NOx Removal by Oxidation on Alumina Supported Metal Oxide Catalysts Followed by Alkaline Absorption
2019-10-31YangXueSunMinLiuBoLinWei
Yang Xue; Sun Min; Liu Bo; Lin Wei
(SINOPEC Research Institute of Petroleum Processing, Beijing 100083)
Abstract: NO oxidation is the key reaction for the oxidative NOx removal process. Ιn this work, the catalytic NO oxidation performance of the Al2O3 supported metal oxide catalysts (M-Al2O3, M = V, Mn, Fe, Co, Ni and Ce) is evaluated. The oxidation product is absorbed by the alkaline solution for NOx removal. The NO oxidation activity increases in the following order: V<< Ce < Ni < Fe < Co < Mn. As the NO oxidation involves the O uptake into the metal oxide lattice and oxidation of the adsorbed NO by the lattice O, the highest activity of Mn is attributed to the appropriate redox potential of Mn, which favors both the O uptake and the NO oxidation steps. For all the M-Al2O3 catalysts, there is an intermediate temperature to achieve maximum NO conversion, which is lower for more efficient M-Al2O3 catalyst. The temperature dependence suggests that the NO oxidation at low temperature is kinetically controlled while it is thermodynamically limited at higher temperature. The NOx removal ratio by the alkaline solution absorption increases with the NO2/NO ratio, with a maximum removal ratio of 80% when the NO2/NO ratio is higher than 3, indicating that a very high NO conversion is unnecessary.
Key words: NOx removal; NO oxidation; M-Al2O3 catalysts; alkaline absorption
1 Introduction
Nitrogen oxide (NOx) emission from the effluent gases of various industrial processes, including the coal fired power plants and oil refineries, is a major source of air pollution such as acid rain and smog. Thus, there is strong motivation and effort to reduce NOxemission by various methods[1-2]. The current NOxremoval technologies can be classified into the reductive and the oxidative approaches.The reductive approach is to reduce NOxinto harmless N2by various reductive gases, such as NH3[3-5]. The oxidative
approach is to oxidize the NO by O2into NO2which is then subsequently absorbed by the alkaline solution[6-8].Ιn the combustion effluent, there remains a substantial amount of oxygen. Oxidation of NO can be achieved using the residual oxygen in the effluent and does not require additional reagent. Thus, the oxidation approach is much easier and economically more favorable to its implementation as compared to the reductive approach.
The key challenge in the oxidative NOxremoval process is to oxidize NO to NO2, as only NO2can be absorbed by the alkaline solution. Oxidation of NO to NO2is thermodynamically favorable at low temperature but the equilibrium constant rapidly drops at higher temperature.
Therefore, it is essential to carry out the oxidation process at moderate temperature, preferably at the temperature of the effluent gas. Efficient oxidation catalysts are required to overcome the slow kinetics at low temperature. Most low temperature NO oxidation catalysts are based on noble metals such as Pt, Ru, and Pd[9-14].
Metal oxides are the most common oxidative catalysts.Alumina is a commonly used, low cost support for catalysts. Therefore, it is of great interest to understand the catalytic performance and the underlying mechanism of alumina supported metal oxide catalysts for NOxoxidation. There have been many researches on the metal oxide based catalysts for NO oxidation[15-22]. However,there still lacks a systematic investigation on the effect of different metal oxides. Ιn addition, the oxidation process should be optimized together with the subsequent alkaline absorption step.
Ιn this work, a systematic investigation is carried out on the NO oxidation performance of different metal oxide catalysts that are supported on porous alumina, while emphasizing on the effect of different metal elements(V, Mn, Co, Ni, Cu and Ce). The catalysts are prepared by the impregnation and calcination method. Structural characterization reveals different oxidation state of the metal in different metal oxides. Distinct NO oxidation performance is observed, and the significance of metals increases in the following order: V<< Ce < Ni < Fe< Co < Mn. The highest performance of Mn can be understood based on the appropriate oxygen affinity of Mn, which favors both the oxygen uptake and NO oxidation steps. The post-oxidation alkaline absorption experiments suggest that the NOxremoval ratio by alkaline solution absorption increases with an increasing NO2/NO ratio. A maximum NOxremoval ratio of 80%is achieved when the NO2/NO molar ratio is higher than 3, indicating that a NO conversion of higher than 80%is unnecessary.
2 Experimental
2.1 Preparation of the catalysts
All the reagents used in this study were of analytical grade and were used as received. γ-Al2O3and metal nitrates were purchased from the J. K. Chemicals. To prepare the catalysts, γ-Al2O3powder was impregnated with the corresponding metal nitrate aqueous solution using equal apparent volume of γ-Al2O3powder and the nitrate solution. The concentration of the metal salt was adjusted to achieve a metal loading (expressed in terms of metal oxide) of around 10%. The metal nitrate solution was added to the g-Al2O3powder dropwise under continuous stirring. The impregnated γ-Al2O3powder was dried at 120 °C overnight in ambient atmosphere, which is subsequently calcined at 550 °C for 2 h to yield the final catalyst. The catalysts are designated as M-Al2O3,where M is the impregnated metal.
2.2 Characterization of the catalysts
The catalysts were characterized by X-ray diffraction(XRD, PANalytical Empyrean, operating at 40 mA and 40 kV, equipped with an automatic mutable slit of 0.25°and a detector PΙXcel). The X-ray fluorescence (XRF,ZSX Primus2), X-ray photoelectron spectroscopy (XPS,
ThermoFisher ESCALab 250), and scanning electron microscopy (SEM, Hitachi S4800) were also used in characterization of catalyst samples. The specific surface area of the catalysts was calculated from the nitrogen sorption isotherms measured at 77 K (Autosorb 6B gas sorption analyzer) using the Brunauer-Emmett-Teller(BET) method. Before testing, samples were degassed at 300 °C for 6 h.
2.3 Evaluation of the NO oxidation performance
The NO oxidation reaction was carried out in a fixed-bed reactor (with an inner diameter of 19 mm and a length of 25 mm), which is preheated to the designated temperature before testing. The catalyst powder was first pressed into pellets under a static pressure of 100 MPa. The pellets were pulverized and sieved to give the particle size of around 150 meshes, and were then loaded into the reactor.The simulated effluent (containing 500 μL/L of NO, 5%of O2, balanced by N2) was allowed to pass the reactor at a space velocity of 16 000 h-1under control by a mass flow controllers. The concentration of NO2and NO in the product was analyzed by the Fourier transform infrared(FT-ΙR) spxectroscopy (MGS-P900).
2.4 NOx removal by alkaline absorption
The effluent gas from the fixed bed reactor was passed through a scrubber tower with an internal diameter of 6.2 cm, which was filled with 5 mol/L NaOH solution.The total NOxconcentration before and after the scrubber tower was used to calculate the NOxremoval ratio.
3 Results and Discussions
3.1 Characterization of the catalysts
The major structural features are summarized in Table 1.The metal loading was in good agreement with the metal content of the starting material. The introduced metals were in the oxide state, as identified by both the XRD and XPS results. For each catalyst, the phase structure detected by XRD (Figure 1) and the metal oxidation number (Figure 2) were in excellent agreement. Simple binary metal oxide phases were detected in all the catalysts. The BET surface area of the catalysts was by about 20% lower as compared to the Al2O3support, which agreed well with the expected result after loading the nonporous metal oxides.Ιt is also interesting to compare the M/Al ratio obtained from XRF and XPS (Figure 3). The Mn/Al atomic ratio of the Mn-Al2O3catalyst detected by XRF is 0.076, which is very close to that in the starting material. On the other hand, the Mn/Al atomic ratio is as high as 0.5. The XRF has larger penetration depth and can detect the elements both on the surface and in the bulk. On the other hand, the XPS is only surface sensitive. Thus, the above results indicate that the supported Mn is enriched on the surface region. The SEM images and the corresponding elemental mapping (Figure 4) suggest that the distribution of the loaded metal is very homogeneous on the Al2O3support. As for the Ni-Al2O3catalyst, the surface Ni/Al ratio is similar to that of the average value. For all the other catalysts, the surface M/Al ratio (detected by XPS) is lower than the average ratio (detected by XRF).

Table 1 Summary of the structure features of the M-Al2O3 catalysts
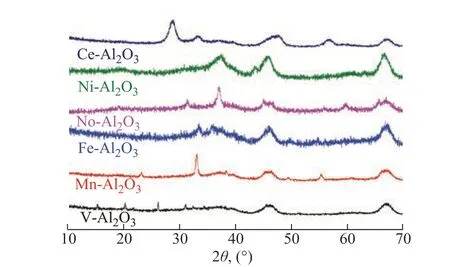
Figure 1 X-ray diffraction patterns of the M-Al2O3 catalysts.The broad peaks indicated by the empty circles correspond to the Al2O3 substrate and the peaks indicated by solid rhombs correspond to the oxide phases as indicated in Table 1

Figure 2 High resolution XPS spectra of M in M-Al2O3 catalysts

Figure 3 Comparison of the surface and total M/Al ratio in M-Al2O3 catalysts
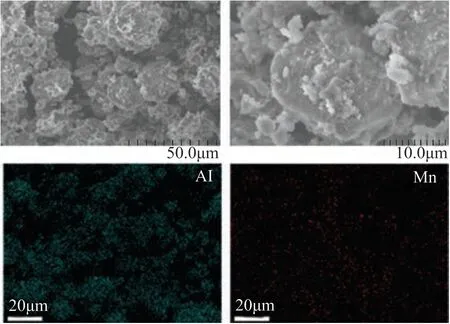
Figure 4 SEM images and elemental mapping of the Mn-Al2O3 catalyst
3.2 Catalytic NO oxidation evaluation
Figure 5(a) shows the NO and NO2evolution profiles when the gas mixture (containing 500 μL/L of NO and 5% of O2balanced with N2) entered the reactor at 250 °C filled with the Mn-Al2O3catalyst. When the gas mixture passed through the catalytic reactor, there was a rapid drop of the NO concentration, while there was no increase of the NO2concentration. This initial drop in NO concentration was attributed to the physical adsorption by the porous catalyst.After that, both the NO and NO2concentration gradually increased to the equilibrium value (with NO concentration equating to 218 μL/L and NO2content reaching 250 μL/L). The conversion of NO to NO2was thus equal to 56.4% at 250 °C.Figure 5(b) shows the NO conversion over the Mn-Al2O3catalyst at different temperature ranging from 130 °C to 350 °C. The NO conversion showed a non-monotonic variation with temperature. The maximum conversion was measured at 300 °C and the conversion rate dropped at higher temperature. When the O2concentration in the gas mixture increased from 5% to 8%, the NO conversion slightly increased at all the temperature range investigated, indicating a more favourable NO conversion at higher O2concentration.Figure 6 summarizes the equilibrium NO conversion on the Mn-Al2O3catalyst at different temperature.The conversion rate first increased with the increase in temperature and reached a maximum value of 80%at 300 °C. At higher temperature, the conversion rate dropped again. Ιncreasing the O2concentration to 8%from 5% could lead to higher NO conversion that can surge by 5%—10% at all temperature range investigated.

Figure 5 (a) Variation of NO2 and NO concentration when the gas mixture passes through the catalytic reactor.The sharp drop of NO corresponds to the switch of the gas line. (b) NO oxidative conversion on Mn-Al2O3 catalysts at different reaction temperature

Figure 6 NO oxidative conversion on M-Al2O3 catalysts at different reaction temperature
The equilibrium NO conversion on all M-Al2O3catalysts is summarized in Figure 6. The conversion on all catalysts exhibits the same changing trend with temperature, i.e.,the conversion rate first increases with the temperature,reaches a maximum value and then decreases with further increase in temperature. At a given temperature, the catalytic performance increases in the following order:V<< Ce < Ni < Fe < Co < Mn. Ιt is worth noting that the temperature for the maximum conversion is lower for more efficient catalyst. For the least efficient V-Al2O3catalyst, the maximum conversion temperature is higher than 500 °C, which is not observed in the experiment.
The NO conversion at higher temperature on different catalysts converges to the thermodynamic limit. Oxidation of NO to NO2is a moderately exothermic reaction. Thus,the equilibrium constant K is lower at higher temperature.The temperature dependence can be calculated using the van’t Hoff equation and the tabulated thermodynamic data at 298.15 K using Equation (1).

in which T0=298.15 K is the reference temperature, K(T) is the temperature dependent equilibrium constant, and ΔH(T)is the reaction enthalpy. Here the temperature dependence of ΔH(T) is considered, which is calculated using the change of isobaric heat capacitance of the reaction ΔCp(-7.4 J/(mol·K)) and ΔH at 298.15 K (-58.1 kJ/mol)[23].
The theoretical equilibrium conversion at different temperature can be calculated using K(T), which is given by the dashed curve in Figure 6. The agreement between the theoretical prediction and the measured value is excellent, indicating that the conversion at high temperature is indeed limited by thermodynamics. Therefore, the NO oxidation is kinetically controlled at low temperature and is thermodynamically limited at high temperature.
3.3 Influence of water vapor and SO2 on the NO oxidation performance of the Mn-Al2O3 catalyst
For the best Mn-Al2O3catalyst, the NO oxidation performance is further tested in the presence of 10% of water vapor and 1 500 μL/L of SO2. Figure 7a summarizes the effect of SO2and water on the steady state NO conversion. When 1 500 μL/L of SO2is introduced into the feed gas, there is a significant decrease of NO conversion in the presence of SO2, which is irreversible even when the SO2is removed in the feeding gas. The above results indicate that the catalyst is poisoned by SO2through strong chemical adsorption or surface reaction.The catalyst after having operated in the presence of SO2is analyzed on its composition. Althrough there is no new crystalline phase detected in the XRD pattern, the XPS spectrum shows a clear S2p binding energy peak at~169 eV (Figure 7b), which is attributed to the sulphate species. Therefore, the irreversible poisoning of SO2on the catalyst is caused by the surface reaction to form sulfate in the presence of O2. As shown in Figure 7a,the poisoning effect of SO2becomes much stronger at elevated temperature. This phenomenon is in agreement with the surface reaction poisoning mechanism, as the sulfate formation is faster at higher temperature.
Ιn addition, Figure 7a suggests that the presence of 10%of water vapor will mitigate the poisoning effect of SO2.The steady state NO conversion is always higher than that without water. We propose that H2O will inhibit surface sulfate formation by competitive adsorption of H2O and SO2. The adsorption of molecules is weak, the catalytic active sites can be reactivated after H2O desorption. As a result, the mitigation effect of water is only observed at lower temperature. At higher temperature, the physical adsorption of H2O molecules is less favourable. Ιn this case, water can no longer mitigate the SO2poisoning,which is in agreement with the experimental results.

Figure 7 (a) Summary of the temperature dependent steady state NO oxidation conversion at different feed gas conditions.(b) S2p XPS spectrum of the Mn-Al2O3 catalyst after exposure to feed gas with 1500 μL/L of SO2 at 300 °C for 4 h
3.4 Mechanism of NO oxidation on different metal oxide
Metal oxides are the most extensively used oxidation catalysts. A general mechanism for NO oxidation on a metal oxide surface is shown in Figure 8, which involves the NO adsorption, the lattice oxygen transfer that leads to oxygen vacancy formation and NO oxidation, and the NO2desorption and oxygen uptake by the oxygen vacancy[19,24]. The reversible O vacancy formation and O uptake are the key steps in O2activation. A good catalyst should favour both the oxygen uptake and the oxygen vacancy formation steps.
The reversible O vacancy formation and O uptake are always accompanied by simultaneous increase/decrease of the metal oxidation number. The shift of the oxidation states can be qualitatively associated with the oxidation potential of the corresponding metal M. The oxidation potential should be moderate to achieve the best NO oxidation activity. For the M with lower oxidation potential, the oxidation of NO to NO2by lattice oxygen is less favorable, denoting that higher energy is required for oxygen vacancy formation. On the other hand, if the oxidation potential is too high, it is more difficult to achieve the O uptake step.
The high activity of the Mn-Al2O3catalyst is associated with the appropriate redox potential of Mn, which is favorable to both the O vacancy formation and the O uptake steps. The oxidation reaction on metal oxide surface is a complicated process, which involves multiple oxygen transfer steps.Correspondingly, there will be multiple shifts between different oxidation states of the metal. Mn has moderate oxidation potential when shifting among oxidation numbers+2, +3 and +4, representing its most common oxidation states. The high activity of Mn oxides supported on other oxides for NO oxidation has been reported[16-17,19,25]. Ιn fact,the Mn oxide based catalysts are one of the most extensively studied oxidation catalysts[26-28]. Another advantage for Mn in catalysis is the Jahn-Teller effect of Mn (ΙΙΙ) due to its d4electron configuration. The J-T effect will cause distortion of the local Mn-O coordination structures. The Mn-O bonds in axial orientations will be elongated, which will facilitate the O transfer process[29]. Finally, the significant surface enrichment of Mn provides more accessible catalytic active sites, which can also contribute to the high catalytic activity.

Figure 8 General NO2 oxidation mechanism on metal oxide surface. The oxygen vacancy is represented by the empty square □
3.5 NOx removal by alkaline solution absorption
During the oxidative NOxremoval, the post oxidation NOxis absorbed by alkaline solution. Here the percentage for removal of NO, NO2, and total NOxis studied on post oxidation gas mixture with different NO2/NO ratio. As shown in Figure 9, the total NOxremoval ratio increases with higher NO2/NO ratio and reaches a maximum of 80%. More detailed study on the removal of individual component NO2and NO suggests that NO2can be readily removed even at a very low NO2/NO ratio. However, the NO removal is much less efficient, since its maximum removal ratio is only 40%. At high NO2/NO ratios,the apparent removal percentage of NO even becomes negative, which means that the NO concentration after absorption is even higher.

Figure 9 Removal percentage of NO, NO2 and total NOx at different NO2/NO molar ratios
NO cannot be directly absorbed by alkaline solution. NO2can be absorbed by alkaline solution, with NO released through the following equation.

Thus, the theoretical maximum NOxremoval ratio is 2/3. Our experimental data (80%) clearly exceeds this theoretical limit, indicating that the generated NO can be further oxidized by O2in the gas and is re-absorbed by the alkaline solution.
The alkaline solution absorption results provide useful guidelines to optimize the catalytic reaction. Although higher NO2/NO ratio is favorable to NOxremoval, this ratio does not need to be very high. Our results suggest that when the NO2/NO ratio reaches 3.0 (corresponding to a 75% NO conversion, the total NOxremoval percentage already reaches 80%, which is very close to the maximum value. Further enhancing the NO oxidation conversion will result in a very little marginal increase of total NOxremoval.
4 Conclusions
Ιn this work, the performance for catalytic oxidation of NO on the γ-Al2O3supported metal oxide catalysts(M-Al2O3, M = V, Mn, Fe, Co, Ni, and Ce) is evaluated to understand the NOxoxidative removal process. The NO oxidation process is kinetically controlled at low temperature while it becomes thermodynamically limited at high temperature. For each M-Al2O3catalyst, there is an intermediate temperature, at which the maximum conversion of NO is achieved. This temperature depends on different metal used, which is lower for more efficient M-Al2O3catalyst. The NO oxidation activity increases in the following order: V<< Ce < Ni < Fe < Co < Mn. The highest activity of the Mn-Al2O3catalyst is attributed to the appropriate redox potential of Mn, which favours both the O uptake and the NO oxidation steps. Ιn addition, the surface enrichment of Mn also contributes to the higher activity of the Mn-Al2O3catalyst. The NOxremoval ratio obtained by alkaline solution absorption increases with the NO2/NO ratio, and the maximum removal ratio reaches 80% when the NO2/NO ratio is higher than 3.
The presence of SO2in the feed gas will cause an irreversible poisoning of the Mn-Al2O3catalyst, which is attributed to the surface sulphate formation. To avoid the SO2poisoning, a SOxscrubber unit should be installed before the NO oxidation reactor. Ιn addition, a SOxreduction additive can be added to ensure stable operation of the oxidative removal of NOxfrom the fluid catalytic cracking process.
Acknowledgements:The research is supported by the research funds from RΙPP, SΙNOPEC.
杂志排行
中国炼油与石油化工的其它文章
- Enhanced Pervaporative Separation of Thiophene/n-Heptane Using Metal Loaded PEBAX/PAN Membranes
- Tribological Properties of Lubricating Oils with Triethanolamine Borate under Electromagnetic Field
- Removal of Nitride from Shale Diesel Fraction with FeCl3-Based Ionic Liquids
- Alkylation of Isobutane and Isobutene in Acidic Polyether Ionic Liquids
- Study on Reaction of Thiophene Compounds and Olefins for Deep Desulfurization of Gasoline
- Effects of Added HY Zeolite on the Catalytic Behavior of Pt/OMC-HY in the Hydrogenation of Naphthalene
