Antimicrobial peptides:new hope in the war against multidrug resistance
2019-10-31JamesMwangiXueHaoRenLaiZhiYeZhang
James Mwangi,Xue Hao,Ren Lai,3,4,5,6,Zhi-Ye Zhang,*
1 Key Laboratory of Bioactive Peptides of Yunnan Province/Key Laboratory of Animal Models and Human Disease Mechanisms of Chinese Academy of Sciences and Yunnan Province,Kunming Institute of Zoology,Chinese Academy of Sciences,Kunming Yunnan 650223,China
2 Kunming College of Life Science,University of Chinese Academy of Sciences,Kunming Yunnan 650204,China
3 Sino-African Joint Research Center,Kunming Institute of Zoology,Chinese Academy of Sciences,Kunming Yunnan 650223,China
4 Institutes for Drug Discovery and Development,Chinese Academy of Sciences,Shanghai 201203,China
5 KIZ-CUHK Joint Laboratory of Bioresources and Molecular Research in Common Diseases,Kunming Institute of Zoology,Chinese Academy of Sciences,Kunming Yunnan 650223,China
6 Center for Biosafety Mega-Science,Chinese Academy of Sciences,Wuhan Hubei 430071,China
ABSTRACT The discovery of antibiotics marked a golden age in the revolution of human medicine. However,decades later,bacterial infections remain a global healthcare threat,and a return to the pre-antibiotic era seems inevitable if stringent measures are not adopted to curb the rapid emergence and spread of multidrug resistance and the indiscriminate use of antibiotics. In hospital settings, multidrug resistant(MDR) pathogens, including carbapenem-resistant Pseudomonas aeruginosa, vancomycin-resistant enterococci (VRE), methicillin-resistant Staphylococcus aureus (MRSA), and extendedspectrum β-lactamases (ESBL) bearing Acinetobacter baumannii, Escherichia coli, and Klebsiella pneumoniae are amongst the most problematic due to the paucity of treatment options,increased hospital stay, and exorbitant medical costs. Antimicrobial peptides (AMPs) provide an excellent potential strategy for combating these threats. Compared to empirical antibiotics, they show low tendency to select for resistance,rapid killing action, broad-spectrum activity, and extraordinary clinical efficacy against several MDR strains.Therefore, this review highlights multidrug resistance among nosocomial bacterial pathogens and its implications and reiterates the importance of AMPs as next-generation antibiotics for combating MDR superbugs.
Keywords: Multidrug resistance; Nosocomial infections; Antimicrobial peptide; Antibiotic alternatives
INTRODUCTION
A perturbing prediction by the World Health Organization(WHO) is that by the year 2050, drug-resistant infections,largely exacerbated by the indiscriminate use of antibiotics,will kill 10 million people annually,ignite a financial cataclysm,and impose extreme poverty upon millions of people (de Kraker et al.,2016).The increasing incidence and prevalence of antibiotic resistance among nosocomial bacterial pathogens and the rapid spread of resistance genes in the environment are major global healthcare threats due to the associated increase in morbidity and mortality and huge burden on the economy(Aslam et al.,2018;Zaman et al.,2017).
Since the discovery of penicillin in 1928 by Alexander Fleming,antibiotics have saved countless lives and marked an important medical revolution in plant,animal,and human prophylaxis. Unfortunately, the continued use of antibiotics has been accompanied by the rapid emergence and spread of multidrug resistant (MDR) strains and many medical specialists are warning of an inevitable return to the preantibiotic era(Adedeji,2016).For example,bacterial genomic analysis has revealed the presence of more than 20 000 genes associated with resistance(Liu&Pop,2009),with a higher number expected over the coming years.Therefore,stringent measures are necessary to curb the spread of resistant strains as they present a major challenge to global public health not only in the war against microbial infections,but also in other clinical applications such as cancer treatment,invasive surgery,and graft transplantation(Gudiol&Carratalà,2014;Lupei et al.,2010).
Several factors that have been implicated in the current upsurge of antimicrobial resistance(AMR)in hospitals and the community,including the indiscriminate use of antibiotics in human and animal medicine and in agricultural practices involving growth promoters(Silveira et al.,2009;van Boeckel et al.,2015),lack of proper regulation regarding over-thecounter antibiotics,especially in developing countries where they are easily available without proper medical prescription(Ayukekbong et al., 2017), and poor sanitation practices leading to the introduction of unmetabolized antibiotics into the environment through human and animal waste(Davies&Davies,2010).
Natural selection is an inherent process and key driver of evolution, conferring organisms with traits for increased environmental adaptability and survival(Jesus et al.,2002).Over the years,the widespread use of antibiotics has led to the selection of diverse strains of microorganisms possessing MDR traits or genes(Martínez&Baquero,2002;Ochoa et al.,2009). Such resistance-conferring properties, through bacterial and genomic associations,can be easily transferred across different ecological niches, e.g., from animals to humans and vice versa,and from hospital settings to the community, where they present an unprecedented crisis(Groisman&Ochman,1996;Kümmerer,2004;Phillips et al.,2004).
In bacteria,mechanisms conferring resistance to almost all available classes of antibiotics have been studied extensively and described in detail in previous literature(Blair et al.,2014;Dever&Dermody,1991;Lin et al.,2015;Lombardi et al.,2019; Morita et al., 2012). These mechanisms include enzymatic degradation of antibiotics,drug-target modification,altered membrane permeability,and enhanced expression of efflux pumps that actively eliminate antibiotics(Alanis,2005;Laxminarayan&Brown,2001;Munita&Arias,2016).
Initially associated with severe nosocomial infections in immunocompromised patients, multidrug resistance has spread to the wider community,resulting in severe infections associated with growing death tolls and huge economic burdens due to increased disability and high medical costs(Jing et al.,2019;Peters et al.,2019).Currently,common problematic MDR bacteria include methicillin-resistant S.aureus (MRSA), vancomycin-resistant MRSA, MDR P.aeruginosa,carbapenem-resistant A.baumannii,E.coli,and K.pneumoniae,vancomycin-resistant enterococci(VRE),and extensively drug-resistant(XDR)Mycobacterium tuberculosis(Levin et al.,1999;Miller et al.,2005).Recent reports have also indicated cases of bacterial strains completely resistant to all available antibiotics.For example,the extensive use of colistin, a drug of last resort for the treatment of MDR pathogens such as P. aeruginosa, A. baumannii, and K.pneumoniae in both human medicine and agriculture,has led to the emergence of a plasmid-mediated MCR-1 gene that encodes for its resistance(Liu et al.,2016;MacNair et al.,2018;Paterson&Harris,2016).
The increasing incidence of infections resulting from MDR pathogens in clinical settings has intensified the demand for alternative therapies. Antimicrobial peptides (AMPs) with potent antimicrobial activities and diverse mechanisms of action(MOA)are considered important alternatives to solving the issues of multidrug resistance.
IMPLICATIONS OF MULTIDRUG RESISTANCE IN NOSOCOMIAL PATHOGENS
Epidemiological surveillance data worldwide indicate that anomalous use of antibiotics has resulted in the evolution of several human pathogens into MDR strains that are highly tolerant or resistant to antibiotic therapies, thus posing a serious threat to public health(Davies&Davies,2010;WHO,2014). Multidrug resistance, especially in nosocomial pathogens,is of great clinical concern due to the increased morbidity and mortality and enhanced virulence and transmissibility(Bhat et al.,2006;Khan et al.,2017).Such pathogens are implicated in severe infections such as ventilator-associated pneumonia(Koenig&Truwit,2006)as well as bloodstream(Martinez&Wolk,2016),surgical site(Anderson, 2011), and implant-associated urinary tract infections(Nicolle,2014).These infections occur often and are severe in immunocompromised patients,although recent evidence also suggests the spread of MDR genes into the general community(Khan et al.,2017).
For gram-negative nosocomial pathogens, especially A.baumannii, P. aeruginosa, K. pneumoniae, S. aureus, and Enterobacter spp.,the emergence of multidrug resistance to several available classes of antibiotics,such as penicillins,aminoglycosides,cephalosporins,and fluoroquinolones,has increased over the years as a result of extensive use,especially in intensive care units (Richard et al., 1994;Struelens,1998).In many cases,these MDR strains show reduced susceptibility to all available antibiotic therapies and are implicated in serious high mortality rate-nosocomial infections (Hirsch &Tam, 2010; Manchanda et al., 2010).Colistin,an AMP consisting of two polypeptides(polymyxin A and B),is often used as a drug of last resort for MDR strains,notwithstanding its adverse side effects such as nephrotoxicity and neurotoxicity, due to the paucity of treatment options(Falagas et al., 2005; Yamamoto et al., 2018). However,current reports indicate the emergence of MDR strains that exhibit reduced susceptibility to colistin following long-term clinical or laboratory exposure (Jeannot et al., 2017).Resistance to colistin is attributed to the presence of the MCR-1 gene,which is highly transmissible across different bacterial strains and has been isolated from strains on hospital surfaces and in animal and clinical human samples(Yamamoto et al., 2018). These strains also exhibit low susceptibility to almost all other antibiotics,including those designated as"last-resort"such as polymyxins(colistin)and carbapenems,thus raising a new crisis in the war against multidrug resistance(Liu et al.,2016;Rapoport et al.,2016;Yamamoto et al.,2018).
Gram-positive S.aureus is another important nosocomial pathogen and is transmitted through direct contact with contaminated surfaces, clinical waste, open wounds, and clinical staff(Denis,2017;Price et al.,2017).It is a major cause of skin infections, sepsis, and pneumonia in hospitalized patients, with recent data indicating increased incidences of community-acquired S.aureus infections due to improper disposal of hospital waste,poor hygiene,and the spread of resistant genes to the community (Dotel et al.,2017).The rising incidence of MRSA infections is a growing healthcare threat associated with increased mortality rates in hospitals and the community (Othman et al., 2019).Additionally,most MRSA strains exhibit resistance to all other antibiotics, including the beta-lactams, and recent reports indicate the emergence and spread of strains with reduced susceptibility to glycopeptide compounds such as vancomycin(vancomycin-resistant MRSA), therefore making treatment almost impossible(Bhat et al.,2006;Centers for Disease&Prevention,1997;Othman et al.,2019).
Over the past few years,enterococci,which are normal flora of the gut and genital tract, have become problematic nosocomial pathogens and a growing clinical predicament.This trend is concomitant with their inherent resistance to several commonly used drugs,including penicillin,ampicillin,cephalosporin,and clindamycin(Gold&Moellering,1996).In addition to inherently acquired resistance, enterococci can rapidly acquire resistance to virtually any antibiotic either through rapid mutation or the acquisition of foreign genetic material, thus expressing a repertory of resistance mechanisms to antibiotics such as enhanced expression of efflux pumps, modification of drug targets, and enzymatic degradation of drug agents(Linden,2002).MDR enterococci exhibit high adaptability under antibiotic pressure and can rapidly acquire resistant genes to enhance their survival.One notable example is the rapid transfer of resistant genes associated with the extensive use of vancomycin among several strains of enterococci, especially Enterococcus faecalis and Enterococcus faecium,two important nosocomial pathogens(Arias&Murray,2012).Resistance to vancomycin presents a major clinical crisis as most of these strains are also resistant to most other antibiotics and the transfer of vancomycin-resistant genes can occur from enterococci strains to even more lethal pathogens such as MRSA,therefore leaving very few or no therapeutic options(Chang et al.,2003;Franchi et al.,1999;Noble et al.,1992).
Bacterial biofilms are of great clinical significance to global healthcare due to their important role in nosocomial and implant-related infections. Most nosocomial pathogens produce biofilms, which complement disease pathogenicity and resistance(Dunne,2002;Gurung et al.,2013).Biofilmproducing organisms such as A.baumannii and P.aeruginosa exhibit extreme resistance to almost all antibiotics compared with non-biofilm-producing microorganisms. Biofilms form a protective coating around bacterial cells,thus hindering the killing action of antibiotics(Stewart,2002).Other factors that contribute to resistance in biofilm-producing organisms include the formation of persister cells(dormant and highly resilient to almost all available antibiotics), slow bacterial growth within the biofilm, and adaptability to stressful conditions(Keren et al.,2012;Lewis,2008).
Multidrug resistance to useful classes of antibiotics has increased gradually among several bacterial nosocomial pathogens.Thus,great efforts have been expended on the discovery of novel antibiotic alternative therapies.Currently,several AMPs with potent efficacy are under clinical trial and present excellent mitigation strategies for the multidrug resistance crisis(de Breij et al.,2018;Koo&Seo,2019;Stefania et al.,2015).Moreover,compared with traditional antibiotics,AMPs possess many important qualities that make them excellent candidates for therapeutic exploitation. For instance, AMPs are multifunctional with diverse MOA,including membrane disruption,inhibition of DNA and protein synthesis,and impairment of key cellular processes such as metabolism and cell wall synthesis(Kumar et al.,2018;Tzong-Hsien et al.,2016;Wimley,2010).Their diverse MOA are important as they minimize the tendency of pathogens to select for resistance, as observed in most conventional antibiotics that only act on single targets unless used in combination (Alanis, 2005; Bechinger & Gorr, 2017).Moreover,most AMPs exhibit potent antimicrobial properties against both gram-negative and gram-positive bacteria,fungi,and viruses in the nanomolar and micromolar range(Frecer et al.,2004;Wakabayashi et al.,1996;Yamauchi et al.,1993),rapidly kill pathogens within minutes,and have a low proclivity to select for resistance compared with conventional antibiotics(de Breij et al.,2018;Nagarajan et al.,2019).
AMPs:ORIGIN AND PROSPECTS
AMPs or host defense peptides are a notably class of compounds of the innate immune system with both microbicidal and immunomodulatory activities,providing a first line of defense against pathogenic invasion (Falanga &Galdiero,2018;Kang et al.,2017;Pasupuleti et al.,2012).AMPs are polypeptides of varying molecular weight and amino acid residues(ranging from five to over 100)and are found in virtually all living organisms,from simple prokaryotes to complex eukaryotes(Figure 1).They play important roles in the direct killing of infectious agents,viruses,bacteria,fungi,and parasites and through the modulation of immune processes such as activation and recruitment of immune cells,wound healing, angiogenesis, and inflammation (Haney &Hancock, 2013; Kumar et al., 2018; Maróti et al., 2011;Oppenheim et al.,2003;Zhao et al.,2013).

Figure 1 Number of natural and synthetic antimicrobial peptides from different kingdoms(total 3 011)as of July 2019
In most organisms, high concentrations of AMPs are expressed on surfaces that are constantly exposed to pathogens. For example, the mucosal lining and skin of vertebrates display broad-spectrum antimicrobial activities(Brogden et al.,2003;Rinaldi.,2002).In general,AMPs are either produced continuously or up-regulated following exposure to a threat(Ganz,2003).For example,human skin is continuously protected by an abundance of psoriasin,dermcidin, and lactoferrin, with cathelicidin LL-37 also upregulated following infection(Harder et al.,2013).In bacteria,AMPs play an important role in enhancing adaptability and survival during antagonistic competition for resources with other bacteria occupying the same ecological niche(Kumar et al.,2018).Several peptides with potential therapeutic activity have been isolated from bacteria and demonstrate potent antimicrobial activities against both gram-negative and grampositive bacteria and even fungi.These AMPs are known as bacteriocins and play important roles in inhibiting or killing antagonistic bacterial strains with no self-harm, thus conferring a critical survival strategy (Yang et al., 2014).Bacteriocins provide important prospects for the development of alternative antibiotic strategies against different bacterial pathogens and are also useful in the food industry as preservatives(Mills et al.,2011;Silva et al.,2018).Bacteriaderived AMPs include nisin,a 34-amino acid residue peptide and an important food preservative isolated from Lactococcus lactis (Tong et al., 2014), and colistin, isolated from Paenibacillus polymyxa and used extensively in agriculture and human prophylaxis(Poirel et al.,2017).Colistin is an important last-resort peptide drug used against several MDR nosocomial pathogens such as MRSA,MDR P.aeruginosa,and carbapenem-resistant pathogens, although its use is somewhat limited due to its side effects(Falagas et al.,2005;Levin et al.,1999;MacNair et al.,2018).
In vertebrates,AMPs can directly kill microorganisms and play a role in immunomodulation through the activation and recruitment of immune cells during infection(Kumar et al.,2018;Mahlapuu et al.,2016).Several different classes of AMPs, such as cathelicidins and defensins, have been isolated and characterized from immune cells, bodily secretions,and epithelial tissues of amphibians and marine and terrestrial animals(Lu et al.,2008;Wang et al.,2016).For example,several AMPs have been identified from amphibian skins,where they are produced in glands of the dermal skin layer and released following pathogenic exposure,inducing a microbicidal action through membrane disruption (Rollins-Smith et al., 2005; Woodhams et al., 2007). In humans,psoriasin,dermcidin,and lactoferrin are continuously secreted by the sweat glands to form an important barrier against infection,and cathelicidin LL-37 is secreted following microbial introduction(Harder et al.,2013).Nearly 30 cathelicidins have been characterized from domestic and wild mammals(Kościuczuk et al., 2012), and are stored as inactive precursors in neutrophil granules and activated following microbial exposure (Treffers et al., 2005). Apart from cathelicidins,several other classes of antimicrobial peptides have been isolated and identified in vertebrates(Brogden et al.,2003;Zhang,2006;Zhang et al.,2008).These AMPs have diverse MOA and play multifunctional roles, including modulation of immune responses, prevention of excessive tissue damage,alleviation of inflammation,and destruction of invading pathogens to mitigate infection onset (Haney &Hancock,2013;Maróti et al.,2011;Oppenheim et al.,2003).
Several vertebrate AMPs show excellent therapeutic potential due to their antibacterial,antiviral,antifungal,and anti-inflammatory properties(Oppenheim et al.,2003;Qi et al.,2019),whereas others show improved clinical efficacy,structural stability, and potent antimicrobial activity against several MDR pathogens following modification(Kumar et al.,2018;Schmidtchen et al.,2014;Zhang et al.,2019).
In contrast to vertebrates, insects and plants lack an adaptive immune system, relying entirely on the innate immune system for defensive purposes,with AMPs playing a pivotal role in the protection against invading pathogens.Several AMPs have been isolated and analyzed from the epithelial cells, hemocytes, and hemolymphs of insects(Brown et al.,2009;Hancock et al.,2006;Philippe&Reto,2005).Peptides isolated from insects are usually cationic and kill bacteria through permeabilizing bacterial membranes,and further possess potent microbicidal activity either singly or synergistically with traditional antibiotics (Yi et al., 2014).Notable examples of insect-based AMPs include cecropins,defensins,drosocins,and diptericins,which exhibit activities against both gram-negative and gram-positive bacteria as well as fungi(Mylonakis et al.,2016;Rozgonyi et al.,2009;Wu et al.,2018;Zhang&Gallo,2016).
AMPs also play important defensive roles in plants(which also lack an innate immune system).Several peptides have been isolated and characterized from the leaves,roots,seeds,and tubers of plants(Tam et al.,2015).Generally,they exhibit high proteolytic,thermal,and chemical stability due to the presence of multiple disulfide bonds conserved within their rich cysteine residues(Hammami et al.,2009;Hilchie et al.,2013;Stotz et al.,2009;Tam et al.,2015).Plant AMPs have been extensively studied and discussed (Craik, 2012;Hammami et al.,2009;Nawrot et al.,2014;Stec,2006).Much like AMPs from vertebrates, plant-based AMPs confer protection against invading pathogens through membrane disruption and pore formation and by targeting key microbial processes such as DNA and protein synthesis(Nawrot et al.,2014). Notable examples of plant AMPs include plant defensins and thionins(Hilchie et al.,2013;Stotz et al.,2009).Plant defensins are small cationic peptides with 45-55 amino acid residues and exhibit potent antibacterial and fungal activities(Gao et al.,2000;Tavares et al.,2008).Thionins are cationic peptides with 45-48 amino acid residues and three or four disulfide bonds(Stec,2006).They exhibit potent activities against bacteria and fungi and cytotoxicity on animal cells(Evans et al.,1989;Fernandez De Caleya et al.,1972).
Used singly or in combination with traditional therapeutics,AMPs are quite effective at combating infectious agents,including MDR bacteria. They exhibit potent microbicidal activity in the micromolar range,rapid killing action,and low selection of resistance,thus constituting an important strategy for curbing MDR pathogens(Deslouches&Di,2017;Loeffler et al.,2001;van't Hof et al.,2001).In contrast to empirical antibiotics that target single or specific bacterial processes,AMPs exhibit multifunctional bacterial killing effects,including disruption of the plasma membrane,and also target microbial intracellular processes,including inhibition of transcription and translation,protein synthesis,and bacterial cell wall formation(Gee et al.,2013;Hurdle et al.,2011;Reddy et al.,2004).
Presently,much effort is being directed toward obtaining novel antimicrobial agents with broad-spectrum activity against pathogenic microorganisms.Naturally occurring AMPs have proven to be invaluable templates for the design and synthesis of synthetic AMPs with increased potency,which are easier to produce and less sensitive to proteolytic degradation(Falanga et al.,2016;Hurdle et al.,2011).
CLASSIFICATION AND STRUCTURE OF AMPs
With more than 3 000 sequences reported to date,AMPs are often classified based on their secondary structures and MOA.Based on structure,the three currently established classes include α-helical,β-sheet, and extended-coiled peptides(Figure 2)(Falanga&Galdiero,2018;Lombardi et al.,2019).The α-helical AMPs are unordered in aqueous solution but once in contact with a biological membrane,they assume an amphipathic α-helical structure(Kumar et al.,2018).Important α-helical AMPs include magainin,a 23-amino acid residue peptide isolated from the skin of the African clawed frog(Xenopus laevis),which exhibits highly potent tumoricidal and broad-spectrum antimicrobial activities against bacteria,fungi,and protozoa due to membrane disruption(Matsuzaki,1999;Zerweck et al.,2017).The cathelicidin family of AMPs are also important members of this group and are found widely in nature in all vertebrates (Kościuczuk et al., 2012). For example,LL-37,a prominent cathelicidin in humans,exhibits broad-spectrum microbicidal activities against gram-positive and gram-negative bacteria by plasma-membrane disruption(Björstad et al.,2009).LL-37 has been extensively studied and used as a template for the design of several synthetic AMPs,many of which are currently under clinical trial(de Breij et al.,2018;Dürr et al.,2006;Kościuczuk et al.,2012).Other α-helical AMPs include aurein from the granular dorsal glands of the frog Litoria aurea(Rai&Qian,2017),melittin from honey-bee venom,and cecropin derived from the hemolymph of Hyalophora cecropia(Yi et al.,2014).
The β-sheet peptides,which constitute the largest group of AMPs, are produced in many organisms such as marine invertebrates, amphibians, plants, and animals (Haney &Hancock,2013;Maróti et al.,2011;Oppenheim et al.,2003;Wang et al.,2016)and show both antibacterial and antifungal properties (Harwig et al., 1996). In contrast to α-helical peptides,these peptides are ordered in aqueous solution and contain conserved cysteine residues that form disulfide bonds that enhance structural stability and minimize proteolytic degradation (Tzong-Hsien et al., 2016). They exhibit boundless therapeutic prospects, e. g., as antifungal,antibacterial, antiviral, and anti-inflammatory agents, and primarily kill bacteria through plasma-membrane disruption(Kumar et al., 2018; Panteleev et al., 2015). Prominent members of this family include defensins, protegrins, and tachyplesins.Defensins are found in plants,invertebrates,and vertebrates and exhibit activity against both gram-positive and gram-negative bacteria,fungi,and viruses(Panteleev et al.,2015;Yamaguchi&Ouchi,2012).Tachyplesins,a class of peptides isolated from hemocytes of the horseshoe crab,exhibit strong microbicidal activity, although their use is hampered by their potential toxicity to mammalian cells(Edwards et al.,2017;Liu et al.,2018;Miyata et al.,1989).
The third class of AMPs include those with an extended-coil structure.These AMPs lack secondary structures present in other AMPs such as α helices and β sheets and mostly consist of specific amino acid residues including arginine(Arg),proline,or tryptophan(Mahlapuu et al.,2016).They exhibit broad-spectrum activity against gram-negative bacteria via membrane disruption and targeting internal processes and also possess antitumor activity(Falla et al.,1996).Important extended-coil AMPs include indolicidin, a 13-amino acid peptide produced by bovine leukocytes that shows potent antimicrobial activity(Falla et al.,1996),as well as tritrpticin(Yang et al.,2002)and human salivary histatin(Du et al.,2017).
MECHANISM OF ACTION(MOA)OF AMPs

Figure 2 Structural diversity and helical wheel projections of representative AMPs
For AMPs, there is strong evidence suggesting a close correlation between their MOA and physical features or primary structure,which are characterized by a net positive charge and hydrophobicity(Figure 2)(Dathe&Wieprecht,1999;Matsuzaki,1999).The cationic charge influenced by amino acid residues such as Arg and lysine(Lys)enhances selectivity for negatively charged bacterial plasma membranes while exhibiting low electrostatic interactions with the uncharged outer layer of the eukaryotic cell membrane(Guilhelmelli et al.,2013).Current efforts at improving the selectivity for and electrostatic interaction of AMPs with bacterial membranes involve the substitution of amino acids with positively charged amino acid residues like Arg and Lys(Arias et al.,2018;Yeaman&Yount,2003).For example,Jin et al. (2016) reported that substitution of amino acids improved antimicrobial efficacy, increased stability, and reduced the hemolytic activity of the designed peptide ZY13 compared to its precursor peptide cathelicidin-BF15.
Previous studies have also indicated a strong correlation between the cationic charge of an AMP and its antimicrobial activity,with data showing improved antimicrobial activity of several AMPs against both bacterial and fungal pathogens paralleled with an increase in cationic charge(Gagnon et al.,2017;Hong et al.,2001;Lyu et al.,2016);however,increased hemolytic activity has been observed for several AMPs following an increase in net charge(Chen et al.,2005;Jiang et al., 2008). Secondly, hydrophobicity is an important parameter of all AMPs and is attributed to a substantial proportion(almost 50%)of hydrophobic residues in peptide sequences, such as leucine, valine, isoleucine, alanine,methionine, tyrosine, tryptophan, and phenylalanine.Hydrophobicity is crucial for peptide selectivity to biological membranes and increased hydrophobicity is correlated with increased hemolytic activity as highly hydrophobic AMPs have a stronger ability to penetrate the hydrophobic core of erythrocyte membranes (Chen et al., 2007; Tachi et al.,2002).
Understanding the MOA of AMPs is paramount for unlocking their full potential as next-generation antibiotics.Extensive research has been conducted,and is still ongoing,to unveil the MOA of AMPs(Guilhelmelli et al.,2013;Hancock&Lehrer,1998;Ulm et al.,2012).Based on their MOA,AMPs can be classified into those that kill through membrane disruptive mechanisms and non-membrane disruptive mechanisms,as illustrated in Figure 3.For the membrane disruptive killing action,AMPs produce microbicidal activity by targeting and disrupting the bacterial plasma-membrane structure,mostly through permeabilization,thus resulting in leakage of intracellular content (Huang et al., 2010). An important aspect to consider for this MOA is the concentration threshold of peptide molecules on the bacterial membrane surface for effective permeabilization via interaction of positively charged AMP residues with negatively charged moieties on the bacterial membrane surface (Chen et al.,2007;Shai,2002).To drive this membrane disruptive MOA,three models have been postulated,i.e.,barrel-stave,carpetlike,and toroidal pore models.For all models,the MOA begins with the accumulation and organization of AMP molecules parallel to the membrane surface,followed by electrostatic interaction between the AMPs'cationic charged residues and negatively charged phospholipids on the membrane surface(Falanga et al.,2016).
Typical for α-helical AMPs, the barrel-stave model constitutes the formation of hydrophilic pores on the hydrophobic core of the bacteria membrane structure,resulting in membrane disruption and leakage of extracellular content(Kumar et al.,2018).For the carpet-like mechanism,peptide molecules accumulate parallel to the membrane surface in a carpet-like fashion,followed by penetration into the membrane and disruption of the lipid bilayer(Brogden,2005;Falanga et al.,2016;Rodríguez-Vázquez et al.,2014).In contrast, toroidal pore models, e.g., magainin peptide,cause membrane disruption by perpendicular insertion into the lipid bilayer(Shai,2002;Wimley,2010).
Direct killing through non-membrane disruptive mechanisms involves targeting microbial processes or physiological functions other than the cell membrane,utilizing similar killing mechanisms as those by conventional antibiotics such as inhibition of cell wall,protein,and DNA synthesis and reduced enzymatic activity(Hancock&Rozek,2002;Hancock&Sahl,2006).Firstly,the AMP interacts with the plasma membrane before penetrating with or without membrane permeabilization(e.g., activity of buforin II on E. coli) and accumulating intracellularly,where it targets and acts on key processes such as transcription and translation, protein synthesis,enzymatic activity,and microbial death(Brogden,2005;Park et al.,1998).For example,human α-defensin 1,human αdefensin 5, human β-defensin 4, and indolicidin all have intracellular targets(Falla et al.,1996;Lehrer et al.,1989;Sharma&Nagaraj,2015).
In addition to direct microbial killing,AMPs are required for other immunomodulatory functions.They are secreted by a wide range of immune cells, including phagocytes,neutrophils, and macrophages, and are involved in the mitigation of infection such as controlled secretion of proinflammatory cytokines to prevent cytokine storm and tissue damage,recruitment and activation of immune cells,promotion of angiogenesis, and suppression of excessive reactive oxygen species release(Hancock et al.,2012;Hilchie et al.,2013;Nicole et al.,2012;Nijnik&Hancock,2009).For example, human cathelicidin LL-37 exhibits immunomodulation properties such as chemoattraction and activation of various immune cells such as monocytes,neutrophils,and mast cells by using formyl peptide receptorlike 1(De et al.,2000;Nijnik&Hancock,2009).A thorough understanding of the antimicrobial and immunomodulatory properties of AMPs provides excellent opportunities for the discovery and design of novel therapies for bacterial infection as well as inflammatory and cardiovascular diseases such as atherosclerosis and thrombosis(Bei et al.,2019;Zhang et al.,2015).
ACTIVITY OF AMPs AGAINST MDR NOSOCOMIAL BACTERIAL PATHOGENS
The rapid spread of antibiotic resistance presents a daunting clinical challenge due to the recalcitrance of pathogens to traditional antibiotics, especially among nosocomial pathogens. In both developing and developed countries,hospital-acquired infections, commonly referred to as nosocomial infections,are a growing concern often associated with prolonged hospital stay,increased mortality rates,and huge economic burden. Therefore, the discovery of novel alternative therapies is paramount.Several AMPs have been found to show potent microbicidal activity in the micromolar range against several bacteria strains,with a low likelihood for selection of resistance,therefore creating hope in the war against MDR pathogens(Deslouches&Di,2017;Loeffler et al.,2001;van't Hof et al.,2001).
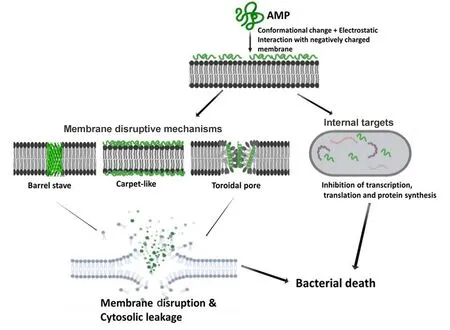
Figure 3 Schematic of membrane disruptive and non-membrane disruptive bacterial killing mechanisms of AMPs
Natural and synthetic peptides have proven to be effective in combating several pathogens,including MDR gram-positive and gram-negative bacteria (see Table 1), with continued effort aimed at designing peptides with improved therapeutic potential and reduced side effects(Chou et al.,2008;Liu et al., 2015). Such strategies have focused on improving efficacy, structural stability, and protection from proteolytic degradation, and include chemical modifications such as acetylation, enrichment with non-natural D-amino acids,substitution of amino acid residues, and use of delivery systems(Nordström&Malmsten,2017;Zhang et al.,2019;Zhao et al.,2016).

Table 1 Select antimicrobial peptides with potent activity against MDR pathogens
Cathelicidin AMPs,which are found in humans and other animals,are excellent candidates for therapeutic agents.They display multifunctional roles,including potent broad-spectrum antimicrobial activity against infectious agents such as fungi,viruses, and bacteria, and can trigger specific immune responses such as activation and recruitment of immune cells(Gennaro et al.,1989;Kościuczuk et al.,2012).The human cathelicidin LL-37, expressed in various tissues and circulating cells,is the most extensively studied peptide and exhibits potent bactericidal activity against bacterial strains and fungi(Björstad et al.,2009).Furthermore,it has been used as a template for the design of several synthetic peptides with improved antibiotic activity against nosocomial pathogens,biofilms,and persister cells(Dürr et al.,2006;Xhindoli et al.,2016).A notable LL-37 derivative(SAAP-148)designed by de Breij et al. (2018) through amino acid substitution of the C-terminal chain of LL-37 shows potent microbicidal activity(minimum inhibitory concentration(MIC)0.4 to 12.8 μm)against several ESKAPE pathogens(e.g.,E.faecium, S. aureus, K. pneumoniae, A. baumannii, P.aeruginosa,and Enterobacter species)without selection of resistance. This derivative can also eliminate biofilms and persister cells of S.aureus,A.baumannii and P.aeruginosa in the micromolar range.
Cathelicidins make up the bulk of naturally occurring AMPs in snake venom. Several snake venom-based AMPs have been established,which potently kill both fungal and bacterial pathogens(Jin et al.,2016;Wang et al.,2011;Zhang,2015).Zhao et al.(2018)identified a novel 34-amino acid residue cathelicidin(OH-CATH)from the king cobra,with its analog DOH-CATH30 found to exhibit potent microbicidal activity(MIC 1.56 to 12.5 μg/mL)against several gram-negative and grampositive bacteria, including MDR A. baumannii, MDR P.aeruginosa,MRSA,and E.coli,compared to nine commonly used antibiotics.Remarkably,bacteria-killing kinetics revealed that D-OH-CATH30 rapidly killed E. coli within 6 min and exhibited low hemolytic activity on red blood cells at high concentration.Furthermore,in vivo toxicity testing using mice revealed a high lethal dose,with no deaths observed at high concentrations of 160 mg/kg(Li et al.,2012;Zhao et al.,2018),thus highlighting their potential as excellent therapeutic candidates.
Cathelicidin-BF is a 30-amino acid residue cathelicidin isolated from the venom of Bungarus fasciatus and possesses potent antimicrobial activity, even against MDR clinical isolates(Wang et al.2008).Its designed and shortened 15-amino acid residue peptide cathelicidin-BF15 also shows strong efficacy against fungal species such as Candida albicans(Jin et al.,2016;Wang et al.,2008)and against several bacterial strains including MDR clinical isolates of E.coli, P. aeruginosa, and S. aureus through membrane permeabilization (Wang et al., 2008), with low hemolytic activity on red blood cells and therapeutic potential against acne vulgaris(Wang et al.,2011).
Several AMPs with potent antimicrobial properties have also been isolated from amphibians such as salamanders,frogs,and toads(Patocka et al.,2018;Liu et al.,2011;Xiao et al.,2011).Such peptides are viable targets for the development of therapeutic agents.For example,Qi et al.,(2019)identified two novel cathelicidin peptides,OL-CATH-1 and-2,from the frog Odorrana livida,which both show potent antimicrobial and anti-inflammatory activities.Wang et al.(2013)isolated five novel AMPs from Limnonectes kuhlii frog skin secretions,which exhibited potent antimicrobial activity against several gram-negative and gram-positive bacterial strains, but with low hemolytic activity on mammalian cells.
Other extensively studied cathelicidins include human-β defensin 3 (hBD-3), sheep myeloid peptide (AMP-29), rat cathelin-related antimicrobial peptide(rCRAMP),and bovine myeloid antimicrobial peptide 27 (BMAP-27), which demonstrate potent microbicidal activity against pathogenic strains such as E. coli, P. aeruginosa, MRSA, and A.baumannii, inhibition of biofilm formation, and immunomodulation activity(Dhople et al.,2006;Giacometti et al.,2004;Guo et al.,2018;Kościuczuk et al.,2012;Kurosaka et al.,2005).
Besides cathelicidins,other AMPs with diverse structural scaffolds and properties have been reported.Indolicidin,a natural cationic peptide from bovine neutrophils,also exhibits potent bactericidal activity through membrane permeabilization against gram-negative and gram-positive nosocomial pathogens such as E.coli,P.aeruginosa,and S.aureus(Falla et al.,1996).Several synthesized derivates of indolicidin have shown improved antimicrobial activities against a wide panel of MDR nosocomial pathogens with low MIC values,e.g.,RN7-IN10 and RN7-IN9(Jindal et al.,2015).Omiganan,a novel peptide currently under phase III clinical trials as a therapeutic agent for bacterial-caused acne and catheter-associated bloodstream infection(Melo&Castanho,2007), also exhibits potent broad-spectrum antimicrobial activity(Sader et al.,2004).
Also currently under preclinical trial, Ci-MAM-A24, a synthetic peptide derivative of a peptide precursor isolated from Ciona intestinalis, exhibits potent bactericidal activity through membrane permeabilization at low concentration(MIC<10 μg/mL)against MDR nosocomial pathogens,including MRSA, VRE, MDR P. aeruginosa, ESBL-producing E. coli(Fedders et al.,2010).Thus,it is an important candidate as a therapeutic agent against MDR nosocomial pathogens.The pexiganan peptide,a synthetic 22-amino acid residue analog of magainin isolated from the African clawed frog Xenopus laevis(Ge et al.,1999;Zasloff,1987),also exhibits potent broad-spectrum bactericidal activity against several pathogens at low concentration.It is currently in phase III clinical trials as an agent for diabetic foot ulcers caused by bacterial infections and can rapidly kill pathogens such as P.aeruginosa and several other gram-positive and gramnegative bacteria,with a low tendency to induce resistance selection(Ge et al.,1999;Mahlapuu et al.,2016).However,several reports demonstrate selection of resistance to pexiganan following long-term laboratory exposure of pathogens(Habets&Brockhurst,2012;Perron et al.,2006).
Thanatin is another potential therapeutic candidate for MDR pathogens. It is a 21-amino acid residue peptide and its analog,S-thanatin,exhibits low hemolytic activity and potent antimicrobial activity against several strains of gram-negative and gram-positive bacteria(Wu et al.,2011).Furthermore,with improved therapeutic activity,S-thanatin shows potent activity against nosocomial pathogens K.pneumoniae and E.coli at very low concentrations,and can alleviate sepsis in mice models, thus making it an excellent therapeutic candidate(Ding et al.,2009;Wu et al.,2013).
Colistin is an important AMP used as a last-resort drug for the treatment of MDR pathogen infections.Recent reports of selection of resistance to colistin thus present a concerning global healthcare predicament in the fight against MDR bacterial infections(Falagas et al.,2005;MacNair et al.,2018;Yamamoto et al.,2018).Remarkably,two novel AMPs(AA139 and SET-M33)with similar MOA as colistin are currently in development and have shown excellent therapeutic potential both in vitro against several MDR gram-pathogens and in-vivo in animal disease models(van der Weide et al.,2019).AA139 is a 21-amino acid amphipathic peptide with reduced cytotoxic and hemolytic activity. It was designed from arenicin-3, a peptide with potent microbicidal activity against several MDR bacterial strains and isolated from the marine lugworm Arenicola marina(Sierra et al.,2017;Wang et al.,2018).SETM33 is a synthetic AMP exhibiting potent microbicidal activity against gram-negative bacteria through membrane disruption and an excellent candidate for in vivo application, as evidenced from animal models of infectious diseases having both anti-inflammatory and immunomodulatory activities(Brunetti et al.,2016b;Falciani et al.,2012;van der Weide et al.,2017).
Several AMPs with potent activity against gram-positive and gram-negative MDR and showing excellent prospects as therapeutic agents have also been isolated and characterized from marine animals(Destoumieux-Garzón et al.,2016;Tincu&Taylor.,2004).For example,mytimacin-AF,an 80-cysteinerich amino acid residue isolated from marine mollusks,shows potent activity against both gram-positive and gram-negative bacteria,especially against nosocomial pathogens S.aureus and K.pneumoniae(MIC<2 μg/mL)(Zhong et al.,2013).EChepcidin3,cloned from marine fish Epinephelus coioides,is a novel cysteine-rich AMP with rapid and potent microbicidal activity against S.aureus and Pseudomonasstutzeri(MIC<3 μmol/L)(Qu et al.,2013).Tachyplesins,a class of peptides isolated from horseshoe crab hemocytes, are cationic βhairpin structured peptides with potent antimicrobial activities against MDR gram-negative and gram-positive bacteria at low concentrations (Falanga et al., 2016; Liu et al., 2018;Nakamura et al.,1988),although with increasing hemolytic and cytotoxic activity at higher levels(Edwards et al.,2017;Liu et al.,2018;Ohta et al.,1992).
Continued research on AMPs has yielded a new class of highly potent peptides called "Selectively Targeted AMPs"(STAMPs),which demonstrate increased sensitivity to target pathogens without harming normal flora and increased bacterial killing potency and kinetics (Chung & Khanum,2017).Such AMPs have been designed to target pathogens through specific determinants followed by selective killing(Sarma et al., 2018). Notable STAMPs include synthetic peptide M8(KH)-20,a multi-headed peptide specifically targeting and potently killing P.aeruginosa and Streptococcus mutans in vitro with very little effect on other pathogens(He et al.,2009).Oritavancin,another synthetic STAMP designed from the naturally occurring glycopeptide chloroeremoycin and currently in clinical development, is reported to be highly selective and potently kills MDR pathogens such as methicillinresistant S. aureus and vancomycin-resistant S. aureus(VRSA) more rapidly than vancomycin through membrane permeabilization and inhibition of cell wall synthesis(Allen&Nicas,2003;Chung&Khanum,2017).
CHALLENGES FACING EXPLOITATION OF AMPs AS THERAPEUTIC AGENTS AND SOLUTION STRATEGIES
To be considered for approval as a therapeutic agent,effective AMPs need to show broad-spectrum activity,high selectivity for bacterial pathogens,and low cytotoxicity on mammalian cells(Dathe&Wieprecht,1999).Currently,various AMPs are under clinical trial;however,several factors curtail their full utilization and approval as antibiotic alternatives(Falanga et al.,2016;Lombardi et al.,2019;Wang et al.,2016).The main factor limiting the systemic application of AMPs is their sensitivity to proteolytic digestion. For instance, host proteolytic enzymes in intestinal mucosa, gastrointestinal tract, and blood plasma can readily degrade antimicrobial peptides,which negatively impacts their in vivo stability and pharmacokinetics(Moncla et al.,2011;Starr&Wimley,2017).Therefore, as a result, many AMPs are limited to topical application rather than oral or intravenous administration.Moreover,studies have demonstrated that,as a defensive mechanism in the presence of AMPs,certain bacteria will upregulate the secretion of proteolytic enzymes and metalloproteases (MMPs) that can partially or completely degrade AMPs (Lai et al., 2007; Sieprawska-Lupa et al.,2004).
Toxicity and efficacy of AMPs is another major drawback of their use.Several AMPs have adverse side effects in vivo,which limits their use to topical application only(McPhee&Hancock,2005).Moreover,in addition to the low correlation between in vitro-and in vivo-based results in some cases,physiological conditions such as high salt concentration and serum components can negatively affect the antimicrobial activity of many AMPs(Cantisani et al.,2014;de Breij et al.,2018;Han et al.,2016).For most AMPs,antimicrobial activity is dependent on the electrostatic interactions between positively charged peptides and negatively charged plasma membranes(Guilhelmelli et al.,2013).Such interactions can be affected by elevated salt levels or binding of AMPs to serum components (e.g., lipoproteins) (Li et al., 2017).Therefore, efficacy screening of AMPs under different physiological conditions and in the presence of host components is necessary to affirm their activity.
Compared to conventional antibiotics, AMPs are also expensive to produce.Therefore, current efforts are being directed at designing shorter peptides with reduced side effects and improved stability under different physiological conditions(Kim et al.,2013;Zhao et al.,2015).For example,Li et al.(2017)pioneered an important strategy for designing AMPs with trypsin inhibitor activity based on a peptide isolated from the frog Odorrana grahami.
Although AMPs have a low likelihood to select for resistance,reports already exist detailing the development of resistance against some AMPs (Andersson et al., 2016;Omardien et al., 2016); for example, the development of resistance to colistin by A. baumannii following long-term clinical application(Jeannot et al.,2017;Liu et al.,2016).Colistin is a last-resort drug used for the treatment of MDR nosocomial pathogens,and thus resistance to colistin is an important clinical issue(Liu et al.,2016;MacNair et al.,2018;Paterson&Harris.,2016).Several mechanisms have been reported to be responsible for resistance to AMPs,including expression of efflux pumps,surface charge modification to impede membrane-peptide electrostatic interactions, and increased secretion of proteolytic enzymes(Andersson et al.,2016; Bechinger & Gorr, 2017; Morita et al., 2012). For example,S.aureus can alter surface charge by adding basic amino groups from D-alanine from the cytoplasm to teichoic acid on the membrane surface (Peschel et al., 1999).Resistance to AMPs also raises other critical issues such as the potential development of cross-resistance to antibiotic regimens and host AMPs(Conlon,2015;Kubicek-Sutherland et al.,2017),which could create unprecedented detrimental consequences.
Presently,studies are focusing on developing strategies to improve the efficacy of AMPs in vivo,enhance selectivity for microbial cells while reducing cytotoxicity,increase stability,and minimize proteolytic degradation.Chemical modifications such as the addition of D-amino acids, cyclization, or acetylation are important strategies used to improve the stability of AMPs and reduce sensitivity to proteolytic degradation(Gao et al.,2018;Zhao et al.,2016).However,additional modifications add to production costs.To solve this,efforts are currently being directed at the design and synthesis of shorter peptides with increased potency and stability and reduced toxicity(Jin et al.,2016;Kim et al.,2013).
The use of delivery systems is another important strategy for improving the stability and efficacy of AMPs(Nordström&Malmsten, 2017). Of particular interest is the use of nanocarriers,which are designed and covalently attached to AMPs to prevent self-aggregation,improve chemical stability,and release profiles of AMPs to target sites.Importantly,such nanocarriers are synthesized from materials that are easily biodegradable, including lipids (such as triglycerides and cholesterol)and polymers such as gels,cellulose,and fiber(D'angelo et al.,2015;Mahlapuu et al.,2016;Yüksel et al.,2016).
FUTURE PROSPECTS AND CONCLUSIONS
In this review, we reiterated the importance of AMPs as potential next-generation antibiotics to mitigate a wide array of microbial infections,including those caused by MDR strains.Natural and synthetic AMPs are unique and exhibit boundless therapeutic potential. Remarkably, AMPs display diverse MOA,with a low tendency to select for resistance,rapid killing ability, and multifunctional activities, thus conferring great advantages over empirical antibiotics.Extensive research on the origin,structure,and biological properties has improved our understanding and use of AMPs.Of particular importance is the potential of these peptides to act as templates and precursor molecules for the development of novel anticancer and antimicrobial agents for bacterial, fungal, and viral infections where most available therapies have been rendered ineffective due to the rapid emergence and spread of resistance. Furthermore, these studies allow for the generation of models mimicking the interaction of these peptides with biological membranes,a key element in their microbicidal activity.Such insight is important in the design and synthesis of AMPs with increased potency against MDR strains and improved chemical stability.
Presently,substantial effort is being dedicated to the design and synthesis of shorter peptides with improved therapeutic activity and reduced cytotoxicity.Such efforts are bearing fruit,as evidenced in the presence of short synthetic AMPs with potent and rapid microbicidal activity against several MDR pathogens, even those showing resistance to last-resort drugs, thus presenting hope in the war against multidrug resistance.
COMPETING INTERESTS
The authors declare that they have no competing interests.
AUTHORS'CONTRIBUTIONS
J.M.,X.H.,R.L.,and Y.Z.Z.conceived the review and prepared the manuscript.All authors contributed to the discussions.All authors read and approved the final version of the manuscript.
杂志排行
Zoological Research的其它文章
- From our roots,we grow Celebrating the 60th anniversary of the Kunming Institute of Zoology,Chinese Academy of Sciences
- Genomic insights into ruminant evolution:from past to future prospects
- Chromosomal level assembly and population sequencing of the Chinese tree shrew genome
- Superior intestinal integrity and limited microbial translocation are associated with lower immune activation in SIVmac239-infected northern pig-tailed macaques(Macaca leonina)
- Conserved sequences identify the closest living relatives of primates
- Systematic relationships of Chinese freshwater semisulcospirids(Gastropoda,Cerithioidea)revealed by mitochondrial sequences
