FAT1, a direct transcriptional target of E2F1, suppresses cell proliferation, migration and invasion in esophageal squamous cell carcinoma
2019-09-10YuWangGuangchaoWangYunpingMaJingleiTengYanWangYongpingCuiYanDongShujuanShaoQiminZhanXuefengLiu
Yu Wang, Guangchao Wang, Yunping Ma, Jinglei Teng, Yan Wang, Yongping Cui, Yan Dong,Shujuan Shao, Qimin Zhan,, Xuefeng Liu
1Institute of Cancer Stem Cell, Dalian Medical University, Dalian 116044, China; 2State Key Laboratory of Molecular Oncology, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College,Beijing 100021, China; 3Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education/Beijing), Laboratory of Molecular Oncology, Peking University Cancer Hospital & Institute, Beijing 100142, China; 4Shenzhen Peking University-The Hong Kong University of Science and Technology (PKU-HKUST) Medical Center, Peking University Shenzhen Hospital, Shenzhen 518036, China; 5College of Stomatology, Dalian Medical University, Dalian 116044, China; 6Key Laboratory of Proteomics, Dalian Medical University, Dalian 116044, China Correspondence to: Xuefeng Liu. Institute of Cancer Stem Cell, Dalian Medical University, Dalian 116044, China. Email: ixuee@sina.com; Qimin Zhan. Institute of Cancer Stem Cell, Dalian Medical University, Dalian 116044, China; Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education/Beijing), Laboratory of Molecular Oncology, Peking University Cancer Hospital &Institute, Beijing 100142,China. Email: zhanqimin@bjmu.edu.cn.
Abstract Objective: Growing evidence indicates that FAT atypical cadherin 1 (FAT1) has aberrant genetic alterations and exhibits potential tumor suppressive function in esophageal squamous cell carcinoma (ESCC). However, the role of FAT1 in ESCC tumorigenesis remains not well elucidated. The aim of this study was to further investigate genetic alterations and biological functions of FAT1, as well as to explore its transcriptional regulation and downstream targets in ESCC.Methods: The mutations of FAT1 in ESCC were achieved by analyzing a combined study from seven published genomic data, while the copy number variants of FAT1 were obtained from an analysis of our previous data as well as of The Cancer Genome Atlas (TCGA) and Cancer Cell Line Encyclopedia (CCLE) databases using the cBioPortal. The transcriptional regulation of FAT1 expression was investigated by chromatin immunoprecipitation(ChIP) and the luciferase reporter assays. In-cell western, Western blot and reverse transcription-quantitative polymerase chain reaction (RT-qPCR) were used to assess the indicated gene expression. In addition, colony formation and Transwell migration/invasion assays were employed to test cell proliferation, migration and invasion.Finally, RNA sequencing was used to study the transcriptomes.Results: FAT1 was frequently mutated in ESCC and was deleted in multiple cancers. Furthermore, the transcription factor E2F1 occupied the promoter region of FAT1, and depletion of E2F1 led to a decrease in transcription activity and mRNA levels of FAT1. Moreover, we found that knockdown of FAT1 promoted KYSE30 and KYSE150 cell proliferation, migration and invasion; while overexpression of FAT1 inhibited KYSE30 and KYSE410 cell proliferation, migration and invasion. In addition, knockdown of FAT1 led to enrichment of the mitogen-activated protein kinase (MAPK) signaling pathway and cell adhesion process.Conclusions: Our data provided evidence for the tumor suppressive function of FAT1 in ESCC cells and elucidated the transcriptional regulation of FAT1 by E2F1, which may facilitate the understanding of molecular mechanisms of the progression of ESCC.
Keywords: E2F1; ESCC; FAT1; tumor suppressor
Introduction
Worldwide, esophageal cancer is one of the most aggressive tumors, and is highly prevalent in China (1,2). It is the 4th leading cause of cancer-related mortality in China, where esophageal squamous cell carcinoma (ESCC)is the major histological type (3,4). There are advances in the early detection and treatment of patients with ESCC,but the 5-year survival of global ESCC patients is still very poor (5,6). Several large-scale analyses demonstrated that some genes and pathways, such as TP53, RB1, CDKN2A,PIK3CA, Notch and FAT atypical cadherin 1 (FAT1), may contribute to ESCC tumorigenesis (7-11). Among these aberrant genes, the “sleeping giant” (12) FAT1 is inactivated by a two-hit model in ESCC tumors, where somatic mutations are often accompanied by the loss of heterozygosity or homozygous deletion (9,11), showing an anti-tumor activity (9,13,14). FAT1 encodes a cadherin-like trans-membrane protein and regulates the cell-cell contact,polarization, migration and growth of mammalian cells (15-17). Interestingly, FAT1 may function as both a tumor suppressor and an oncogene depending on different cell context (18-21). The inactivation of FAT1 via somatic mutations or deletions is also observed in multiple human cancers to promote Wnt/β-catenin signaling and tumorigenesis (18). Other mechanisms regulating FAT1 expression remain poorly understood and need further investigation.
Transcriptional regulation by transcription factors is a critical way to affect gene expression. E2Fs are a large family of transcription factors that modulate gene expression by acting as either activators or repressors of transcription based on their structures and functions(22,23). In Homo sapiens, E2F transcription factor 1(E2F1), which is the first member of the E2F family, is characterized as an activating transcription factor to mediate various biological processes, including cell cycle progression, apoptosis, DNA-damage response and metastasis (24-27). E2F1 binds to promoters of target genes, such as G1/S regulated genes and apoptosis-related genes, to regulate their expression (28-30), predominantly by interacting with the RB pocket protein (31). Advances in high-throughput technologies reveal that E2F1 also occupies a large fraction of gene promoters (32), suggesting that it has a wide spread regulation role in the human genome. However, the transcriptional regulation of E2F1 on the FAT1 locus remains unknown.
In this study, we comprehensively analyzed the genetic alterations of FAT1 in ESCC, as well as its copy number variants (CNVs) in other cancers. More importantly, the transcriptional regulation of FAT1 by E2F1 was elaborated.In addition, the effects of FAT1 on cell proliferation,migration and invasion were also monitored in ESCC cells.Finally, RNA sequencing (RNA-seq) was performed to investigate the gene expression profile upon FAT1 knockdown.
Materials and methods
Cell lines
ESCC cell lines, KYSE30, KYSE150 and KYSE410, were generous gifts from Prof. Y Shimada of Kyoto University,Japan. All cell lines were cultured at 37 °C with 5% CO2in RPMI 1640 medium (Gibco) with 10% fetal bovine serum(FBS).
Cell transfection
Cells at the logarithmic growth phase were transfected with siRNAs or plasmids using Lipofectamine 2000 (Invitrogen,USA) according to the manufacturer's instructions. All siRNAs (25-mer duplex Stealth siRNAs) were obtained from Invitrogen, and the sequence information is as follows: siFAT1: HSS103568 and HSS176716 and siE2F1:HSS103016 and HSS103017. The FAT1-Trunc plasmid,which contains all key functional domains of FAT1 (18),was a generous gift from Dr. Luc GT Morris (Human Oncology and Pathogenesis Program, Memorial Sloan-Kettering Cancer Center).
Chromatin immunoprecipitation (ChIP)
Pierce Magnetic ChIP Kit (Thermo Fisher, Waltham,USA) was used according to the manufacturer's instructions. Briefly, KYSE30 cells were cross-linked with formaldehyde and the nucleus was isolated. Chromatin DNA was fragmented and incubated with E2F1 antibody(ab179445; Abcam, Cambridge, UK) followed by addition of magnetic beads. After washing and purification, the precipitated chromatins were analyzed by quantitative polymerase chain reaction (qPCR). The primers targeting the FAT1 promoter were synthesized by Invitrogen, and the sequences are as follows: forward 5'-GGAGCTCACCC GCCGTCTCA-3' and reverse 5'-GCTCGTGCGGC AGGTACCA-3'.
Luciferase reporter assay
To obtain the pGL3-FAT1 vector, the promoter region of FAT1, ranging from -2000 bp to +500 bp, was synthesized and inserted into the pGL3-Basic vector by Generay(Shanghai, China). Then, KYSE30 cells were cotransfected by pGL3-FAT1 vector and pRL-TK Renilla vector together with E2F1 siRNA. After 36 h of transfection, the luciferase activity was detected by the Dual-Luciferase Reporter Assay System (Promega, USA),and the Firefly luciferase activity was normalized to the Renilla luciferase activity.
RNA extraction and reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA from ESCC cells was purified using RNAiso plus (Takara, Dalian, China). Complementary DNA(cDNA) was synthesized from 1 μg of total RNA using a PrimeScript®RT reagent Kit with gDNA Eraser (Takara).TB Green®Premix Ex Taq®II kit (Takara) was used to detect the indicated RNA levels on the QuantStudio Real-Time PCR System (Applied Biosystems, USA) or the CFX96 Real-Time System (Bio-Rad, USA). The relative expression levels of the target genes were normalized to endogenous GAPDH. The primers synthesized by Invitrogen are listed in Supplementary Table S1.
Western blot
Total protein from ESCC cells was extracted using RIPA lysis buffer (Proteintech Group, Wuhan, China) that was supplemented with the protease inhibitor PMSF and was quantified by Enhanced BCA Protein Assay Kit (Beyotime Institute of Biotechnology, Shanghai, China) following the manufacturer's protocols. Equal quantities of proteins were separated by 10% SDS-PAGE and were transferred onto PVDF membranes. After blocking in 5% non-fat milk for 1 h, the membrane was then incubated with E2F1 primary antibody (ab179445; Abcam) at 4 °C overnight. Protein bands were detected using a horseradish-peroxidase(HRP)-conjugated IgG secondary antibody (Proteintech Group), and images were captured with the BioSpectrum imaging system (UVP, USA). GAPDH (60004-1-lg;Proteintech Group) was used as loading control.
In-cell western assay
Cells grown in 96-well plates were fixed with 3.7%formaldehyde for 20 min and were permeabilized using 0.1% Triton X-100. After blocking with 5% non-fat milk for 1 h, cells were incubated with a mixture of FAT1 primary antibody (ab190242; Abcam, UK) and GAPDH primary antibody (60004-1-lg; Proteintech Group, China)overnight at 4 °C. Then, cells were washed with PBST and were incubated with 680 nm and 800 nm infrared-labeled secondary antibody solutions (LI-COR Biosciences, USA)for 1 h at room temperature. Fluorescent signals for FAT1 and GAPDH were captured using the Odyssey infrared imaging system (LI-COR Biosciences, Lincoln, Nebraska,USA). Finally, the relative abundance of the FAT1 protein was normalized to GAPDH.
Transwell migration/invasion assays
Transwell migration and invasion assays were performed using a Transwell plate (Corning, New York, USA) that was coated with (for invasion) or without (for migration)Matrigel Basement Membrane Matrix (BD). Briefly,1.5×105cells were seeded into the upper chamber of Transwell with serum-free medium, while culture medium with 20% FBS was added to the lower chamber. After culturing for 12-24 h, cells that migrated or invaded though the membrane were fixed with methanol for 10 min, stained with crystal violet solution for 5 min and finally photographed under a microscope (Leica,Germany).
Colony formation assay
A total of 1,000-3,000 transfected cells per well were seeded into plates containing medium with 10% FBS on d 0, and the cells were incubated at 37 °C with 5% CO2for 14 d. On d 14, the cells were washed with PBS, fixed with methanol for 10 min and then stained with crystal violet solution for another 10 min. After washing with water and drying at room temperature, the colonies were photographed and counted.
RNA sequencing (RNA-seq)
The transcriptome sequencing profiles of KSYE30-control and KYSE30-siFAT1 cells were obtained to detect the altered expression of downstream genes. Briefly,sequencing libraries were constructed with NEBNext Ultra RNA Library Prep Kit for Illumina (NEB, Ipswich, USA)
according to manufacturer's protocols. The libraries were sequenced on the Illumina HiSeq platform by Novogene(Beijing, China). Clean data were then mapped to the human reference genome hg19. The Fragments Per Kilobase of exon per Million mapped fragments (FPKM) of each gene was calculated and differential expression analysis was performed. The significantly differentially expressed genes between the control cells and the FAT1 depleted cells were input to DAVID database (33,34) and enriched using Kyoto Encyclopedia of Genes and Genomes(KEGG) (35) and Gene Ontology (GO) analyses.
Statistical analysis
The data are presented as the. P<0.05 was considered statistically significant. The data were statistically analyzed using GraphPad Prism 6 (GraphPad Software, San Diego,CA, USA) by means of a two-sided Student's t-test.
Results
FAT1 exhibits a high frequency of genetic alterations in ESCC
To comprehensively explore the genetic alterations of FAT1 in ESCC, we first analyzed the somatic mutations of FAT1 from a study by Du et al. (36), which consisted of a total of 41 whole-genome sequences and 449 whole-exome sequences from seven published ESCC genomic data (7-10,37-39). We observed that FAT1 was frequently mutated in ESCC (Figure 1A). The mutation rate of FAT1 was 10%(49/490), and most of the mutations, including missense,truncating or inframe, occurred in the cadherin domains.
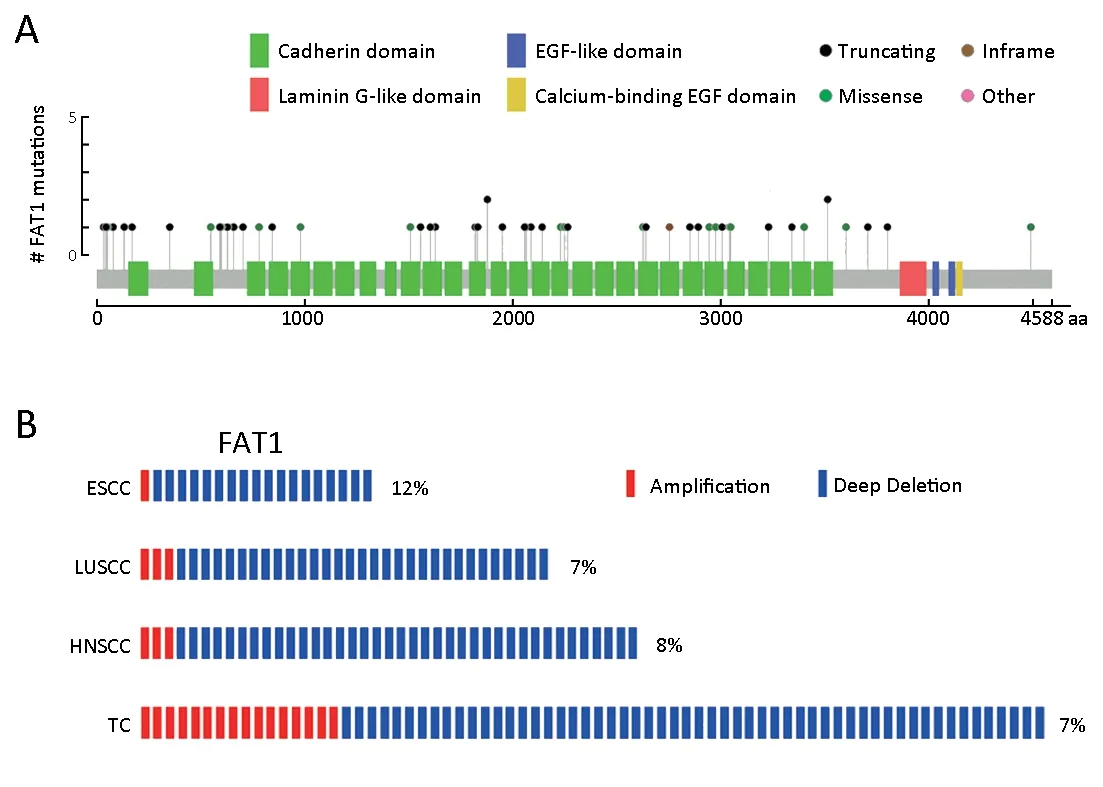
Figure 1 FAT1 exhibits genetic alterations with high frequency in esophageal squamous cell carcinoma (ESCC). (A) Frequency of FAT1 mutations in ESCC. Green box, cadherin domain; Red box, laminin G-like domain; Blue box, epidermal growth factor (EGF)-like domain;Yellow box, calcium-binding EGF domain; Black ball, truncating mutation; Green ball, missense mutation; Brown ball, inframe mutation;Pink ball, other mutation; (B) Frequency of FAT1 copy number variants (CNVs) in three types of squamous cell carcinomas and tumor cell lines. The percentage represents the approximate ratio of CNVs. Red box, amplification; Blue box, deletion. LUSCC, lung squamous cell carcinoma; HNSCC, head and neck squamous cell carcinoma; TC, tumor cell lines.
Furthermore, we analyzed the CNV features of FAT1 in 154 ESCC patients from our previous studies (7,10) and found that CNVs of FAT1 were altered in ESCC (Figure 1B).The total CNV rate, including the amplification of 1/154(0.6%) and the deletion of 18/154 (11.7%), was approximately 12.3% (19/154). We also consulted The Cancer Genome Atlas (TCGA) database (40) and observed similar CNV features for FAT1 in lung squamous cell carcinoma (LUSCC) and head and neck squamous cell carcinoma (HNSCC) using the cBioPortal (41,42) (Figure 1B). In LUSCC, the total CNV rate was approximately 6.8% (34/501), consisting of the amplification of 3/501(0.6%) and the deletion of 31/501 (6.2%). Consistently, in HNSCC, the total CNV rate was approximately 7.9%(41/522), consisting of the amplification of 3/522 (0.6%)and the deletion of 38/522 (7.3%). In addition, we analyzed the CNV rate of FAT1 in 995 tumor cell lines (TC) from the Cancer Cell Line Encyclopedia (CCLE) database (43)using the cBioPortal and found that the total CNV rate of FAT1 in the pan tumor cell lines was approximately 7.2%(72/995), including the amplification of 16/995 (1.6%) and the deletion of 56/995 (5.6%) (Figure 1B). Taken together,our results revealed frequent inactivation of FAT1 via mutations or deletions in ESCC and multiple other human cancers.
FAT1 expression is regulated by transcription factor E2F1
It is reported that FAT1 expression is downregulated in ESCC tissues (9,13,14). In addition to the genetic alterations described above, we hypothesized that transcriptional regulation may also play an important role in this process. To test this, we consulted the encyclopedia of DNA elements (ENCODE) database (44) and observed a putative binding site for the transcription factor E2F1 on the promoter region of FAT1 with the following identifiers:ENCFF000XCY, ENCFF000XEQ and ENCFF000ZLB(Figure 2A). Thus, a ChIP assay, using an E2F1 antibody,was then employed to confirm this occupancy. As expected,we observed a strong enrichment of E2F1 at the promoter region of FAT1 when compared with that in IgG control(Figure 2B).

Figure 2 E2F1 regulates FAT1 transcription. (A) Putative binding sites of E2F1 on the FAT1 promoter region from different cohorts in encyclopedia of DNA elements (ENCODE) database. Red box represents binding reads of E2F1; (B) Enrichment of E2F1 on FAT1 promoter in KYSE30 cells shown by chromatin immunoprecipitation (ChIP) and quantitative polymerase chain reaction (qPCR).Enrichment is determined as the amount of FAT1 promoter associated to E2F1 relative to immunoglobulin G (IgG) control; (C) Luciferase activity of pGL3-FAT1 vector was measured in KYSE30 cells upon E2F1 knockdown. Data are presented as ratio of the firefly luciferase activity to Renilla luciferase activity; RT-qPCR (D) and Western blot (E) analyses of E2F1 expression in KYSE30 cells upon E2F1 knockdown. Relative RNA levels of E2F1 were normalized to endogenous GAPDH; (F) RT-qPCR analysis of FAT1 expression in KYSE30 cells as described in (D). Relative RNA levels of FAT1 were normalized to endogenous GAPDH. *, P<0.05; **, P<0.01; ***, P<0.001 vs.control.
To ascertain whether E2F1 regulates the transcription of FAT1, we constructed a luciferase reporter, namely pGL3-FAT1, which contained the promoter region of FAT1. The luciferase activity of pGL3-FAT1 was much higher than the control vector upon transfection into KYSE30 cells,and this increased luciferase activity was inhibited by siRNA-mediated E2F1 knockdown (Figure 2C).Meanwhile, knockdown of E2F1 reduced FAT1 mRNA levels in KYSE30 cells (Figure 2F). The knockdown efficiency of E2F1 at the mRNA and protein levels was confirmed by RT-qPCR (Figure 2D) and Western blot(Figure 2E), respectively. Collectively, these results showed that E2F1 binds to the FAT1 promoter to activate its transcription in ESCC cells.
Downregulation of FAT1 promotes ESCC cell proliferation,migration and invasion
To explore the biological roles of FAT1, we performed a loss-of-function study using two small interfering RNAs(siRNAs) to knock down the FAT1 in KYSE30 and KYSE150 cell lines. RT-qPCR and In-cell western assays showed that the two siRNAs effectively downregulated both mRNA and protein levels of FAT1, when compared with the control siRNA (Figure 3A,B). The colony formation and Transwell assays were used to elucidate the effects of FAT1 knockdown on ESCC cell proliferation,migration and invasion. As shown in Figure 3C,D, the cells transfected with siRNAs targeting FAT1 exhibited an enhanced colony formation ability compared with the control siRNA transfected cells. Taking advantage of the Transwell system, we validated that knockdown of FAT1 enhanced the migration and invasion abilities of KYSE30 and KYSE150 cells (Figure 3E-H). Taken together, these results demonstrated that FAT1 knockdown strengthened the aggressive potential of ESCC cells.
Overexpression of FAT1 inhibits ESCC cell proliferation,migration and invasion
To test the gain-of-function of FAT1 in ESCC cell lines,we transfected FAT1 into KYSE30 and KYSE410 cell lines using an expression vector with all the key functional domains of FAT1 (truncated FAT1, which was named FAT1-Trunc) (18). The mRNA and protein levels of FAT1 were monitored by RT-qPCR and In-cell western assays,respectively (Figure 4A,B). FAT1 overexpression reduced the colony formation abilities of KYSE30 and KYSE410 cells compared with the control empty vector (Figure 4C,D). Furthermore, the Transwell assays revealed that FAT1 overexpression greatly attenuated the migration and invasion abilities of KYSE30 and KYSE410 cells (Figure 4E-H). Collectively, the results of both the loss-of-function and gain-of-function studies demonstrated that FAT1 may act as a tumor suppressor in ESCC.
FAT1 is involved in mitogen-activated protein kinase(MAPK) signaling pathway and cell adhesion process in ESCC cells
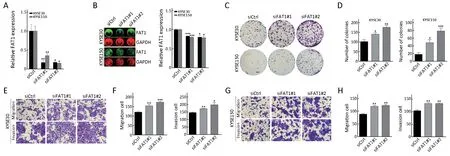
Figure 3 FAT1 knockdown promotes KYSE30 and KYSE150 cell migration, invasion and proliferation. Reverse transcription-quantitative polymerase chain reaction (RT-qPCR) (A) and In-cell western analyses (B) of FAT1 expression in KYSE30 and KYSE150 cells upon FAT1 knockdown. Relative RNA and protein levels of FAT1 were normalized to endogenous GAPDH; (C, D) KYSE30 and KYSE150 cells were transfected with the indicated siRNAs. After 24 h of transfection, cells were subject to colony formation assay. Representative results (C)and corresponding quantification (D) are shown (Scale bar =2 mm); (E-H) Representative results of Transwell migration/invasion assays and corresponding quantification in KYSE30 (E, F) and KYSE150 (G, H) cells after transfected with indicated siRNAs as described in (C,D) (Scale bar =100 μm). *, P<0.05; **, P<0.01; ***, P<0.001 vs. control.
To investigate the molecular mechanism of FAT1 in ESCC oncogenesis, we performed a RNA-seq analysis using control and FAT1 knocked-down KYSE30 cells, and subsequently analyzed the altered gene expression profiles(Supplementary Table S2). The KEGG pathway enrichment analysis revealed that upon FAT1 knockdown, the significantly differentially expressed genes were enriched in multiple pathways, including the MAPK signaling pathway and cell adhesion molecules (CAMs) (Figure 5A). The GO functional analysis showed that these genes were enriched in biological processes, including the cell adhesion, cell-cell signaling and epidermis development (Figure 5B). Genes involved in the MAPK signaling pathway and the cell adhesion process were selected and validated by RT-qPCR after the knockdown of FAT1 in KYSE30 cells. As expected,FAT1 knockdown induced the mRNA levels of MAP3K8,MAP2K2 and MAP2K6 and decreased the mRNA level of MAPK inactivator DUSP6 (Figure 5C). Meanwhile, FAT1 knockdown enhanced the mRNA levels of L1CAM and CDH5, which are involved in the cell adhesion process(Figure 5D). In conclusion, these results indicated that FAT1 is involved in the MAPK signaling pathway and the cell adhesion process in ESCC cells.
Discussion
ESCC is one of the most prevalent malignant tumors in China, with an elusive cancerogenesis and a poor prognosis(3,6). To study the molecular mechanism of ESCC tumorigenesis, several large-scale genomic sequencing studies have attempted to uncover the driving genomic variations (7-11). Interestingly, FAT1 is frequently subject to inactivating mutations in ESCC (8-11), which is accompanied by loss of heterozygosity or homozygous deletion (9,11). Given that the case number of ESCC in each of these studies is relatively small, we analyzed the mutations of FAT1 by using data that was combined from different cohorts by Du et al. (36) and revealed that FAT1 was indeed frequently mutated in ESCC. Furthermore, we analyzed the FAT1 CNV using our previous data (7,10) and databases, and the results showed a deletion of FAT1 in ESCC and other cancers. Although the function of FAT1 in tumorigenesis is still under debate in different types of tumors (18-21), it is considered a tumor suppressor gene in ESCC (9,13,14). Consistently, in this study, the in vitro experiments revealed that upregulation of FAT1 inhibited ESCC cell proliferation, migration and invasion, while FAT1 exhaustion resulted in the opposite effects. Thus, our study supplements the current theories that FAT1 shows mutations and deletions in ESCC and acts as a tumor suppressor gene.
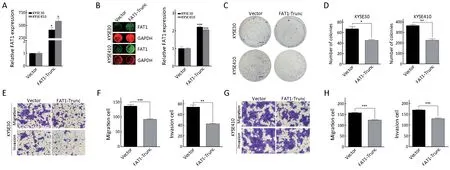
Figure 4 FAT1 overexpression suppresses KYSE30 and KYSE410 cell proliferation, migration and invasion. Reverse transcriptionquantitative polymerase chain reaction (RT-qPCR) (A) and In-cell western analyses (B) of FAT1 expression in KYSE30 and KYSE410 cells upon FAT1 overexpression. Relative RNA and protein levels of FAT1 were normalized to endogenous GAPDH; (C, D) KYSE30 and KYSE410 cells were transfected with indicated vectors. After 24 h of transfection, cells were subject to colony formation assay.Representative results (C) and corresponding quantification (D) are shown (Scale bar =4 mm); (E-H) Representative results of Transwell migration/invasion assays and the corresponding quantification in KYSE30 (E, F) and KYSE410 (G, H) cells after transfected with the indicated vectors as described in (C, D) (Scale bar =100 μm). *, P<0.05; **, P<0.01; ***, P<0.001 vs. control.
In addition to the dysregulation caused by the genetic alterations, an alternative possibility is that transcription is changed during tumorigenesis. To illustrate the transcriptional regulation of FAT1 mRNA expression in ESCC cells, we consulted the ENCODE database and found that the FAT1 promoter region was occupied by transcription factor E2F1 in several ChIP-seq projects. E2F1 is an important transcription factor that regulates the gene expression in multiple types of human cancers and has both oncogenic and tumor-suppressive properties (45-48).Matrix metalloproteinase (MMP) genes are direct transcriptional targets of E2F1 in non-small cell lung cancer (NSCLC) cell lines (49). E2F1 binds to the promoter region of the phosphatase of activated cells 1(PAC1) and actives its transcription in breast cancer cells(50). Nevertheless, little is known about the transcriptional regulatory effects of E2F1 in ESCC. Here, we characterized the binding and transcription activity of E2F1 upon the FAT1 promoter region, as well as the influence of E2F1 on FAT1 mRNA levels, and thus identified FAT1 as a transcriptional target of E2F1 in ESCC cells.
The results of RNA-seq and RT-qPCR revealed that FAT1 knockdown resulted in the altered expression of package of genes that are involved in the MAPK signaling pathway, which is consistent with previous report (13). The MAPK signaling pathway is an important signaling component that consists of a set of evolutionarily conserved kinase cascades from yeast to human, including MAPK kinase kinase kinase (MKKK), MAPK kinase kinase (MKK)and MAPK (51,52). It is reported that the MAPK pathway is dysregulated in ESCC and may function as an oncogene in ESCC tumorigenesis (53,54). On the other hand, the GO functional analysis and RT-qPCR also revealed that loss of FAT1 subsequently upregulated the expression of genes that participate in the cell adhesion process,including L1CAM and CHD5, which may play roles in the progression or metastasis of multiple cancers and correlate with poor outcomes (55-58). Collectively, the RNA-seq results revealed that FAT1 knockdown led to the abnormal expression of genes in the MAPK signaling pathway and cell adhesion process, further indicating that FAT1 is involved in ESCC tumorigenesis
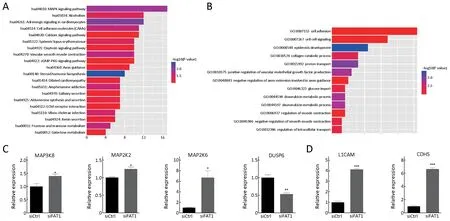
Figure 5 Global transcriptional identification of genes regulated by FAT1. KYSE30 cells were transfected with FAT1 siRNA or scramble control siRNA. After 48 h of transfection, cells were collected and subject to RNA-seq. Kyoto Encyclopedia of Genes and Genomes(KEGG) pathway enrichment analysis (A) and Gene Ontology (GO) functional analysis (B) of significantly differently expressed genes upon FAT1 knockdown in KYSE30 cells; Reverse transcription-quantitative polymerase chain reaction (RT-qPCR) showing the expression of genes involved in mitogen-activated protein kinase (MAPK) signal pathway (C) and cell adhesion process in KYSE30 cells after 48 h of transfection with the indicated siRNAs (D). Relative RNA levels of genes were normalized to endogenous GAPDH. *, P<0.05; **, P<0.01;***, P<0.001 vs. control.
Conclusions
In this study we identified FAT1 as the transcriptional target of E2F1. Moreover, we also validated that FAT1 exhibits tumor suppressive function in ESCC and participates in the MAPK signaling pathway and cell adhesion process. These results advance our understanding of mechanisms by which FAT1 is inactive and regulated in ESCC, and the data also highlight the importance of FAT1 in ESCC tumorigenesis.
Acknowledgements
This work was supported by the National Basic Research Program of China (973 Program) (No. 2015CB553906 and 2015CB553904), the National Natural Science Foundation of China (No. 81490753 and 81830086) and the Education Department of Liaoning Province in China (Scientific Research Projects, No. L2016038).
Footnote
Conflicts of Interest: The authors have no conflicts of interests to declare.
杂志排行
Chinese Journal of Cancer Research的其它文章
- Chinese guidelines for diagnosis and treatment of melanoma 2018 (English version)
- An update on biomarkers of potential benefit with bevacizumab for breast cancer treatment: Do we make progress?
- Association of cancer prevention awareness with esophageal cancer screening participation rates: Results from a populationbased cancer screening program in rural China
- Clinical significance of MET gene amplification in metastatic or locally advanced gastric cancer treated with first-line fluoropyrimidine and platinum combination chemotherapy
- A 18FDG PET/CT-based volume parameter is a predictor of overall survival in patients with local advanced gastric cancer
- Radiomics-based predictive risk score: A scoring system for preoperatively predicting risk of lymph node metastasis in patients with resectable non-small cell lung cancer
