基于超高效液相色谱-质谱红花药材指纹图谱的建立
2019-10-09罗春霞祁艳茹
罗春霞,王 瑾,祁艳茹,马 丽,杨 亮,关 明
(新疆师范大学 化学化工学院,新疆 乌鲁木齐 830054)
红花(CarthamustinctoriusL.)系菊科[1],又名刺红花、草红花,一年或两年生草本植物. 红花是我国传统的中药材,具有活血化瘀、舒筋活络、预防和治疗心脑血管疾病等功效[2-4]. 新疆因具有特殊的地理环境和生态条件而成为我国红花的主产区[5]. 由于长期的自然选择和人工选择,红花种内产生了明显分化,形成了丰富的种质资源,不同种质来源的红花药材品质差异明显. 中药材的质量问题直接关系到中药的疗效及安全,因此建立一种反映中药内在质量的评价方法是关键.
近年来,指纹图谱已广泛应用于药材的鉴别和质量评价[6-8]. 目前,关于红花指纹图谱的报道很多[5,9-11],但有些来源地比较有限,有些指纹图谱共有峰较少,很难对红花药材的质量进行全面、客观的评价. 高效液相色谱(high performance chromatography, HPLC)法建立的中药指纹图谱存在灵敏度较低、溶剂消耗量大等问题,使中药中的组分不能被全部识别,进而导致药材质量评价不够准确. 近年来,超高液相色谱(ultra high performance liquid chromatography, UPLC)以其分析速度快、分离性能好等优点,在中药分析中快速发展[12]. 本研究以20批不同产地的红花药材为分析对象,构建红花药材多指标成分超高效液相色谱-质谱(ultra high performance liquid chromatography-mass spectrometry, UPLC-MS)指纹图谱,对20批红花药材进行相似度评价,较为客观地反映药材的内在质量,为新疆地域药材质量评价理论的完善和质量鉴别方法的建立提供一定的参考依据.
1 试验部分
1.1 仪器、试剂与材料
UltiMate3000/QExactive超高效液相色谱质谱联用仪(Thermo Scientific);色谱柱:Hypersil Gold C18,(100 mm×2.1 mm×1.9 μm,Thermo Scientific);微波超声波合成萃取仪(北京祥鹄科技有限公司).
甲醇、甲酸、乙腈(均为HPLC级,Fisher scientific公司);试验用水(广州市屈臣氏牌纯净水).
红花药材采自新疆不同产地,共20批,经新疆农业大学杨晓君副教授鉴定,为菊科红花属草红花. 自然晾干后粉碎,过180 μm筛,放入干燥器中室温下避光保存,样品信息如表1所列.
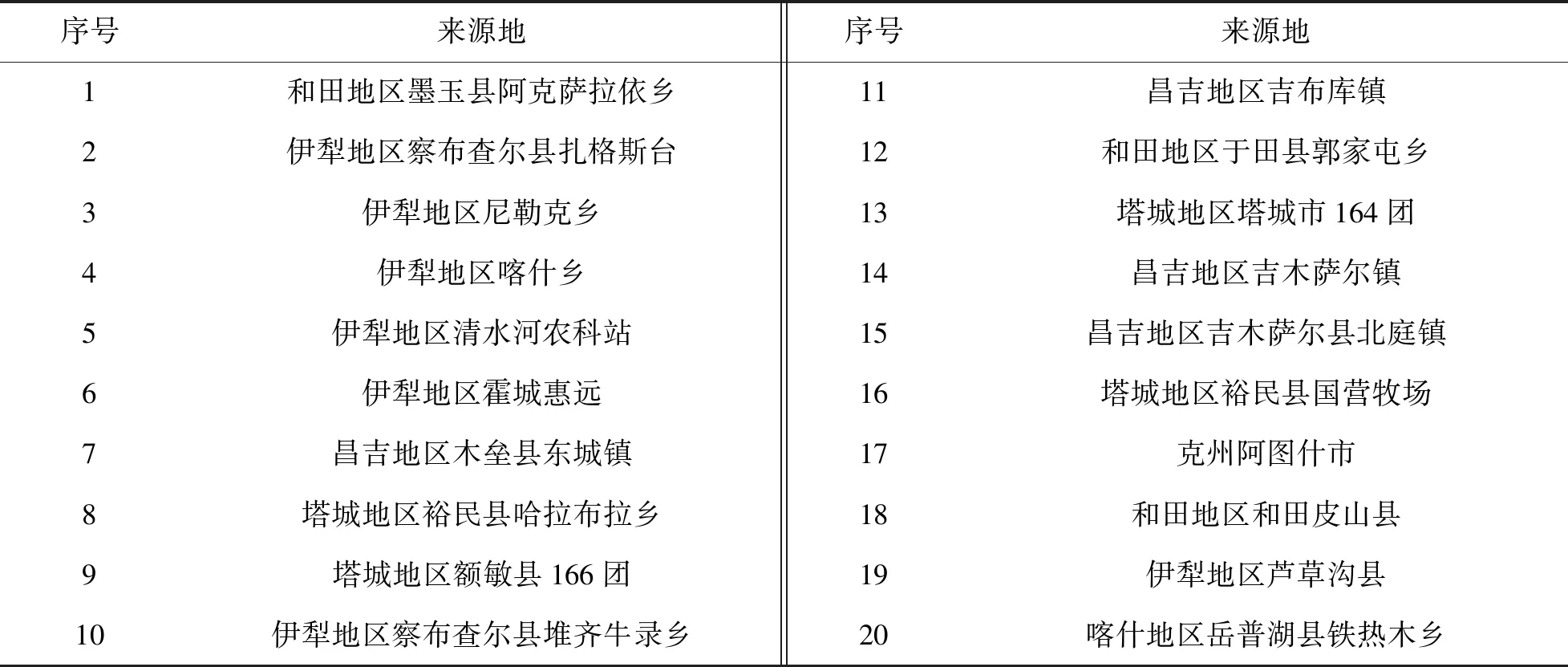
表1 新疆不同地区红花药材采样点Table 1 Sampling sites of Carthamus tinctorius L. in different areas of Xinjiang
1.2 供试品溶液的制备
20批红花样品粉碎,过75 μm筛,室温下称取1.000 0 g红花粉末于微波超声波合成萃取仪的三口瓶中,加15 mL 50%甲醇溶液,微波功率600 W,温度70 ℃,萃取20 min. 冷却至室温,将反应液及残余的红花全部倒入25 mL容量瓶,50%甲醇定容,过0.22 μm滤膜,待测.
1.3 色谱条件
色谱柱:Thermo Hypersil Gold C18,(100 mm×2.1 mm,1.9 μm);流动相:0.1%甲酸水溶液(A)-乙腈(B);流速:0.3 mL/min;柱温: 30 ℃;检测波长:270 nm;进样量:2 μL;梯度洗脱程序为:0~2 min,98% A;2~20 min,98~92% A;20~48 min,92~88% A;48~60 min,88~84% A;60~105 min,84~81% A;105~110 min,81~70% A;110~115 min,70~65% A;115~120 min,65~60% A;120~122 min,60~98% A;122~125 min,98% A.
1.4 质谱条件
鞘气流速:45 arb,辅助气流速:15 arb,雾化电压:3.3 KV,离子传输管温度:350 ℃,辅助气加热温度:400 ℃.
2 结果与讨论
2.1 色谱条件的优化
2.1.1 检测波长的选择
对270、403、390、367 nm 4个检测波长进行考察,不同检测波长下红花样品的出峰情况存在显著差异(如图1所示). 由图1可见,当检测波长为270 nm时,红花样品中指标成分响应最为明显,出峰个数多且峰型较好,能够提供的有效成分信息较为丰富,故选择270 nm为检测波长.
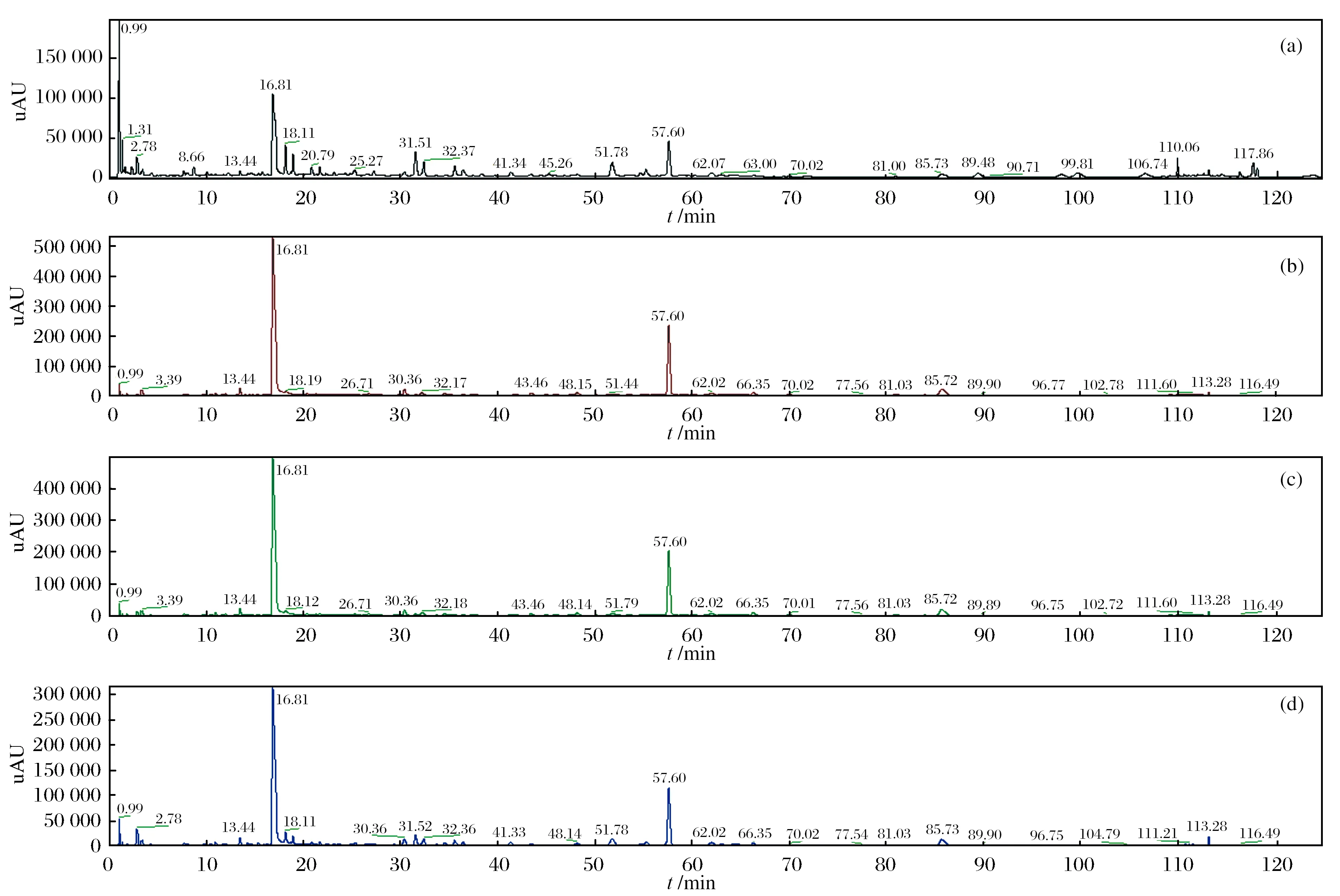
图1 不同检测波长下的红花样品UPLC色谱图Fig. 1 UPLC chromatograms of safflower samples at different detection wavelengths(a) 270 nm, (b) 403 nm, (c) 390 nm, (d) 367 nm
2.1.2 梯度洗脱条件的优化
参考相关文献[13],采用0.1%甲酸水溶液-乙腈为流动相,并对梯度洗脱条件进行优化,最终确认1.3项下为较优色谱条件. 该条件下,红花各组分达到最佳分离,对应样品UPLC色谱图如图2所示.
2.2 提取条件的优化
2.2.1 提取溶剂的考察
分别以甲醇∶水为0∶100(体积比)、甲醇∶水为25∶75(体积比)、甲醇∶水为50∶50(体积比)、甲醇∶水为75∶25(体积比)、甲醇∶水为100∶0 (体积比)作提取溶剂. 结果表明,提取溶剂中无甲醇或甲醇浓度过低时,红花药材中许多脂溶性组分没有出峰或者峰面积较小. 而甲醇浓度过高时,又会导致红花中的水溶性组分无法被提取出来. 故选择甲醇∶水为50∶50(体积比)作为提取溶剂.
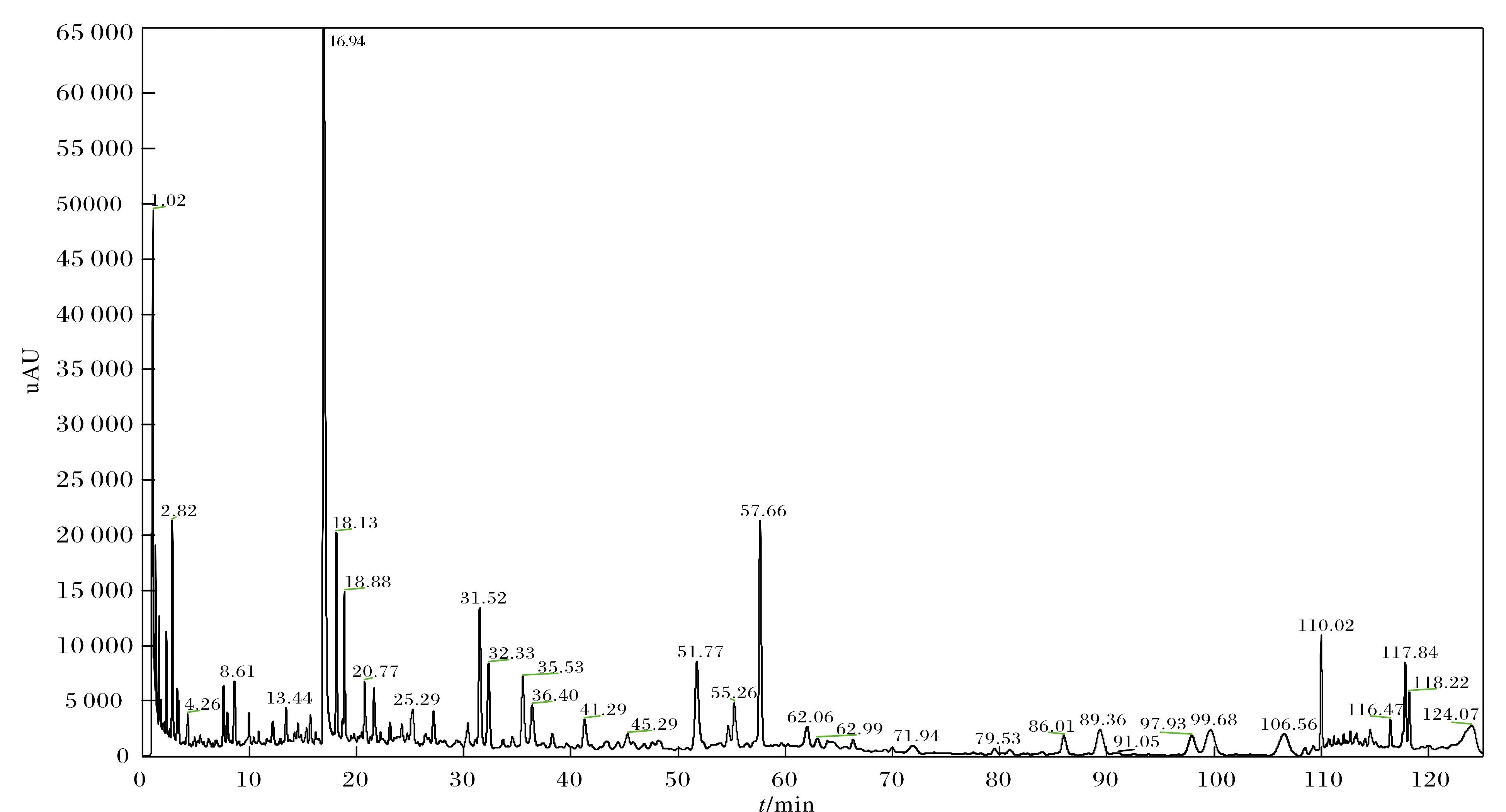
图2 红花样品UPLC色谱图Fig. 2 UPLC chromatogram of safflower sample
2.2.2 提取方法考察
分别以超声辅助提取法、微波辅助提取法、基质固相分散萃取法(MSPD法)、快速溶剂提取法对红花样品进行提取,比较5种指标成分的峰面积、提取时间等(如表2所列). 由表2可见,超声辅助提取法、微波辅助提取法提取出的有效成分峰面积较大,但微波辅助提取法的提取时间仅为20 min. 故选择微波辅助提取红花样品.
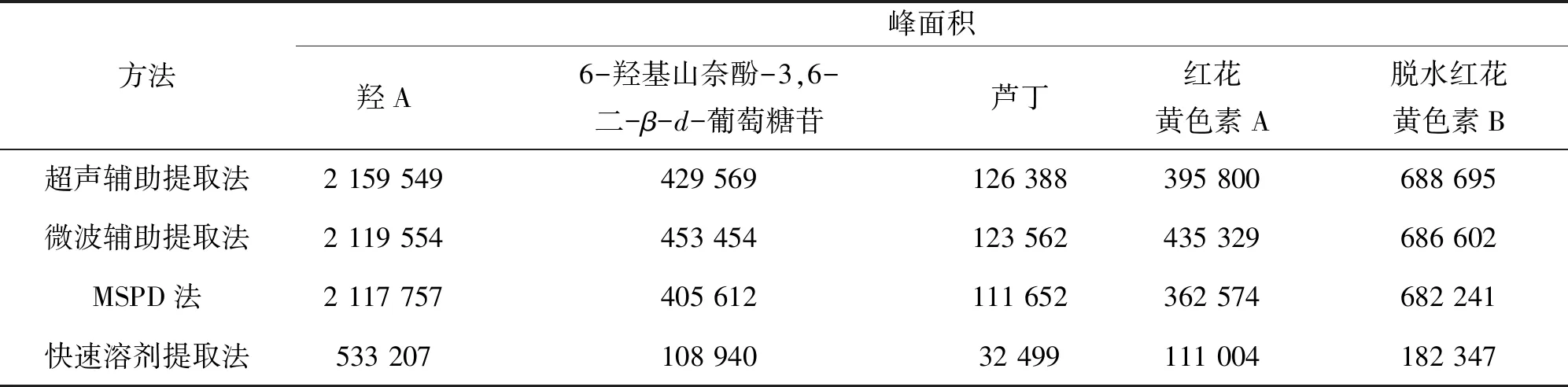
表2 不同样品前处理方法所得指标成分峰面积比较Table 2 Comparison of peak area of different sample pretreatment methods
2.3 指纹图谱的建立
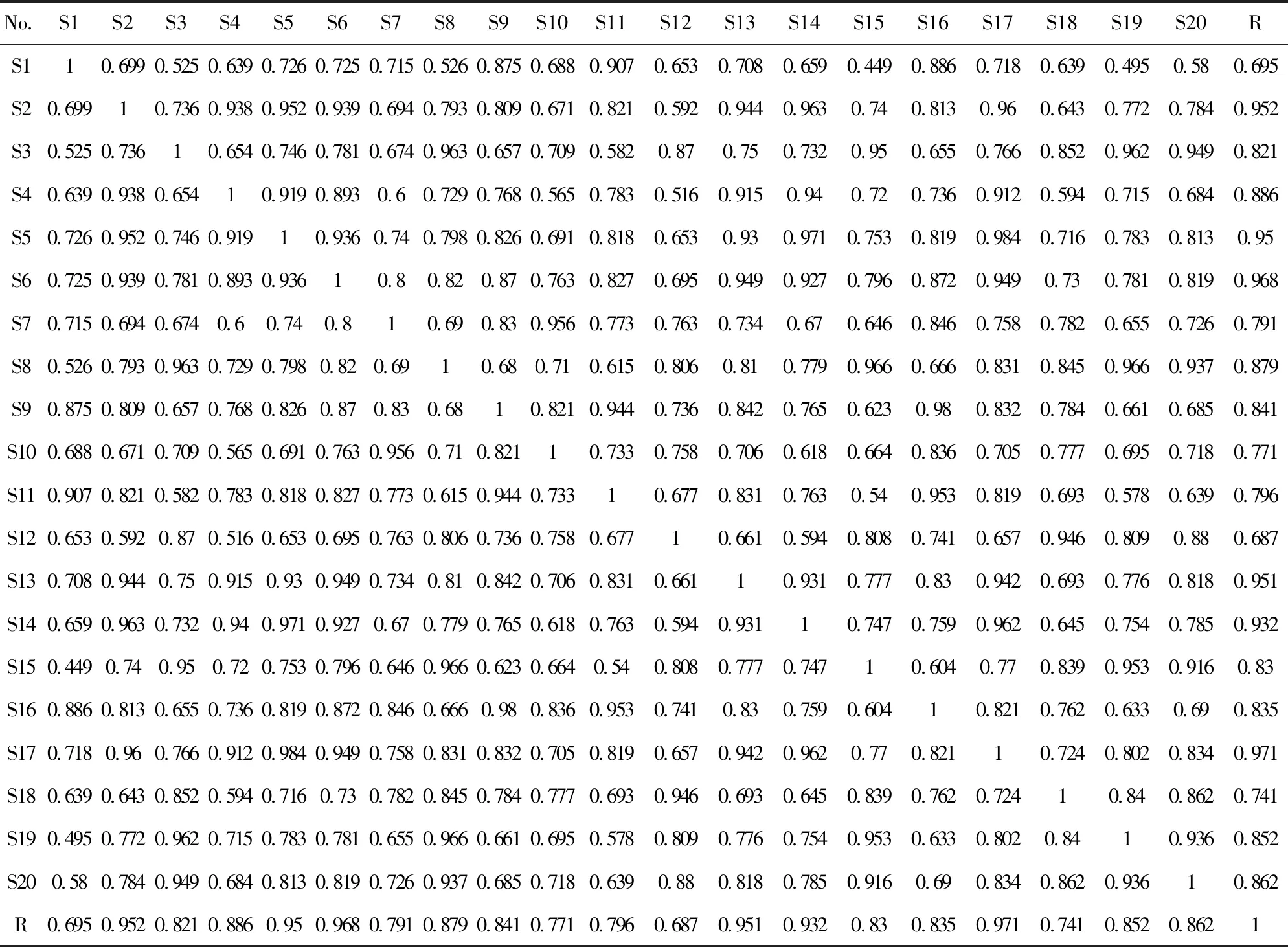
在上述最优条件下,对20个产地的红花样品进行分离分析. 将色谱图导入中药指纹图谱相似度评价(2004A)软件,经过设置参照谱图、系统自动匹配、生成对照谱图、相似度评价4个步骤,即得到对照指纹图谱(采用中位数法,时间窗为1.00),其中R谱即为红花药材的UPLC对照指纹图谱(如图3所示).
2.4 相似度评价
相似度评价结果由2.3中的“相似度评价”步骤得出,将R谱与20张红花样品的UPLC色谱图进行比较(如表3所列). 由表3可见,除样品S1和S12与R谱的相似度低于0.70之外,其余样品与R谱的相似度均大于0.70,其中S7、S10、S11、S18与R谱的相似度均介于0.70~0.80之间,但是其相互之间的相似度都在0.80以上. 总体来看,20个样品与R谱的相似度在0.90以上.

图3 20批红花药材UPLC图谱(R为对照指纹图谱)Fig. 3 UPLC chromatograms of 20 batches of safflowers (R as the control fingerprint)
No.S1S2S3S4S5S6S7S8S9S10S11S12S13S14S15S16S17S18S19S20RS110.6990.5250.6390.7260.7250.7150.5260.8750.6880.9070.6530.7080.6590.4490.8860.7180.6390.4950.580.695S20.69910.7360.9380.9520.9390.6940.7930.8090.6710.8210.5920.9440.9630.740.8130.960.6430.7720.7840.952S30.5250.73610.6540.7460.7810.6740.9630.6570.7090.5820.870.750.7320.950.6550.7660.8520.9620.9490.821S40.6390.9380.65410.9190.8930.60.7290.7680.5650.7830.5160.9150.940.720.7360.9120.5940.7150.6840.886S50.7260.9520.7460.91910.9360.740.7980.8260.6910.8180.6530.930.9710.7530.8190.9840.7160.7830.8130.95S60.7250.9390.7810.8930.93610.80.820.870.7630.8270.6950.9490.9270.7960.8720.9490.730.7810.8190.968S70.7150.6940.6740.60.740.810.690.830.9560.7730.7630.7340.670.6460.8460.7580.7820.6550.7260.791S80.5260.7930.9630.7290.7980.820.6910.680.710.6150.8060.810.7790.9660.6660.8310.8450.9660.9370.879S90.8750.8090.6570.7680.8260.870.830.6810.8210.9440.7360.8420.7650.6230.980.8320.7840.6610.6850.841S100.6880.6710.7090.5650.6910.7630.9560.710.82110.7330.7580.7060.6180.6640.8360.7050.7770.6950.7180.771S110.9070.8210.5820.7830.8180.8270.7730.6150.9440.73310.6770.8310.7630.540.9530.8190.6930.5780.6390.796S120.6530.5920.870.5160.6530.6950.7630.8060.7360.7580.67710.6610.5940.8080.7410.6570.9460.8090.880.687S130.7080.9440.750.9150.930.9490.7340.810.8420.7060.8310.66110.9310.7770.830.9420.6930.7760.8180.951S140.6590.9630.7320.940.9710.9270.670.7790.7650.6180.7630.5940.93110.7470.7590.9620.6450.7540.7850.932S150.4490.740.950.720.7530.7960.6460.9660.6230.6640.540.8080.7770.74710.6040.770.8390.9530.9160.83S160.8860.8130.6550.7360.8190.8720.8460.6660.980.8360.9530.7410.830.7590.60410.8210.7620.6330.690.835S170.7180.960.7660.9120.9840.9490.7580.8310.8320.7050.8190.6570.9420.9620.770.82110.7240.8020.8340.971S180.6390.6430.8520.5940.7160.730.7820.8450.7840.7770.6930.9460.6930.6450.8390.7620.72410.840.8620.741S190.4950.7720.9620.7150.7830.7810.6550.9660.6610.6950.5780.8090.7760.7540.9530.6330.8020.8410.9360.852S200.580.7840.9490.6840.8130.8190.7260.9370.6850.7180.6390.880.8180.7850.9160.690.8340.8620.93610.862R0.6950.9520.8210.8860.950.9680.7910.8790.8410.7710.7960.6870.9510.9320.830.8350.9710.7410.8520.8621
2.5 色谱峰的指认
采用UPLC-MS对红花样品进行检测,对已达到分离要求的组分进行质谱分析. 将各组分的分子量与文献中已报道的组分进行比较,确认红花中11个未知组分(如图4所示). 20批红花的11个共有峰的保留时间及峰面积如表4、5所列.
根据高分辨质谱提供的准确分子量与红花中已知组分的分子量进行对比,将理论分子量和实测分子量进行比较计算,对于质量偏差小于2 ppm的组分予以确认,共确定了11个组分,按照保留时间的先后顺序分别如表6所列.
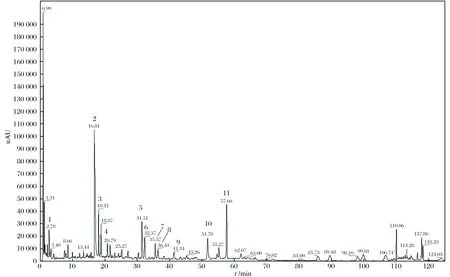
图4 UPLC-MS联机分析红花药材中的11个组分Fig. 4 Analysis of 11 components in safflower by UPLC-MS
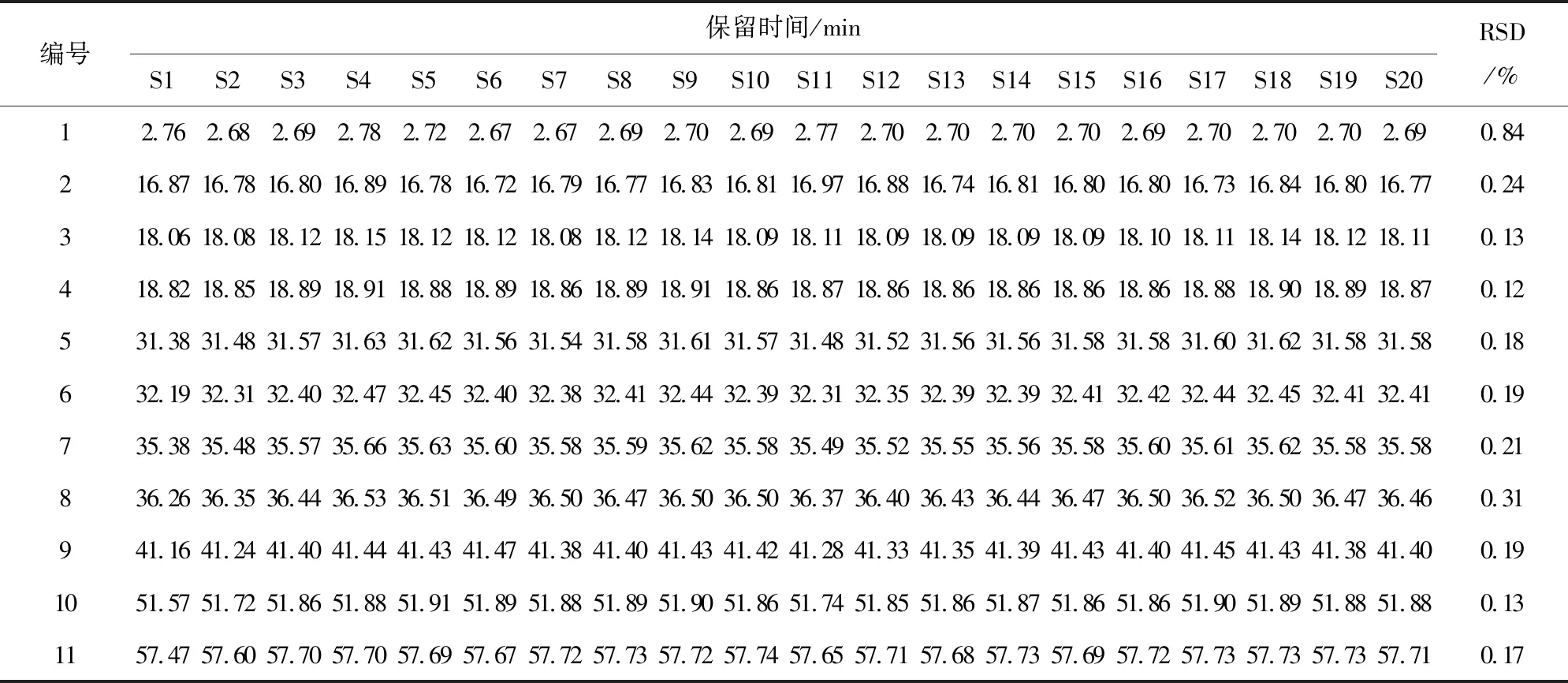
编号保留时间/minS1S2S3S4S5S6S7S8S9S10S11S12S13S14S15S16S17S18S19S20RSD/%12.762.682.692.782.722.672.672.692.702.692.772.702.702.702.702.692.702.702.702.690.84216.8716.7816.8016.8916.7816.7216.7916.7716.8316.8116.9716.8816.7416.8116.8016.8016.7316.8416.8016.770.24318.0618.0818.1218.1518.1218.1218.0818.1218.1418.0918.1118.0918.0918.0918.0918.1018.1118.1418.1218.110.13418.8218.8518.8918.9118.8818.8918.8618.8918.9118.8618.8718.8618.8618.8618.8618.8618.8818.9018.8918.870.12531.3831.4831.5731.6331.6231.5631.5431.5831.6131.5731.4831.5231.5631.5631.5831.5831.6031.6231.5831.580.18632.1932.3132.4032.4732.4532.4032.3832.4132.4432.3932.3132.3532.3932.3932.4132.4232.4432.4532.4132.410.19735.3835.4835.5735.6635.6335.6035.5835.5935.6235.5835.4935.5235.5535.5635.5835.6035.6135.6235.5835.580.21836.2636.3536.4436.5336.5136.4936.5036.4736.5036.5036.3736.4036.4336.4436.4736.5036.5236.5036.4736.460.31941.1641.2441.4041.4441.4341.4741.3841.4041.4341.4241.2841.3341.3541.3941.4341.4041.4541.4341.3841.400.191051.5751.7251.8651.8851.9151.8951.8851.8951.9051.8651.7451.8551.8651.8751.8651.8651.9051.8951.8851.880.131157.4757.6057.7057.7057.6957.6757.7257.7357.7257.7457.6557.7157.6857.7357.6957.7257.7357.7357.7357.710.17
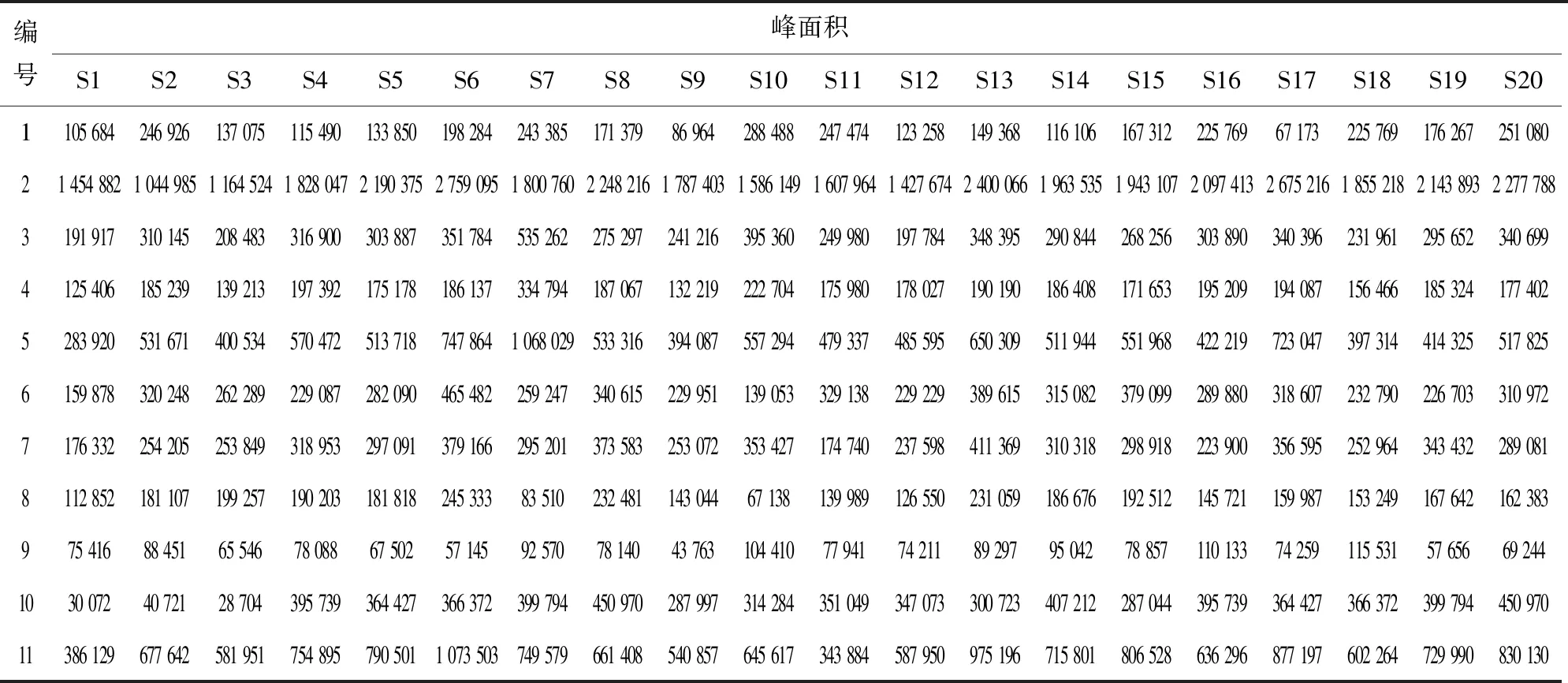
表5 20批红花样品11个共有峰的峰面积Table 5 Peak areas of 11 peaks in 20 batches of safflower samples
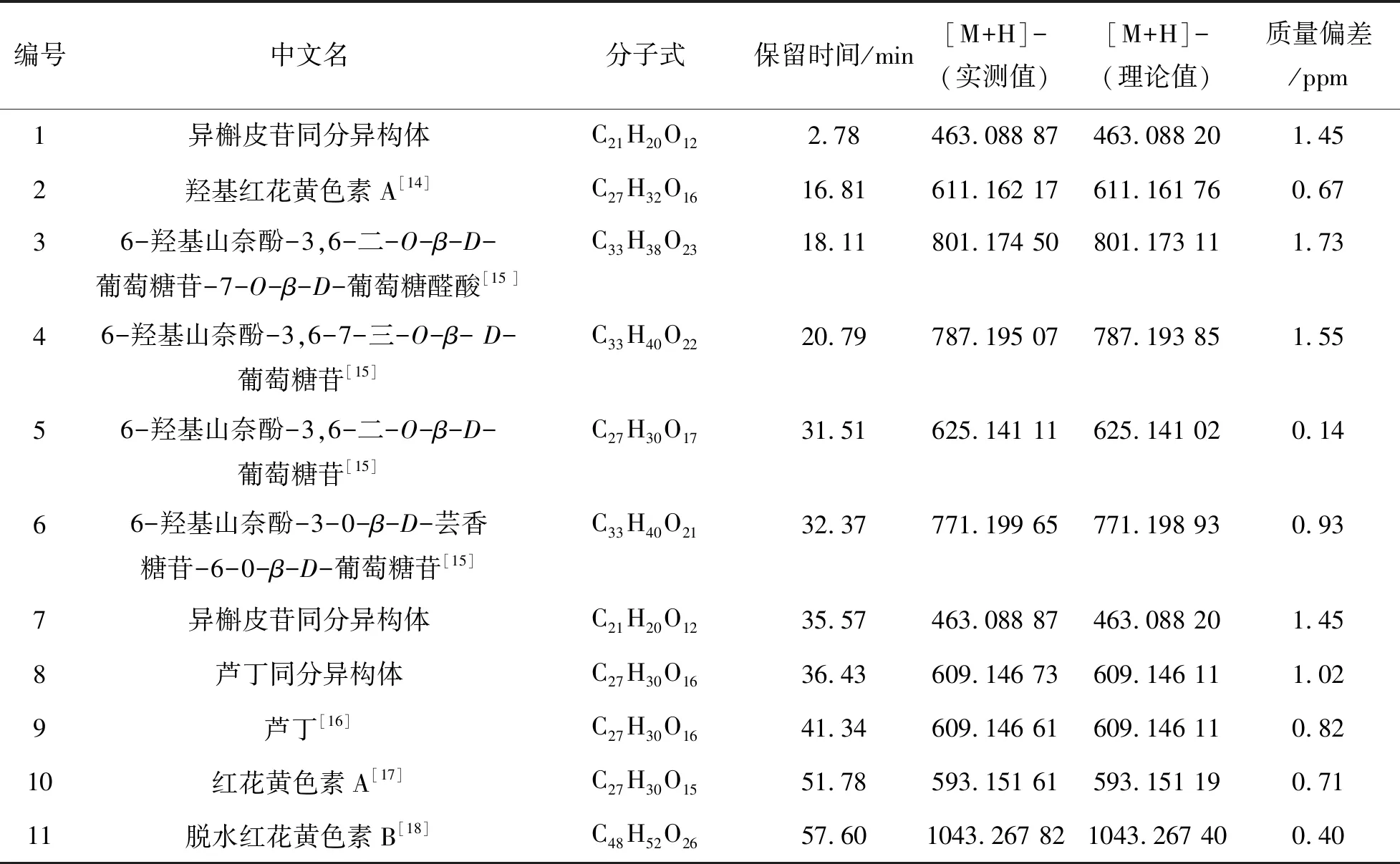
表6 红花药材中11个共有峰具体信息表Table 6 Specific information table of 11 common peaks in safflower
3 结论
本研究以来自新疆伊犁地区、塔城地区、昌吉地区、喀什地区及和田地区等不同产地的20批红花样品为分析对象,建立了一种UPLC-MS指纹图谱分析方法,确定了67个共有峰,并通过UPLC-MS指认了其中的11种组分. 本文建立的UPLC-MS指纹图谱可识别出更多的有效成分,在红花药材的质量评价方面更具优势,可为红花药材质量的全面控制提供一定的借鉴. 同时,该方法可为构建红花药材质量分析体系与优质红花药材种质的筛选奠定基础,为红花药材的质量分析与评价提供较为全面的技术支撑和参考.
