Clinical progress of cell therapy in treating age-related macular degeneration
2019-10-14LingDiaoPanFengFang
Ling Diao , Pan-Feng Fang
1 Institute of Burn Research, Wuxi Third People's Hospital, Wuxi, Jiangsu Province, China
2 Eyecure Therapeutics Inc.(Jiangsu), Wuxi, Jiangsu Province, China
Abstract
Key words: age-related macular degeneration; AMD; ARMD; cell therapy; retinal pigment epithelium; RPE; iPSC-RPE cells; geographic atrophy
INTRODUCTION
The dysfunction of retinal pigment epithelium (RPE) cells is the leading cause of the dysfunction of photoreceptor cells.1,2Therefore, photoreceptor cells physiologically declining in the response of the light during the progress of age-related macular degeneration (AMD).There are two types of AMD, dry AMD,and wet AMD.The dry AMD develops small drusen under the macula in the early stage and leads to geographic atrophy in the late stage.3While the wet AMD (also called neovascular AMD) is involved in the abnormal penetration of new blood vessels into the retinal area, and the vision loss of wet AMD can be abrupt because of leakage of blood into the retina.The abnormal growth of the blood vessels can be blocked by antagonists of vascular endothelial growth factor; therefore,wet AMD can be treated with medicines targeted to vascular endothelial growth factor.4However, there is no cure for dry AMD.Several chemical compounds and few antibodies, which target the complement immune system, are under investigation.No clinical results support the notion that those candidates can reverse the deterioration of vision loss measured by Early Treatment Diabetic Retinopathy Study test.5,6Cell therapy is considered to be a more effective treatment for AMD.Both non-RPE and RPE cells have been tested clinically.The non-RPE cells, including mesenchymal stem cells or iris pigment epithelial cells, could not fully complement the physiological function of RPE cells and some treatments were even reported to cause serious medical malpractice.7Therefore, RPE cell therapy has gradually been accepted as the ideal solution in the treatment of AMD.8There are mainly three types of RPE cells derived from different sources: (1) RPE cells differentiated from human embryonic stem cells (hESC); (2) RPE cells differentiated from induced pluripotent stem cells (iPSC); (3)allogeneic primary RPE cells amplified from fetal or adult eye tissue.We will not emphasize the adult RPE cell therapy due to limitations of the clinical research data.9-11More attention will be drawn to the progress of clinical trials and drug discovery for remaining types of RPE cells.The future direction for cell therapy of AMD treatment will also be discussed.
SEARCH STRATEGY AND SELECTION CRITERIA
A search strategy was developed and undertaken by one reviewer (PFF), supported by the second reviewer (LD).The following databases were searched: The National Center for Biotechnology Information (PubMed).Common keywords relating to AMD and cell therapy were derived from the literature, and refined following search in the database; this created a final set of terms for age-related macular degeneration, (AMD, ARMD, dry AMD, dry-AMD, geographic atrophy) and cell therapy (cell-therapy, cell transplantation).Papers about wet AMD (neovascular AMD) are excluded.We restricted our search studies in English, relating to humans,published after January 1, 1980.Searches were run from August 1, 2016 to May 10, 2019.The full electronic searches are shown in Table 1.
POLICIES INTRODUCTION
The RPE cells are categorized as human cellular and tissue-based product12in the US.Those are regulated under human cellular and tissue-based product regulations (2012) issued by the “US Food and Drug Administration (FDA)” including regulation 21CFR, 1271, 600S, 312, 210 and 211.The US FDA has two different paths for cell therapies according to the product’s “relative risk.” These pathways are commonly referred to as section 361 and section 351.RPE cells are considered as a “361 products” and under more stringent regulations than “351 products.”13Similarly, “China Food and Drug Administration (CFDA)” requires cell therapy products to be registered as new drug according to “Measures for The Administration of Drug Registration (for Trial Implementation, 2017)”; this regulation is further refined by the “Guiding Principles for the Research and Evaluation of Cell Therapy Products (for trial implementation, 2017)” and “Stem Cell Preparations Quality Control and Preclinical Research Guidelines.”14-16In Europe, the cell-based product is one of the advanced therapy medicinal products, which are authorized by the “European Medicines Agency (EMA).”17There are mainly three categories of advanced therapy medicinal product, including tissue engineering product, somatic cell therapy products, and gene therapy medicinal products.The European Commission has adopted the “New Guidelines on Good Manufacturing Practice specific to Advanced Therapy Medicinal Products” in November 2017, and cell therapy needs to comply with the regulations of “EC 83/2001,” “EC 1394/2007” and “EU 536/2014.” All the US FDA, CFDA and EMA’s regulations cover the whole process of manufacturing to ensure the quality and safety of the final product.For example, validation of the supply of starting materials to ensure biosafety and solve potential ethical problems, good manufacturing practice involved manufacturing process,rigorous quality research to ensure the biosafety and efficacy the biomedical products, well-characterized product and consistent yield, and well-designed preclinical/clinical research.Therefore, it is an international consensus that cell therapy products have to comply with drug registration.

Table 1: Search strategy
CLINICAL RESEARCH PROGRESS OF HUMAN EMBRYONIC STEM CELLS-RETINAL PIGMENT EPITHELIUM CELLS
hESC was first reported to be induced into RPE cells (hESCRPE) in 2004.18Many more attempts have been made to conduct clinical studies in treating AMD using hESC-RPE cell transplantation.In 2012, a Lancet paper19reported to the implantation of hESC-RPE cells into the sub-retinal space of one dry-AMD patient (Ocata Therapeutics Inc.).The visual acuity of the patient was slightly increased and NO cells related serious adverse effects were found 4 months later.No hyperproliferation, tumorigenesis or severe immune rejection was found.In 2014, 9 AMD patients and 9 Stargardt’s patients were treated, more than 9 patients had a better corrected visual acuity at 22 months after cell transplantation and remained stable.20In addition, a 4-year follow-up study further confirmed the safety of the cell therapy.21
There are currently 16 clinical AMD trials in the ClinicalTrials.gov,22including regenerative patch technologies,Pfizer, cell cure neurosciences and several groups in Korea23and China.24Scientists at Pfizer tested two subjects for wet AMD cell therapy using hESC-RPE cells patch.Two subjects improved their best visual acuity by 29 and 21 letters, respectively.25Similar research was conducted to treat wet AMD patients with hESC-RPE in China.24However, no significant improvement in visual function was found in the follow-up of 1 year after the operation.The clinical article published by regenerative patch technologies in 201826showed that five dry-AMD patients were treated by hESC-RPE cell cultured in synthetic polymers (p-xylylene).One treated eye improved 17 letters in Early Treatment Diabetic Retinopathy Study and another two eyes improved fixation, which is a sign of potential improvement in the long run.The company of Cell Cure Neurosciences reported part of their data in American Academy of Ophthalmology meeting 2018, and 12 patients were treated with their hESC-RPE cell suspensions, geographic atrophy area, and best corrected visual acuity over time are both stable with the relatively low dose they used.Overall, all of the above clinical research showed no severe side effects related to the explanted cells and are approved to be safe of hESC-RPE cell implantation (Table 2).
CLINICAL RESEARCH PROGRESS OF INDUCED PLURIPOTENT STEM CELLS-RETINAL PIGMENT EPITHELIUM CELLS
The first paper that induces RPE cells from iPSCin vitrowas published in 2009.27This type of RPE cells is referred to as“iPSC-RPE”.Scientists in the RIKEN Institute in Japan had conducted a clinical study on the treatment of AMD by iPSCRPE cell transplantation in 2014.28An autologous cell patch containing cultured iPSC-RPE was used and the cell patch was inserted between the retina and native RPE layer in one eye of the patient.28There was no significant change in visual acuity one year after surgery, and no adverse events related to transplanted cells were reported.Due to the time consuming and high cost of iPSC-RPE, the researchers switched their focus to RPE cells differentiated from common iPSC bank,dramatically reducing the time and cost as compared with their autologous way.The second reason switched to common iPSC bank is to make it easier to choose human leukocyte antigen homozygous donors and reduce T cell response in the host.29Another group at National Eye Institute isolated CD34+cells from peripheral blood of three AMD patients and obtained good manufacturing practice grade RPE cells patch within 15 weeks.30The preclinical animal study has been completed and a formal clinical trial application will be expected to file soon to FDA.
We summarized more clinical trials based on iPSC-RPE cell therapy in Table 3.
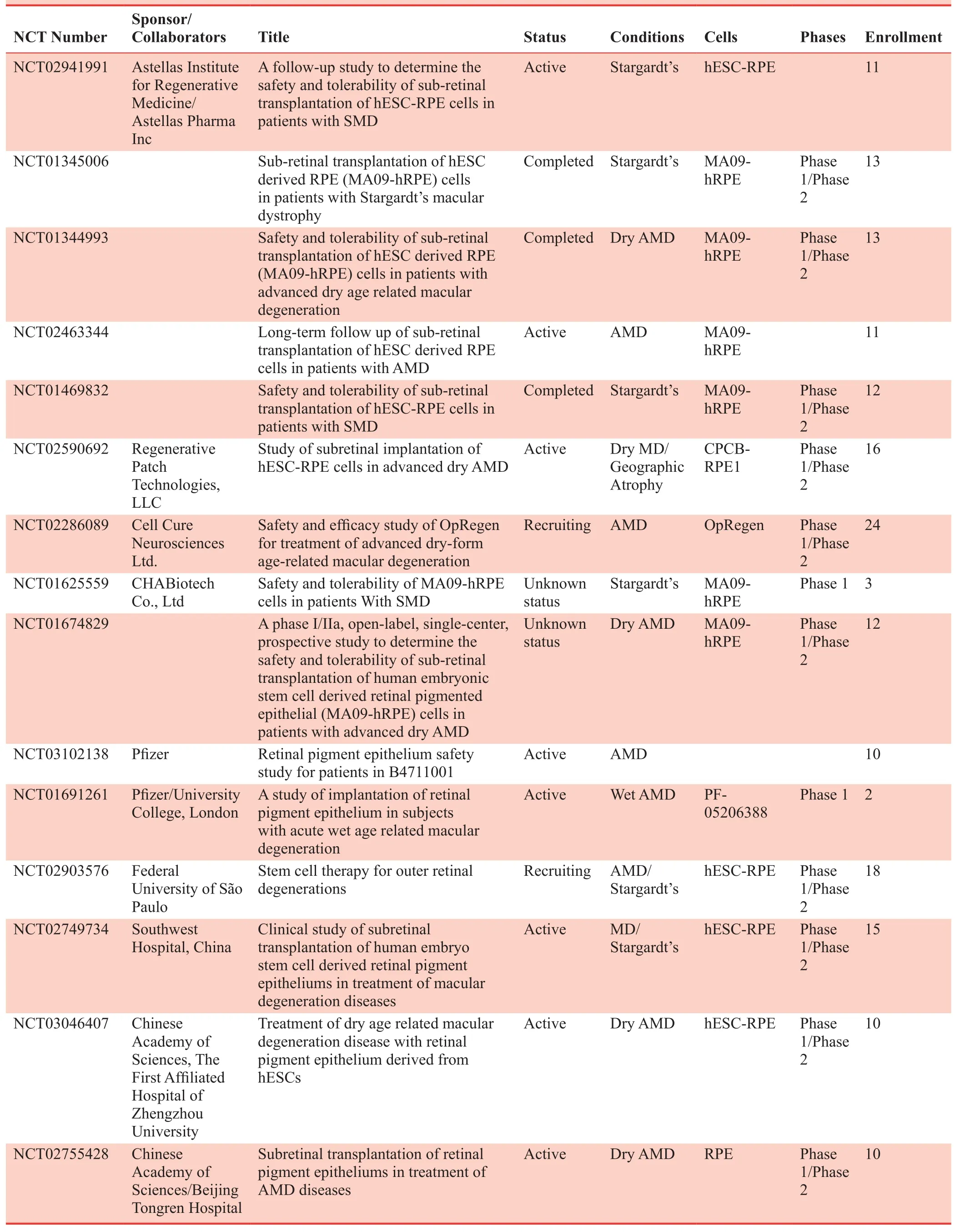
Table 2: Clinical studies on retinal degenerative diseases based on hESC-RPE cell transplantation
CLINICAL RESEARCH PROGRESS OF PRIMARY RETINAL PIGMENT EPITHELIUM CELLS
As early as the 1990s, researchers tried to treat AMD by transplanting the patient’s own RPE cells (autologous RPE cells)31,32or RPE cells from the fetal eye tissue (fRPE).33Some encouraging results emerged that cytokines secreted by fRPE cells can be more effective in improving the visual acuity of the patient than autologous RPE cells.34-36Therefore, the fRPE cells are the best choice for primary RPE cell transplantation once the ethical requirements of fRPE cells are met.Those requirements need to be strictly ful filled in a well-designed process that obtained fRPE cells in compliance with the laws and public ethics of the country.
It was well documented that fRPE cells could only be amplified for a few passages, and then the RPE cells would switch on to a dedifferentiation phenotype through epithelial-mesenchymal transition.Only recently, the yield and quality of primary RPE cells could be well controlled to meet the industrialization standards.37In particular, Dr.Yuan optimized RPE isolation and upgraded the culture process in 2014.38This work built the foundation for the researchers in Eyecure Therapeutics Inc.(Jiangsu, China).They further systematically modified the cell culture of fRPE according to the pharmaceutical standard for drug discovery.The RPE yield was steadily increased to 6 ×109cells per batch; therefore enough for treating thousands of patients for each batch while still adequate to the cell stability test and the packaging compatibility test.Those stable yields of the fRPE cells, to our knowledge, are for the first time to produce enough RPE cells suitable for the allogenic treatment of dry AMD according to the high standard of new drug discovery.They also established the whole set of standards for the quality control of fRPE cells.We summarized clinical trials with primary fRPE cells therapy in Table 4.
SUMMARY
Lots of clinical data have been reported describing cell transplantation of ES-RPE, iPSC-RPE and primary RPE cells by several companies and research institutes.Some complications were related to surgical operation and medicine usedto suppress the immune response.No tumorigenesis or other serious safety problems were observed in transplanted cells.However, most of these studies are still in their early stages and the number of tested subjects is quite limited.Larger sample size and longer observations are needed to verify the safety and efficacy for dry AMD treatment in the follow-up studies.In addition, cell transplantation surgery, optimal dosage, and immune-compatible test and placebo control, all need to be addressed thoroughly.
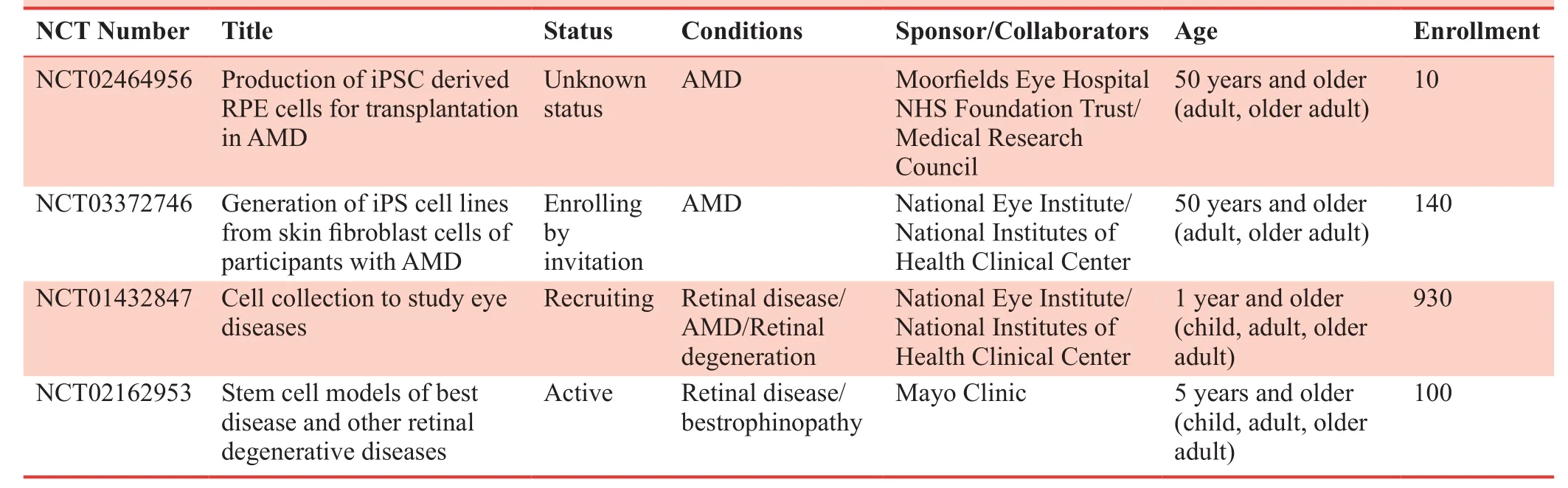
Table 3: Clinical studies on retinal degenerative diseases based on iPS-RPE cell transplantation
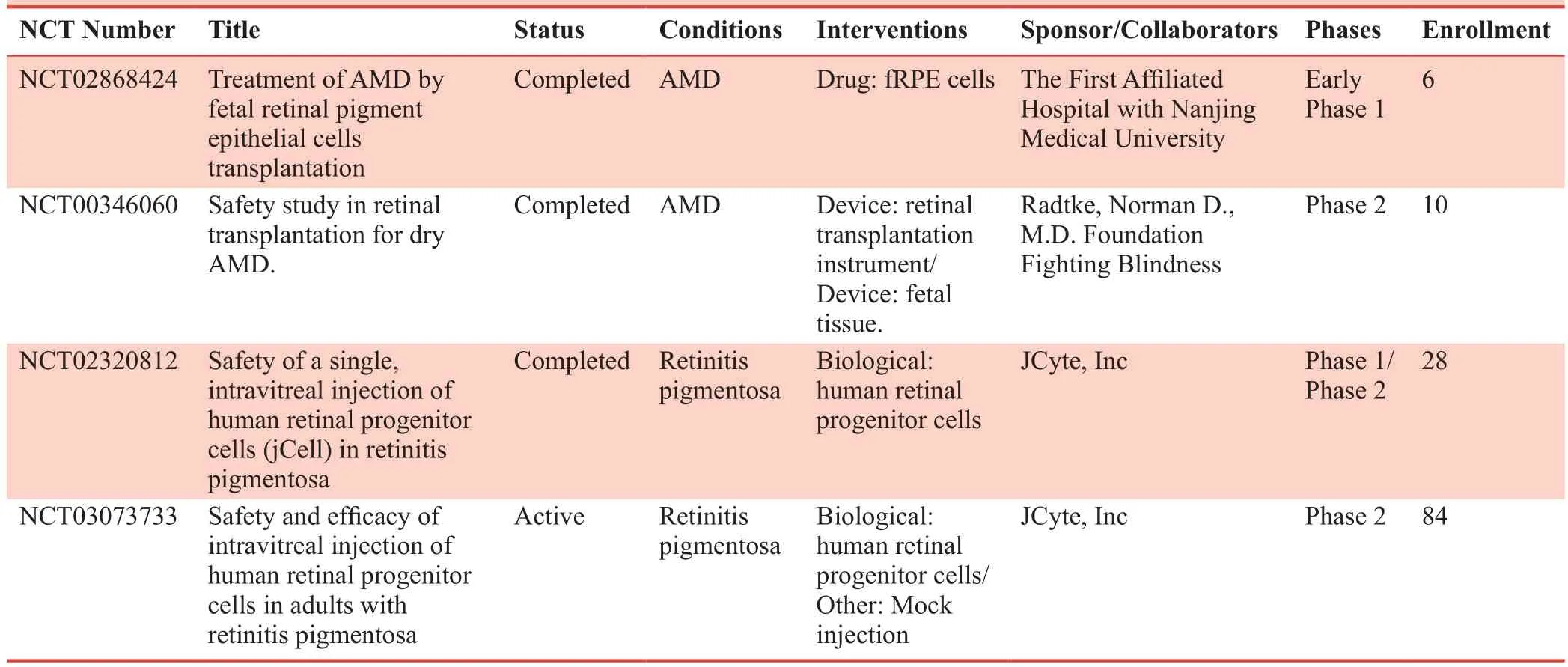
Table 4: Clinical studies on retinal degenerative diseases based on iPS-RPE cell transplantation
Developing a cell-based product as a new drug is an even more challenging task compared to the harsh requirements for conventional new drug applications.Some requirements must be fulfilled before any type of RPE cells to be approved as a new drug for the treatment of AMD.For example, the source of primary RPE cells and other differentiated RPE cells need to be reviewed by their National Ethics Department of each sponsor.The yield of RPE cells needs to achieve stable largescale productivity.Quality studies need to be established and verified throughout the whole production process.The final products of cell therapy need to meet the requirement of the off-the-shelf application.The manufacturing platform needs to meet the good manufacturing practice standard for RPE cells amplification.Effective RPE cell quality standards and quality control measures need to be formulated.39The safety and efficacy of RPE cells need to be tested thoroughly in preclinical research and clinical trials.The clinical trials in human patients would be the most important step.Rigorous and detailed design of the clinical research, reasonable selection of appropriate RPE cells, well-designed pilot study, and perfect compliance with Good Clinical Practice guideline would be the key to the Investigational New Drug Application of RPE cells for the treatment of AMD.
As a new type of drug, cellular drugs have great advantages in the treatment of many degenerative diseases and have become a hot area for the future drug investigation.The US FDA, CFDA, and EMA have developed a series of policies to encourage innovative drugs in cell therapy.For example,the US FDA has four special policies to accelerate the process according to different situations, which include priority review, breakthrough therapy, accelerated approval and fast track.Cell therapy of AMD with RPE cells can utilize all of those policies when detailed requirements are met.As a new member of The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use,CFDA has initiated innovation and quickly adapted their policies accordingly.
ANTICIPATION
To cope with the challenges that utilize live cell products as drugs, various subsystems need to be modified accordingly.Systematic design and reasonable innovation are encouraged to accelerate this process while ensuring cellular products safety and potency.A strong team is needed to engage in talents, technology, management, experts of regulation policy and venture capital.Furthermore, close cooperation within the team is necessary to bridge the huge gap between cellular products and new drug discovery.
RPE cell transplantation is currently the most promising treatment for dry AMD.40RPE cells from different sources are being studied in accordance with the regulations of the IND.All of the different strategies stand a chance to get the approval of New Drug Application.We are looking forward to utilizing RPE cells as a new drug to treat dry AMD and other retinal diseases within a few years.We can also anticipate cell therapy will meet the needs of many life-threating and other unmet medical conditions in the near future.The trend of these new types of drugs based on cell and tissue has emerged.With the aid of clearly defined regulations, it will be developed on the fast track and have a prosperous future.41
Author contributions
Review concept, design and revision: LD, PFF; abstract and table writing: LD; data acquisition and analysis: PFF.Both authors approved the final manuscript.
Conflicts of interest
We declare no conflicts of interest.
Financial support
This work was supported in part by a grant from the Jiangsu Provincial Science and Technology Department (Key Research & Development Project of Jiangsu Province 2017) in China, No.BE2017629; and partially by a grant from the Wuxi Science and Technology Bureau in China, No.CBE01G1735 (to PFF).
Copyright license agreement
The Copyright License Agreement has been signed by both authors before publication.
Plagiarism check
Checked twice by iThenticate.
Peer review
Externally peer reviewed.
Open access statement
This is an open-access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
杂志排行
Clinical Trials in Degenerative Diseases的其它文章
- Relationship between low vitamin D status and extra-skeletal diseases: a systematic review on effects of prophylaxis with vitamin D
- Tanreqing injection with ambroxol hydrochloride for heart failure and pulmonary infection in senile degenerative heart disease: a randomized controlled trial
- Hemodynamic responses of patients with stable coronary artery disease to a comedy film: study protocol for a randomized controlled trial
- Role of trace mineral in periodontal health: a review
- CTDD call for papers
