells and support the survival of photoreceptors[42].On the contrary,pro-angiogenic factors,such as VEGF A-E,insulinlike growth factor-I (IGF-I),platelet-derived growth factor,placental growth factor,hepatocyte growth factor (HGF) and FGF-2,which can be produced by the retina and act paracrinely,are involved in neovascularization and rather worsen retinal diseases[43,44].In the opposite way,the RPE cells produce retinal pigment epitheliumderived factor and trombospondin-1 which have been shown to counterbalance angiogenesis and inflammation[45].
Thus,the retina is a producer of numerous cytokines and factors,which act in a protective way,i.e.they inhibit the inflammatory reaction and support the survival and the growth of retinal cells.However,there are a number of cytokines and factors that attract pro-inflammatory cells,enhance inflammation,increase neovascularization and contribute to the damage of individual retinal layers.The identification of these factors and understanding of the mechanisms of the interplay among them will increase the efficacy of stem cell therapy for retinal degenerative disorders.
CYTOKINES PRODUCED BY INFLAMMATORY CELLS IN THE DISEASED RETINA
All retinal degenerative diseases are accompanied by a local cytokine disbalance,by increased production of chemokines and by infiltration with inflammatory cells.One of the first inflammatory cell populations detected in the diseased retina is macrophages and neutrophils.The accumulation of macrophages can be associated with elevated levels of vitreal granulocyte-macrophage colony-stimulating factor[46].Macrophages are producers of numerous cytokines and factors,and their secretory profile depends on their polarization into M1 or M2 population.With the progression of the disease,increased concentrations of pro-inflammatory cytokines and chemokines,such as IL-1,IL-6,IL-8,TNF-α,interferon (IFN)-γand monocyte chemoattractant protein-1 can be detected in vitreal liquid[47].The deleterious role of these cytokines for the development and spreading of the disease was directly proved by the observation that intravitreal administration of IL-1β and TNF-α in mice induced vessel dilatation,bleading,retinal edema and microglia upregulation[48].Kuttyet al[49]showed that RPE cells exposed to IL-1,TNF-α and IFN-γ,which are secreted by lymphocytes or macrophages in the retina,decreased the expression of key genes involved in the visual cycle,epithelial morphology and phagocytosis.We observed that intravitreal administration of pro-inflammatory cytokines (such as IL-1α,TNF-α and IFN-γ) induced in the mouse retina an enhanced expression of genes for a large number of cytokines and pro-inflammatory molecules (Figure 2).With the progression of the retinal disease,the infiltration with cells of adaptive immunity can be detected[49,50].Johnsen-Sorianoet al[52]showed cytokine disbalance and significantly increased levels of Th1 cytokines IL-2 and IFN-γ and NO in the retina of diabetic rats.These pro-inflammatory molecules could contribute to the development of diabetic retinopathy.This concept is supported by the observation that transgenic mice expressing IFN-γ in the retina develop ocular inflammation and photoreceptor loss[53].
Thus,cytokines produced by cells infiltrating the diseased retina contribute to the development and spreading of retinal disease.They have additive and synergistic effects with cytokines produced by the diseased retina,and these interactions can further deteriorate or attenuate the disease progression.
CYTOKINES PRODUCED BY MSCS
MSC-based therapy has been proposed and tested as a prospective treatment in various models of retinal degenerative diseases[2,3,54-56].MSCs mediate their therapeutic effect by multiple mechanisms involving immunomodulation,production of trophic factors and a possible differentiation into other cell types.The paracrine trophic effects of molecules secreted by MSCs have recently been suggested as the primary mechanisms of MSC action.Some of these immunomodulatory and trophic factors are produced by MSCs spontaneously (such as HGF or CCL2),while others are secreted only after stimulation (for example IL-6 or LIF) or are secreted in lower quantities spontaneously and in increased levels after stimulation (VEGF or IGF-I) (Figure 3).
MSCs are well known as cells with a potent immunosuppressive potential.It has been demonstrated that MSCs inhibit the secretion of cytokines and have the ability to suppress transplantation,anti-cancer or inflammatory reactions[23,57].Mathewet al[58]showed that intravitreal administration of MSCs decreased the intraocular level of inflammatory molecules TNF-α,IL-1β and IL-6 and rescued the retina from ischemic damage.We have shown that MSCs transferred into the damaged ocular surface significantly decreased infiltration with T lymphocytes and attenuated a local inflammatory reaction[8,59].Furthermore,we observed that the cultivation of retinal explants with pro-inflammatory cytokines IL-1β,TNF-α and IFN-γ induced enhanced expression of genes for numerous cytokines and that this enhanced gene expression was attenuated in the presence of MSCs (Figure 4).The suppressive activity of MSCs could be profitable for the therapeutic inhibition of the inflammatory reaction in the diseased retina.Although different mechanisms can be responsible for the suppression,one of the most important effects could be the spontaneous production of TGF-β by MSCs.TGF-β is a strong negative regulator of immune reactions,and it is the main cytokine determining the development of Tregs which can indirectly contribute to immunosuppression mediated by MSCs[60].However,in the presence of pro-inflammatory cytokines,MSCs also produce IL-6,which is a pro-inflammatory cytokine and attenuates the development of Tregs.This plasticity of MSCs should be kept in the mind when MSCs are delivered into the inflamed site with the aim to attenuate the inflammation.
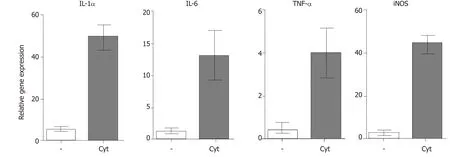
Figure 2 Expression of genes for immunoregulatory molecules and growth factors in the retina after intravitreal injection of pro-inflammatory cytokines.
In addition to the production of immunoregulatory cytokines,MSCs are also potent producers of numerous growth and trophic factors,such as HGF,LIF,VEGF,IGF-I,nerve growth factor,brain-derived neurotrophic factor,CDTF,glial cell line-derived neurotrophic factor or platelet-derived growth factor[56,61,62].After the application of MSCs into the eye,the factors produced by MSCs support the survival and viability of various types of retinal cells[2,3].Ezqueret al[63]showed that intravitreal injection of MSCs in diabetic mice increased intraocular levels of nerve growth factor,basic fibroblast growth factor and glial cell line-derived neurotrophic factor and triggered an effective cytoprotective microenvironment.The factors produced by MSCs have a wider spectrum of trophic effects.For example,it has been shown that HGF supports the growth and differentiation of numerous cell types[64],LIF is a highly pleiotropic factor promoting cell differentiation and tissue growth[65],and IGF-I is also a pleiotropic factor with multiple effects on cell differentiation,survival and growth[66].
The angiogenic effects of VEGF are also well known[67].These molecules and numerous other growth and trophic factors produced by MSCs can contribute to the regeneration of the diseased retina.However,some of these factors have both positive and negative impacts on the diseased retinal tissue.For example,VEGF supports tissue healing by stimulating the formation of blood vessels,but inadequate neovascularization can contribute to the development of some types of retinal degeneration[67].Thus,local concentrations of particular factors decide their effects,and the stage and extent of the disease are very important for the formation of the cytokine microenvironment and for therapeutic possibilities.
CONCLUSION
With the persistent absence of treatment protocols for retinal degenerative diseases,stem cell-based therapy offers a promising approach.Among the various stem cell types,MSCs provide several advantages.They can be prepared in a sufficient number as autologous stem cells and administered repeatedly,and there is no danger of uncontrolled cell growth or a formation of teratomas.Numerous experimental therapeutic protocols and clinical studies using intravitreal administration of MSCs have demonstrated the safety of this therapy without any harmful side effects[68,69].
Retinal degenerative diseases are associated with local inflammation,cytokine disbalance and a loss of specialized retinal cells,and MSCs can display therapeutic effects for all of these dysfunctions by multiple mechanisms.They are the producers of numerous growth and trophic factors that support the survival and growth of the remaining retinal cells.They also possess potent immunosuppressive properties to attenuate inflammatory reactions and have the ability to differentiate into many other cell types.It has been shown that in the presence of retinal cells,supernatant from cultures of retinal cells or in the presence of retinal cell extracts MSCs differentiate into cells expressing genes and markers typical for retinal cells[18,20,70].
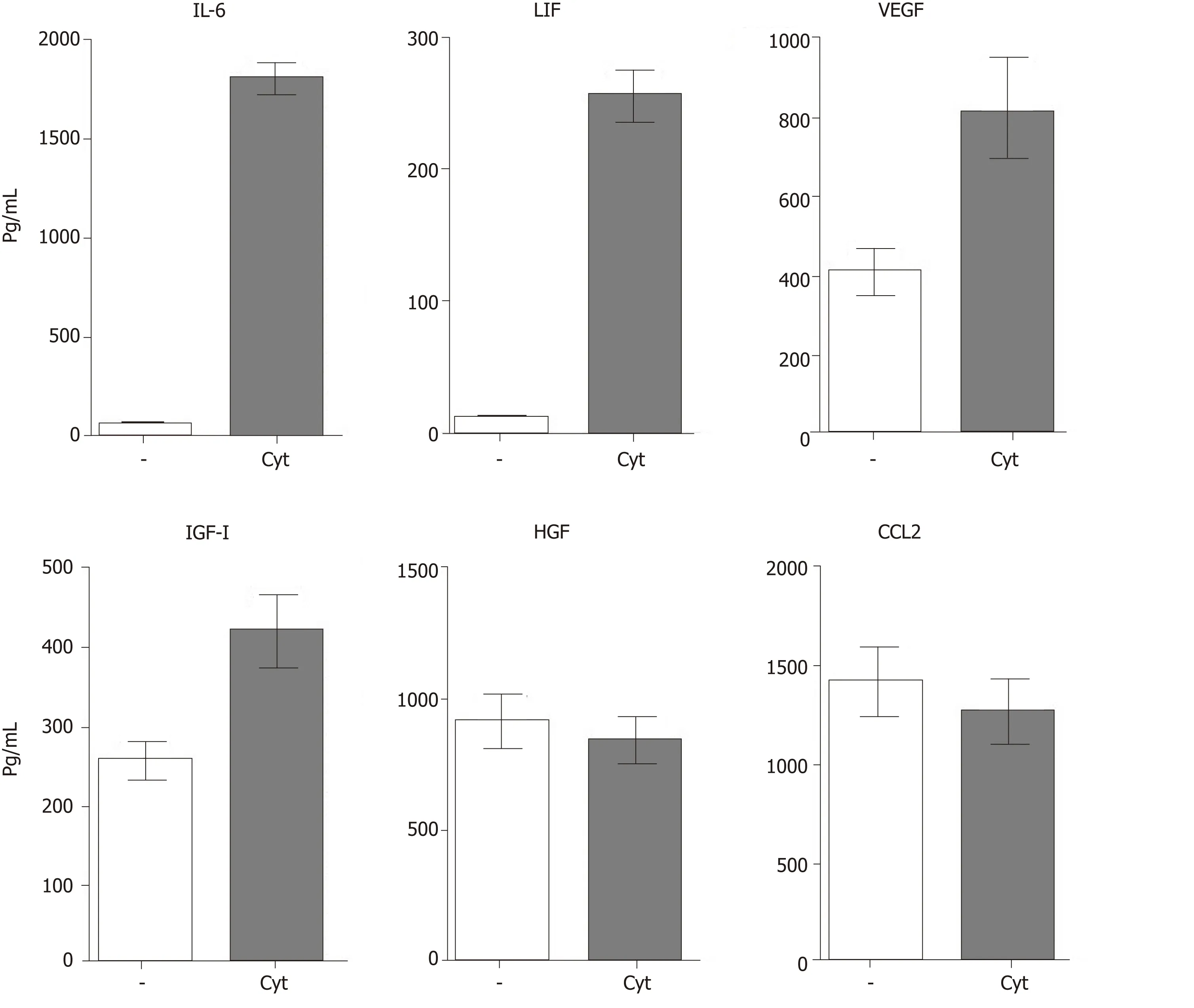
Figure 3 Spontaneous and cytokine induced production of immunoregulatory cytokines and growth factors by mouse mesenchymal stem cells.
While there is abundant data from various experimental models that demonstrate the positive effects of intraocularly administered MSCs on retinal healing,the results still have to be taken with a precaution.In some studies,human MSCs were injected intravitreally in rodents and their biocompatibility and positive therapeutic effects were described[71-73].However,a recent study by Lohanet al[74]demonstrated that human MSCs administered into immunocompetent rats do not have the same therapeutic effects as rat MSCs and that human MSCs do not suppress the proliferation of rat T lymphocytes.Moreover,human MSCs are not activated by rat pro-inflammatory cytokines[74].These interspecies incompatibilities have to be taken into account when the results from preclinical animal studies utilizing human MSCs are considered in the context of translation to clinical trials.
Another issue that deserves attention is the secretory profile of MSCs after their delivery into the inflammatory environment of the diseased retina.Such a secretory profile could be quite distinct from that in MSCs during their cultivationin vitro.We have shown in a mouse model that highly purified MSCs spontaneously produce significant levels of TGF-β but do not produce IL-6.However,in the presence of proinflammatory cytokines,such as IL-1β,TNF-α or IFN-γ,MSCs simultaneously secrete a significant amount of IL-6[25].IL-6 is a pro-inflammatory cytokine,which together with TGF-β determine the development of the highly pro-inflammatory Th17 cells.A crucial role in the shift between inhibitory Tregs and pro-inflammatory Th17 cells is played by the ratio in concentrations of TGF-β and IL-6[25,75].This plasticity in the secretory potential of MSCs represents limitations when cultured MSCs producing TGF-β are transferred into the inflammatory environment of the diseased retina.To prevent activation of pro-inflammatory cells,MSCs could be co-administrated with antibody anti-IL-6 or anti-IL-6 receptor,as this has been tested in patients with kidney allografts[76,77].It has been shown that blocking of the IL-6 pathway can attenuate inflammation,support the activation of Tregs and enhance allograft survival.It can be proposed that such interventions into cytokine pathways or the regulation of cytokine interactions can significantly improve the efficiency of stem cell-based therapy for retinal diseases.
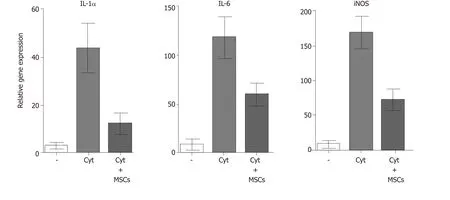
Figure 4 The expression of genes for pro-inflammatory molecules in stimulated retinal explants and suppression of the gene expression by MSCs.
While there is still a debate about the origin and characterization of MSCs[21]and many unknown interactions among cytokines and other growth and trophic factors remain to be recognized,stem cell-based therapy represents a great promise and hope for the patients with visual problems and for the treatment of so far incurable retinal degenerative diseases.
杂志排行
World Journal of Stem Cells的其它文章
- Unexpected encounter of the parasitic kind
- Colon cancer stemness as a reversible epigenetic state: Implications for anticancer therapies
- CRISPR/Cas system: An emerging technology in stem cell research
- Developments in cell culture systems for human pluripotent stem cells
- Monitoring maturation of neural stem cell grafts within a host microenvironment
- Ameliorating liver fibrosis in an animal model using the secretome released from miR-122-transfected adipose-derived stem cells
