Systematic relationships of Chinese freshwater semisulcospirids(Gastropoda,Cerithioidea)revealed by mitochondrial sequences
2019-10-31LiNaDuJunChenGuoHuaYuJunXingYang
Li-Na Du,Jun Chen,Guo-Hua Yu,Jun-Xing Yang
1 Kunming Institute of Zoology,Chinese Academy of Sciences,Kunming Yunnan 650223,China
2 Key Laboratory of Ecology of Rare and Endangered Species and Environmental Protection(Guangxi Normal University),Ministry of Education,Guilin Guangxi 541004,China
3 Key Laboratory of the Zoological Systematics and Evolution,Institute of Zoology,Chinese Academy of Sciences,Beijing 100101,China
4 Yunnan Key Laboratory of Plateau Fish Breeding,Kunming Institute of Zoology,Chinese Academy of Sciences,Kunming Yunnan 650223,China
ABSTRACT The systematics of Semisulcospiridae in China is revised here based on morphological characters and mitochondrial phylogenetics. Phylogenetic relationships within the Chinese semisulcospirids were assessed via DNA sequences from mitochondrial analysis(cytochrome c oxidase I and 16S rRNA). This research contains most morphospecies of semisulcospirids previously recorded in China. Based on these results, the family of Chinese Semisulcospiridae is represented by three genera: i.e., viviparous Semisulcospira Böttger, 1886, oviparous Hua Chen, 1943 and Koreoleptoxis Burch and Jung,1988.These genera can be distinguished from each other by reproductive anatomy, reproductive mode, and radula features. Species of Hua are mainly distributed in southwest China and Guangxi,whereas Koreoleptoxis and Semisulcospira are mainly distributed in south and northeast China.
Keywords:Phylogeny;China;Semisulcospira;Hua;Koreoleptoxis
INTRODUCTION
Semisulcospirids are an ecologically important and taxonomically challenging family of freshwater snails distributed in lakes, rivers, and streams in eastern Asia,including Far East Russia, Japan, Korean peninsula, and China,and in western North America(Köhler,2016,2017;Liu et al., 1993; Strong & Köhler, 2009; Yen, 1939). Basic knowledge on freshwater gastropods in China was first established in the late nineteenth and early twentieth centuries(e.g.,Dautzenberg&Fischer,1906,1908;Fulton,1914; Heude, 1888), with all cerithioidean freshwater gastropods designated under the single genus Melania Lamarck, 1799 (junior synonym of Thiara Röding, 1798).Heude(1888,1889(1882-1890))recorded 24 species of Melania in south China,especially in the Yangtze River Basin.Yen(1939)subsequently identified Chinese freshwater snails from the Naturmuseum Senckenberg,Germany,and recorded 12 Chinese species of Thiaridae and a new genus,Senckenbergia Yen, 1939, for the type species Melania pleuroceroides from Sichuan.Chen(1943)later described two genera,Wanga(type species Melania henriettae Gray,1833)and Hua(type species Melania telonaria Heude,1888),into which were placed eight and eleven nominal species,respectively.In dispute,Morrison(1954)proposed Wanga and Hua to be synonyms of Brotia H.Adams 1866 and Oxytrema Rafinesque 1819, respectively, although the suggestion of Hua being a synonym of Oxytrema was not accepted by subsequent authors (Du et al., 2019; Köhler, 2016, 2017;Strong&Köhler,2009).Abbott(1948)described a new genus,Namrutua, for the type species Melania ningpoensis.However, Senckenbergia and Namrutua were primarily ignored by most Chinese researchers,with all species instead placed in Semisulcospira Böttger,1886(Liu et al.,1979,1993;Xu, 2007; Zhang et al., 1997). Several species of Semisulcospira were subsequently described in China,including S. inflata Tchang & Tsi 1949 recorded in Lake Dianchi,Yunnan,and S.marica Li,Wang&Zhang 1994 and S.crassicosta Liu,Wang&Zhang 1994 recorded in Guizhou Province(Liu et al.,1994;Tchang&Tsi,1949).Liu et al.(1994) also descried two new species of Paludomus, P.qianensis Liu Zhang&Duan 1994 and P.cinctus Liu,Zhang&Duan 1994,in Guizhou Province.Xu(2007)stated that there were 53 species of Semisulcospiridae found in China,including 49 species of Semisulcospira,two species of Hua,one species of Paludomus,and one species of Potodoma.However, Xu (2007) did not revise the validity of these species.The first systematic revision of semisulcospirids was provided by Strong&Köhler(2009)based on morphological characters and molecular phylogenetic analysis.They showed Hua to be a valid genus and distinguishable from Semisulcospira by distinct features of the digestive,reproductive,and nervous systems.Du et al.(2017,2019)designated Semisulcospira trivolvis Yen 1939 as synonym to S.paludiformis Yen 1939 and further stated that it should be assigned to the genus Sulcospira Troschel, 1858(Pachychilidae Fischer&Crosse,1892).More recently,Du et al. (2019) studied semisulcospirids of southwest China by morpho-anatomy and mitochondrial phylogenetics and supported Namrutua and Senckenbergia as viviparous and as synonyms of Semisulcospira. Additionally, the taxonomic status of several species were discussed, in particular M.cancellata Benson 1842 and M.suifuensis Chen 1937 were treated as junior synonyms to S.ningpoensis(I.Lea,1856),and M.dulcis Fulton 1904,M.lauta Fulton 1904,and S.inflata Tchang 1949 were treated as junior synonyms to Hua textrix(Heude, 1888). However, several semisulcospirid species were not contained in Du et al. (2019), including H.kweichouensis (Chen, 1937) (synonym to H. intermedia(Gredler,1885)in this study),Paludomus minensis Chen 1943(synonym to Lithogryphus pallens Bavay & Dautzenberg 1910,valid species name is H.pallens in this study),and P.rotundata(Heude,1888).Furthermore,most semisulcospirids were recorded in the Yangtze River.Thus,based on previous literature,52 species of semisulcospirids have been recorded in China(Du&Yang,2019;Du et al.,2019;Liu et al.,1993;Xu, 2007). However, the systematic and phylogenetic relationships of semisulcospirids remain poorly understood.
From 2014 to 2017,specimens of semisulcospirids were collected from 14 Chinese provinces,including Heilongjiang,Jilin, Liaoning, Anhui, Jiangxi, Hunan, Zhejiang, Fujian,Guangdong, Guangxi, Guizhou, Sichuan, Chongqing, and Yunnan.Based on morphological and molecular analyses,the specimens were used to clarify the phylogenetic and systematic relationships among Chinese semisulcospirids.
MATERIALS AND METHODS
Taxon sampling
Specimens were collected from 2014 to 2017 by L.N.Du et al.in 14 Chinese provinces(Figure 1).The foot muscle tissues were preserved in 99%ethanol.All voucher specimens were deposited at the Kunming Institute of Zoology, Chinese Academy of Sciences(KIZ,CAS)(Table 1).The identification of specimens included verification of origin descriptions and topotypic specimen photos.
We examined 225 sequenced samples from 41 species,including 95 sequenced samples from 22 species of Hua from Yunnan,Chongqing,Guangxi,and Guizhou,90 sequenced samples from 12 species of Koreoleptoxis from Heilongjiang,Jilin, Hunan, Jiangxi, Fujian, Zhejiang, and Anhui, and 40 sequenced samples from seven species of Semisulcospira from Hunan, Jiangxi, Guangxi, Chongqing, Zhejiang, and Liaoning(Table 1).
Morphology
Measurements and descriptions,including shell dimensions,radulae,and shell features,followed Strong&Köhler(2009)and Du et al.(2019).Radulae and embryonic shells were cleaned enzymatically with proteinase K for 30 min, after which they were sonicated for 60 s,mounted on aluminum specimen stubs with adhesive tabs, and coated with gold palladium for analysis via scanning electron microscopy(Hitachi S-3000,Japan)at 15 kV.Soft body anatomy was observed using a microscope(Leica S6D,6.3-40x)and was described following the terminology used by Strong&Köhler(2009)and Köhler(2017).
Molecular analyses
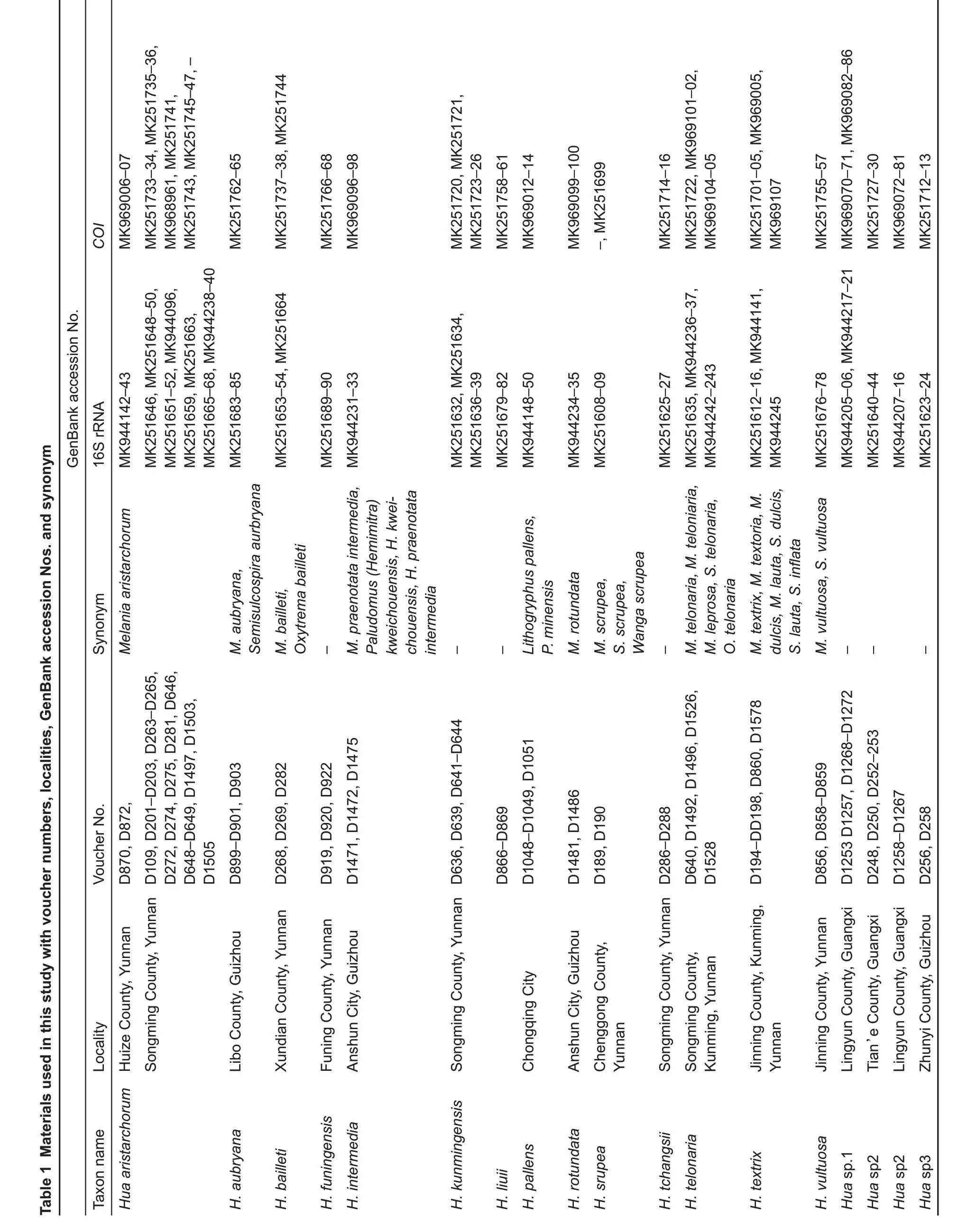
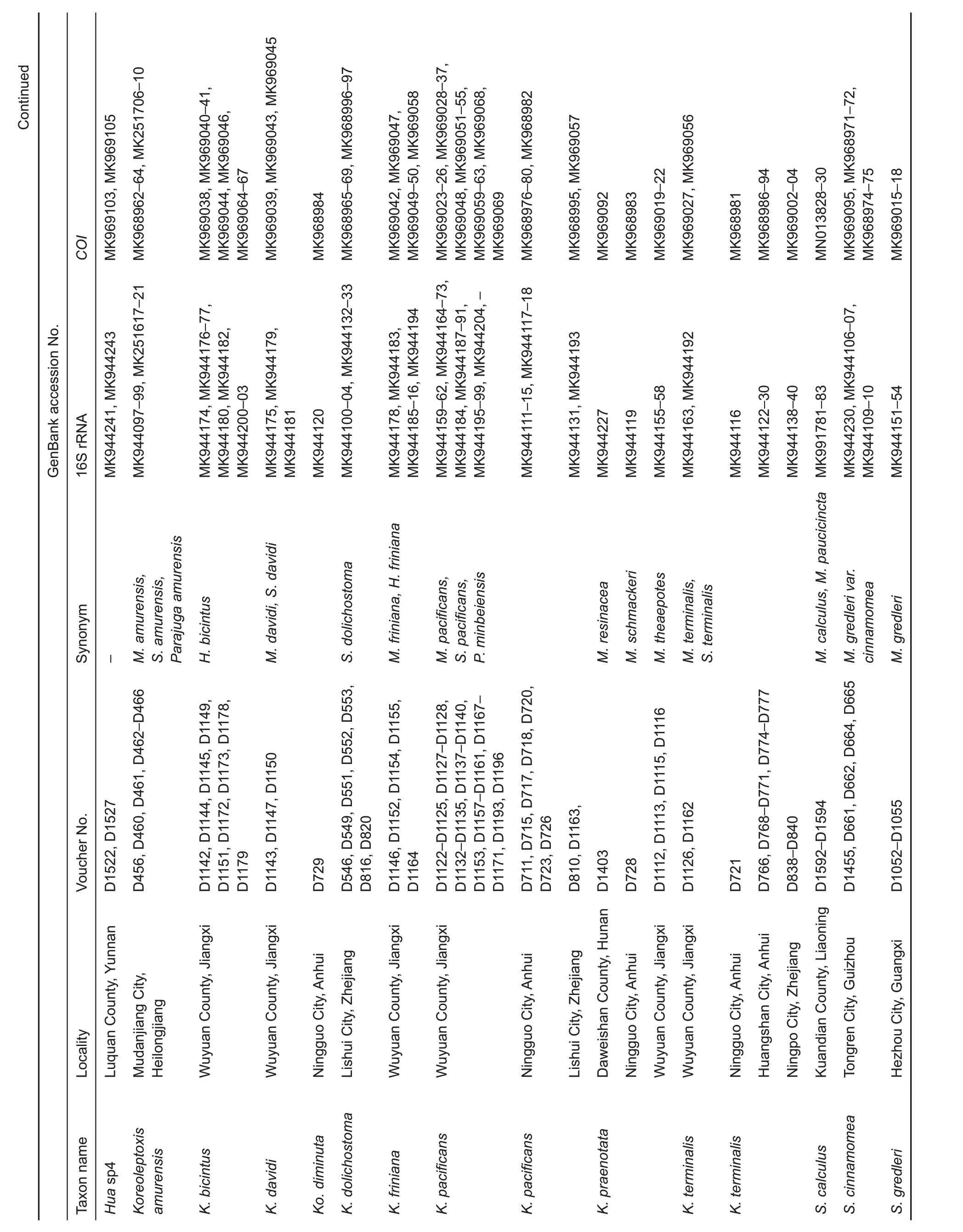
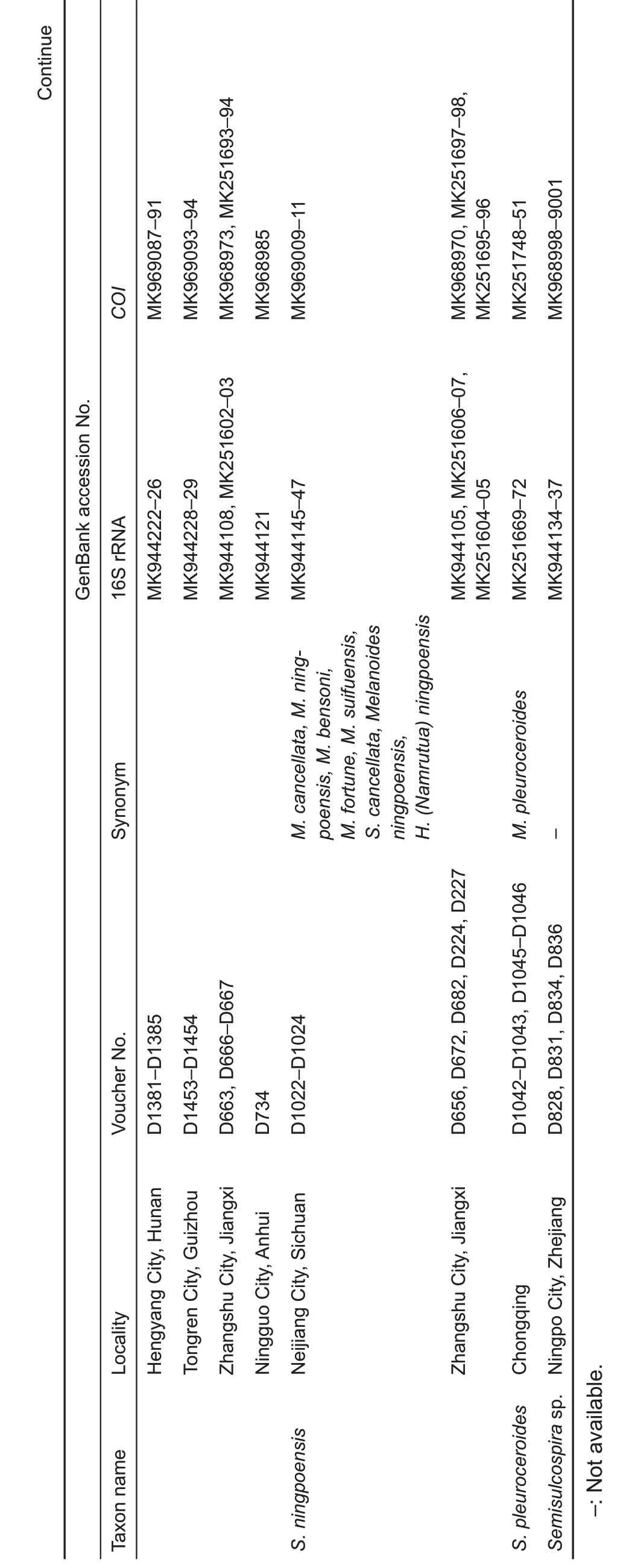

Figure 1 Sampling localities of semisulcospirids in China
Genomic DNA was purified via standard phenol-chloroform isolation and ethanol precipitation. Partial sequences of mitochondrial cytochrome c oxidase subunit I(COI,772 bp)and 16S ribosomal RNA(16S,856 bp)were amplified using primers F2 and R2(Du et al.,2019)for COI,and 16S-F(Wilson et al.,2004)and H3059(Palumbi et al.,1991)for 16S.Homologous sequences of other semisulcospirids were obtained from GenBank,with all new sequences deposited in same under accession Nos. MK944096-MK944245,MK968961-MK969107, MK991781-MK991783, MN013828-MN013830(Table 1).We also acquired various sequences from GenBank for use as the ingroup following Du et al.(2019),including one Koreoleptoxis species(K.amurensis),three Semisulcospira species(S.pleuroceroides,S.gredleri(Böttger,1886),S.ningpoensis),and thirteen Hua species(H.aristarchorum(Heude,1888),H.telonaria(Heude,1888),H.aubryana,H.bailleti(Bavay&Dautzenberg,1910),H.textrix,H. vultuosa (Fulton, 1914), H. scrupea (Fulton, 1914), H.funingensis Du, Köhler, Yu, Chen & Yang, 2019, H.kunmingensis Du,Köhler,Yu,Chen&Yang,2019,H.liuii Du,Köhler,Yu,Chen&Yang,2019,H.tchangsii Du,Köhler,Yu,Chen & Yang, 2019, and two undescribed) (MK251600-MK251768));Elimia clenchi,E.catenaria,and Melanopsis sp.were also obtained from GenBank as the outgroup to root the phylogeny in accordance with the trees in Strong&Köhler(2009)and Köhler(2017).
Sequence alignment of 16S rRNA and COI was performed with default parameters using Mafft v7.305(Katoh et al.,2002)and the CIPRES Science Gateway (Miller et al., 2010).Pairwise distances between species were determined using MEGA 7(Kumar et al.,2016)and the optimal substitution model was selected by corrected Akaike Information Criterion using jModeltest v2.1.10(Posada,2008).MRBAYES v3.2.6(Ronquist et al.,2012)and RAxML-HPC v.8.2.10(Stamatakis,2014) were used for Bayesian inference and maximum likelihood analyses,respectively,in reference to the selected model of sequence evolution.Bayesian posterior probabilities(BPPs)of nodes were determined using Metropolis-coupled Markov chains(one cold chain)for five million generations,with sampling every 100 generations. Node support for maximum likelihood analysis was determined using 1 000 rapid bootstrap replicates.
RESULTS
Sequence analysis and mitochondrial phylogeny
The final concatenated dataset contained 221 16S sequences and 220 COI sequences.The 16S alignment contained 567 nucleotide positions after pruning alignment ends and removing poorly aligned portions.The COI dataset had 762 nucleotide positions and a total length of 1 339 bp,including 553 variable sites and 497 phylogenetically informative sites.The GTR+I+G model was selected as the best-fit model of nucleotide substitution. The dataset contained sequences from previously published studies,which were obtained from GenBank, as well as the new sequences, which were deposited in GenBank(accession Nos.shown in Table 1).Sequences of Western North America Juga,type species of the genera Semisulcospira libertina and Koreoleptoxis globus ovalis,and other published sequences of K.nofifila and S.reiniana from GenBank were included to check the phylogenetic positions of the Chinese semisulcospirids.
Both phylogenetic analyses produced identical,usually wellsupported topologies consisting of two main clades(A and B),which contained sequences of various nominal species previously recorded in China.Clade A could be divided into four subclades:subclade A1 contained species P.rotundata,P. minensis, and L. pallens; subclade A2 contained one undescribed species from Nanpanjiang River,Hua sp.3,H.aristarchorum, and species of Wanga as orphans without genus affiliation from southwest China and Guangxi Zhuang Autonomous Region;subclade A3 contained H.funingensis and an undescribed species,Hua sp.2,from Nanpanjiang River; subclade A4 contained H. telonaria (type species),species of Hua from southwest China and Vietnam,and two undescribed species(Figure 2).Clade B could be divided into three subclades;subclades B1 and B2 contained species of viviparous Semisulcospira from Japan and China;subclade B3 contained oviparous K.globus ovalis,K.nofifila,and K.amurensis,and species from the lower Yangtze River(Figure 3).These clades revealed an approximate phylogeographic structure, which roughly adhered to the major drainage systems in China(Figure 1).Results indicated that clade A(black) occurred predominantly in southwest China and drainages of the upper Yangtze and Pearl rivers,whereas subclades B2 (red) and B3 (blue) mainly occurred in the middle and lower Yangtze River, with a few specimens collected from northeast China(Figure 1).
Comparative morphology
We identified several patterns largely consistent with the phylogenetic clustering in the mitochondrial trees shown in Figure 2 and Figure 3.Subclades B1 and B2 are viviparous and mature females do not possess an ovipositor pore or egglaying groove below the right cephalic tentacle;furthermore,the lower margin of the central teeth is straight in both subclades. Clade A and subclade B3 are both oviparous,but show differences in reproductive anatomy; specifically,mature females in clade A possess an ovipositor pore or egg-laying groove below the right cephalic tentacle,whereas mature females in subclade B3 possess both an ovipositor and egg-laying groove.In addition,the lower margin of the central teeth is w-shaped in clade A, but triangular in subclade B3.
Regarding operculum features,the nucleus is obvious and located in the lower base of the operculum in all subclades,except subclade A1,in which it is not obvious(Figure 4A-B).In subclade A3,the nucleus is very small and located in the lower one sixth of the operculum(Figure 4C-D);in subclades A2 and A4 and clade B,the operculum shows a clear spiral growth line and the nucleus is located in the lower one third of the operculum(Figure 4E-H).
In clade A,species of subclades A1 and A4(except H.aubryana and H. jacqueti), as well as H. sp.3, and H.funingensis,possess smooth shells,whereas all other species possess a sculptured shell.In P.minensis,L.pallens,H.liuii,and H.funingensis,the lateral teeth exhibit a single prominent squarish cusp without small denticles at either side(Figure 5B),whereas,in all other species,the lateral teeth exhibit two to four small denticles at each side(Figure 5A).In P.minensis,P.rotundata,L.pallens,H.sp.3,and H.textrix,the mature females possess an egg-laying groove, whereas mature females in all other species possess an oviparous pore.In subclade B2,S.ningpoensis,S.gredleri,and S.cinnamomea(Gredler,1887)have well-sculptured shells,whereas all other Chinese species possess a smooth shell.In subclade B3,K.amurensis and Melania pacificans Heude,1888 have wellsculptured shells,whereas all other Chinese species have a smooth shell.
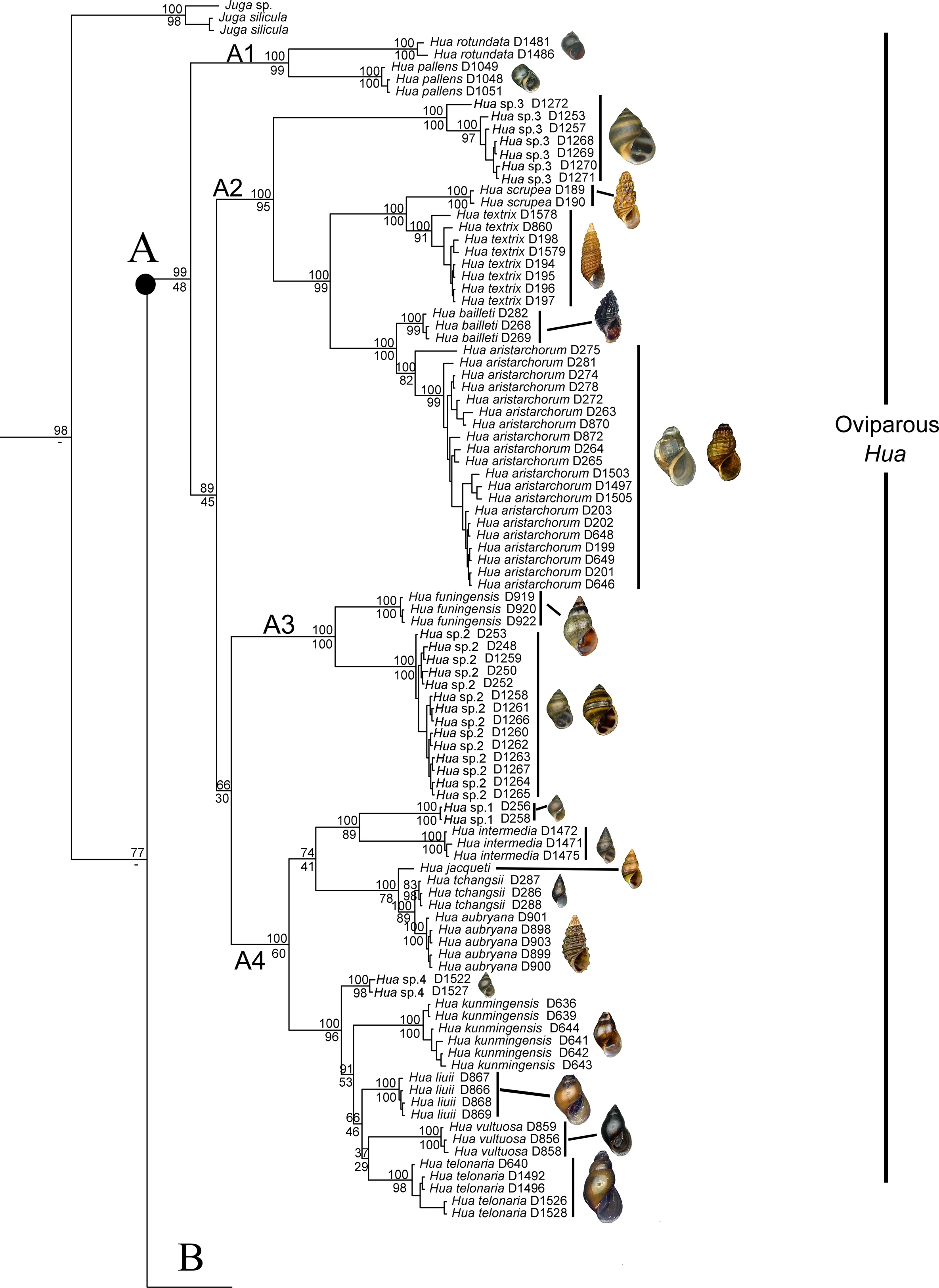
Figure 2 Bayesian phylogram of clade A based on analysis of concatenated dataset of mitochondrial cytochrome c oxidase subunit I(COI)and 16S rRNA sequences
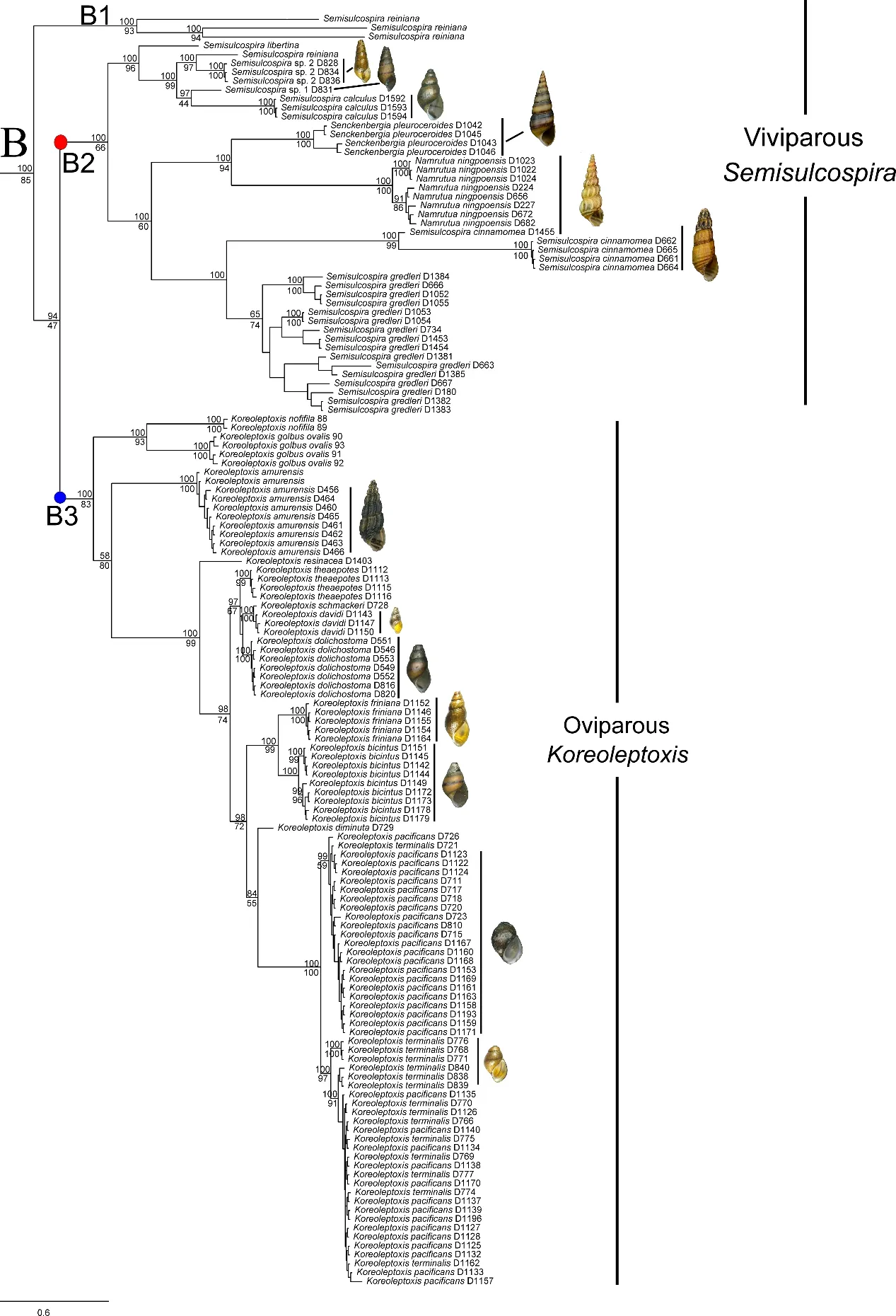
Figure 3 Bayesian phylogram of clade B based on analysis of concatenated dataset of mitochondrial cytochrome c oxidase subunit I(COI)and 16S rRNA sequences.
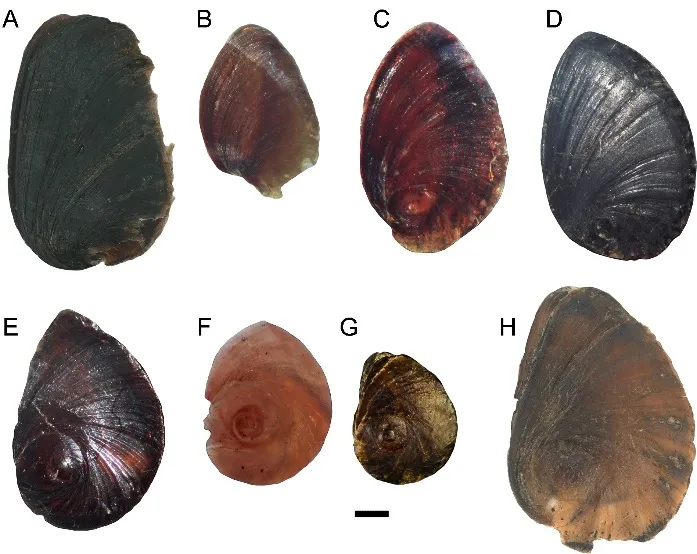
Figure 4 Opercula of semisulcospirids from China
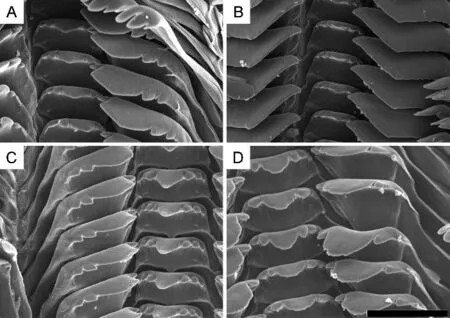
Figure 5 Radulae of semisulcospirids from China
Mitochondrial differentiation
Pairwise comparisons of COI distances revealed that clade A and subclades B2 and B3 differed from each other by average uncorrected P-distances of 14.6%to 14.7%(average 14.6%).Species within clade A differed by an average uncorrected Pdistance of 0.8%to 14.5%(average 11.2%).Species within subclade B2 differed by an average uncorrected P-distance of 10.0%to 14.9%(average 12.0%).Species within subclade B3 differed by an average uncorrected P-distance of 1.2%to 11.2%(average 6.6%).
DISCUSSION
In this study,the Chinese semisulcospirids were divided into two main clades,i.e.,clades A and B.Minton&Lydeard(2003)reported maximum intergeneric differences of 15.5%for COI in Semisulcospiridae.Here,we observed a maximum intergeneric divergence of 14.7%.Hence,clade A,together with subclades B2 and B3,represented the genera of Hua,Koreoleptoxis, and Semisulcospira, respectively.Morphologically,the three genera can be distinguished from each other by reproductive mode, radula, and female reproductive features.
Liu et al.(1993)and Xu(2007)assigned Senckenbergia pleuroceroides and Namrutua ningpoensis into Semisulcospira,but did not provide a reason for this decision.Du et al. (2019) treated Namrutua and Senckenbergia as junior synonyms to Semisulcospira based on morphological and phylogenetic evidence. In addition to three Chinese species, i. e., S. gredleri, S. ningpoensis, and S.pleuroceroides, four other species, i.e., S. cinnamomea(Gredler,1887)from Yangtze River,S.calculus(Reeve,1860)from Liaoning Province in northeast China, and two undescribed species from Zhejiang Province were contained in this study. Semisulcospira cinnamomea was initially described as a subspecies of S. gredleri (Gredler, 1887);however,it is treated as a valid species in this study as it can be distinguished from S.gredleri by its six to nine whorls(vs.four to five) and body whorl not inflated (vs. inflated).Furthermore,M.calculus and M.paucicincta Martens 1894 are virtually identical (shell size, proportion, rate of whorl expansion, and color bands), and are here treated as synonyms,with Semisulcospira calculus considered as the valid species name due to the description of M. calculus predating that of M. paucicincta. The Chinese species of Semisulcospira can be distinguished from each other by their shell features(sculptured or smooth,body whorl inflated or not,number of whorls)and radulae.
Several species were originally described as members of Paludomus, including P. kweichouensis, P. minensis, P.qianensis,and P.cinctus(Chen,1937,1943;Liu et al.,1994).Although the Chinese Paludomus species are similar to P.conica Gray (type species of Paludomus, type location in India)in operculum features,Abbott(1948)stated that the mantle margin in P.conica contains 20 to 25 papillae,whereas the mantle margin in Chinese Paludomus is smooth.Furthermore, in the current study, phylogenetic evidence indicated that Chinese Paludomus species recorded in southwest China could belong to Hua(Semisulcospiridae).In addition,P.minensis and L.pallens,which come from the same locality,are virtually identical,except that the body whorl in L.pallens is more inflated than that in P.minensis;thus,here,they are deemed to be synonyms,with Hua pallens considered as the valid species name as the description of L.pallens predates that of P. minensis. Furthermore, P.kweichouensis is treated as a synonym of M. intermedia Gredler,1884 due to their virtually identical shells and same locality,with Hua intermedia considered as the valid species name due to the description of M.intermedia predating that of P.kweichouensis.
Chen(1943)originally described Wanga(containing eight species)and Hua(containing eleven species).In this study,however, the species of Wanga and Hua from southwest China and Guangxi are placed in Hua, which therefore contains 19 species in China, i.e., H. aristarchorum, H.aubryana, H. bailleti, H. funingensis, H. intermedia, H.jacqueti,H.kunmingensis,H.liuii,H.pallens,H.rotundata,H.scrupea,H.textrix,H.telonaria,H.tchangsii,H.vultuosa,and four undescribed species(Du et al.,2019);other species from south of the middle and lower Yangtze River are placed into Koreoleptoxis,which therefore contains K.diminuta(Boettger,1887),K.friniana(Heude,1888),K.joretiana(Heude,1890),K.naiadarum(Heude,1890),K.oreadarum(Heude,1888),K.parenotata(Gredler,1884),K.schmackeri(Boettger,1886),and K.toucheana(Heude,1888).
Within Hua,species can be distinguished from each other by the shell (sculptured or smooth, elongated or ovate),operculum, radula, and female reproductive features.Additionally, within Koreoleptoxis, species can be distinguished from each other by the shell (sculptured or smooth,elongated or ovate)and radula features.Although K.pacificans and K. terminalis are not monophyletic in the phylogenetic tree,K.pacificans can be distinguished from K.terminalis by morphological characteristics: i.e., shell solid and sculptured(vs.thin and smooth)and same number of inner and outer margin teeth (vs. different). Hence, K.pacificans and K.terminalis are treated as valid species in this study. Incongruence between mtDNA phylogeny and morphospecies is not a rare phenomenon in semisulcospirids(Köhler,2016,2017).Retention of ancestral polymorphisms, introgression, heteroplasmy, recombination,and the presence of morphologically cryptic species or taxonomic misidentification may explain why mtDNA phylogenies do not accurately reflect the relationships of their host organisms(Ballard&Whitlock,2004;Edwards&Beerli,2000;Funk&Omland,2003;Köhler&Deein,2010;Köhler,2016;White et al.,2008).
COMPETING INTERESTS
The authors declare that they have no competing interests.
AUTHORS'CONTRIBUTIONS
L.N.D. and J.X.Y.designed the study.J.C.checked the specimens of Koreoleptoxis and Semisulcospira that preserved in Institute of Zoology, CAS. G.H.Y. supervised the molecular analyses. L.N.D.extracted genomic DNA and wrote the manuscript with the other authors'input.L.N.D.and G.H.Y.sequenced mitochondrial DNA and submitted to GenBank.L.N.D. and G.H.Y. revised the manuscript. All authors read and approved the final version of the manuscript.
ACKNOWLEDGEMENTS
We are grateful to G.H.Cui,S.S.Shu,S.W.Liu,X.A.Wang,Y.E.Jiang,C.Yuan,X.F.Pan,T.Qin(Kunming Institute of Zoology,Chinese Academy of Sciences),J.Yang(Nanning Normal University,China)and J.H.Lan(Du'an Fishery Technique Popularization Station)for specimens collection.Special thanks are due to Dr.V.Héros(Muséum National d'Histoire Naturelle,Pairs,France),Dr.A.Hänggi(Naturhistorisches Museum,Basel,Switzerland),Dr.E.Strong(National Museum of Natural History in Washington DC,USA),and Dr. J.Ablett (Natural History Museum, London, UK) for providing photographs of type specimens. We are grateful to Dr. C. Watts for polishing this manuscript.
杂志排行
Zoological Research的其它文章
- From our roots,we grow Celebrating the 60th anniversary of the Kunming Institute of Zoology,Chinese Academy of Sciences
- Genomic insights into ruminant evolution:from past to future prospects
- Antimicrobial peptides:new hope in the war against multidrug resistance
- Chromosomal level assembly and population sequencing of the Chinese tree shrew genome
- Superior intestinal integrity and limited microbial translocation are associated with lower immune activation in SIVmac239-infected northern pig-tailed macaques(Macaca leonina)
- Conserved sequences identify the closest living relatives of primates
