Chinese guidelines for diagnosis and treatment of melanoma 2018 (English version)
2019-09-10NationalHealthCommissionofthePeopleRepublicofChina
National Health Commission of the People's Republic of China
1. Overview
2. Screening and diagnosis
2.1 Surveillance and screening of high-risk population
2.2 Diagnosis of melanoma
2.2.1 Clinical symptoms
2.2.2 Imaging diagnosis
2.2.3 Laboratory tests
2.2.4 Focus biopsy
2.3 Pathological diagnosis of melanoma
2.3.1 Criteria for pathological diagnosis
2.3.2 Standard pathological diagnosis of melanoma
2.3.3 Pathological report of melanoma
2.4 Clinical diagnostic criteria and route map of melanoma
3. Staging
4. Treatment
4.1 Surgical treatment
4.1.1 Wide excision
4.1.2 Sentinel lymph node biopsy (SLNB)
4.1.3 Lymph node dissection
4.1.4 Treatment of local recurrence or local metastasis
4.1.5 Postoperative adjuvant therapy (prevention and treatment for metastasis and recurrence)
4.2 Radiotherapy
4.2.1 Adjuvant radiotherapy
4.2.2 Plaque radiotherapy
4.3 Systemic therapy
4.3.1 Systemic therapy and response evaluation criteria
4.3.2 Symptomatic supportive therapy
4.3.3 Therapy of special lesions
1. Overview
Although melanoma is a rare malignant tumor in China,the mortality rate is high and the incidence rate is increasing year by year. There is a big difference between melanoma in China and Caucasians in Europe and America. The differences in pathogenesis, biological behavior, histological morphology, treatment methods and prognosis are quite different. Among Asians and other colored people, the primary melanoma of the extremities accounts for about 50%. The common primary sites are more common in the soles of the feet, toes, fingers, and lower extremities. They originate in the mucosa, for example, the melanoma of the rectum, anus, vulva, eye, or nasopharynx accounts for about 20%-30%; for whites, the primary melanoma of the skin accounts for about 90%, and the primary site is common in the back, the skin of the chest, abdomen and lower limbs; and melanoma originating from the mucosa only accounts for 1%-5%.
2. Screening and diagnosis
2.1 Surveillance and screening of high-risk population
Surveillance and screening for high-risk population of melanoma is helpful for early detection, diagnosis and treatment, and is also the key to improve the efficacy of melanoma treatment. In China, the high-risk population of skin melanoma mainly includes the history of severe sunburn, skin cancer, pigmented nevus and chronic inflammation of acral skin, as well as inappropriate treatment, such as salting, cutting, needle picking, rope stranding, etc. The high-risk factors for mucosal melanoma remain unclear. It is suggested that the high-risk population should check themselves regularly and go to a specialized hospital when necessary. They should not deal with it at will.
2.2 Diagnosis of melanoma
Melanoma occurs mostly on the skin, so visual inspection is the easiest way to diagnose early. Visual examination and palpation of primary lesions, affected sites and regional lymph nodes are common methods for the initial diagnosis of melanoma.
2.2.1 Clinical symptoms
Skin melanoma is mostly developed from nevus. The early malignant symptoms of nevus can be summarized as the following ABCDE rules:
A. Asymmetry: These lesions usually present as an asymmetrical pigmented macule.
B. Border irregularity: Irregular edge, or with incision or serration, and it does not have a smooth circular or elliptical contour like normal pigmented nevus.
C. Color variation: Normal pigmented nevus is usually monochromatic, while melanoma often manifests as dirty black, and can also have many different colors such as brown, brownish black, blue, pink, black, and even white.
D. Diameter: It is necessary to pay attention to pigmented nevus with diameter of >5-6 mm or obviously growing pigmented nevus. Melanoma is usually larger than normal nevus. It is better to perform biopsy on pigmented nevus with a diameter of >1 cm.
E. Elevation: Some melanomas usually have a slight bulge in an early stage.
The only downside of the ABCDE rule is that it does not take the growing rate of melanoma into account, which can have a significant change in weeks or months. Dermatoscope can make up for visual observation, and can detect and compare changes in suspected melanoma, and its application can significantly improve the accuracy of early diagnosis of melanoma. Melanoma can further develop satellites, ulceration, repeated unhealed, regional lymph node metastasis and transitional metastasis. Late-stage melanoma has different symptoms according to different metastatic sites, usually lung, liver, bone and brain.Melanoma of the eye and rectum is likely to metastasize to liver.
2.2.2 Imaging diagnosis
Imaging examination should be based on local conditions and the patient's economic situation. The necessary items include regional lymph nodes ultrasound (neck, armpit,groin, popliteal space, etc.), chest computed tomography(CT), abdominal pelvic ultrasound, CT or magnetic resonance imaging (MRI), whole body bone scan and brain CT or MRI. Patients with good economic conditions can take a full-body positron emission tomography-computed tomography (PET-CT), especially those with unknown primary origins. PET is an examination method that is easier to find subclinical metastases. For early-stage melanoma, PET is not sensitive to metastatic lesions and has a low benefit rate. For patients with stage III, PET-CT scans are more useful and can help identify lesions that cannot be clearly diagnosed by CT, as well as sites that are not visible on conventional CT scans (such as limbs). PETCT has advantages over ordinary CT in finding distant lesions.
(1) Ultrasound examination (Ultrasonography, US)
US is the most commonly used imaging examination method in clinical practice because it is easy to use, flexible,intuitive, non-invasive and portable. US of melanoma is mainly used to determine the nature of regional lymph nodes and subcutaneous nodules, and provides important information for the choice of clinical treatment methods and the development of surgical plans. Real-time contrastenhanced ultrasound can reveal hemodynamic changes in metastases, especially in identifying small liver and lymph node metastases.
(2) CT
Conventional plain and enhanced scanning (using iodine contrast agent) are widely used in clinical diagnosis and staging of melanoma. Besides, it is also commonly used in the evaluation efficacy of melanoma, tumor volume measurement, and metastasis evaluation of lung and bone and other organs.
(3) MRI
Conventional plain and enhanced scanning (using contrast agent Gd-DTPA) becomes a common imaging technique for clinical melanoma diagnosis and efficacy evaluation because of its non-radiation, high-tissue resolution, multiazimuth and sequence parameter imaging, as well as morphological binding function (including diffusionweighted imaging, perfusion-weighted imaging and spectral analysis).
(4) PET-CT
The advantages of whole body imaging of fluoro-18-deoxyglucose (18F-FDG) PET-CT are: 1) Tumor staging:it can comprehensively evaluate lymph node metastasis and distant organ metastasis through one examination; 2) Restaging: PET functional imaging is unaffected by the anatomical structure, it can accurately show the recurrence and metastasis of the anatomical structure or the complex part of the anatomical structure; 3) Efficacy evaluation: for the targeted drugs that inhibit tumor activity, the efficacy evaluation is more sensitive and accurate; 4) Guiding delineation of the target area and biopsy of the active area of the tumor lesion; 5) Evaluation of the malignancy and prognosis of the tumor. Conventional CT is less sensitive to the diagnosis of skin or subcutaneous metastasis, and PET-CT can make up for it.
2.2.3 Laboratory tests
Indictors like blood routine, liver and kidney function and lactate dehydrogenase (LDH), are mainly used for the preparation of follow-up treatment, while understanding the prognosis. Although LDH is not a sensitive indicator of metastasis, it can guide the prognosis. There is no specific serum tumor marker for melanoma, and it is not recommended to examine the tumor marker at present.
2.2.4 Focus biopsy
Biopsy methods for cutaneous melanoma include excisional biopsy, incisional biopsy and punch biopsy. Shave biopsy is not usually used. Routine excisional biopsy is recommended. The incision margin is 0.3-0.5 cm. The incision should follow the direction of dermatoglyph (such as the incision along the long axis of limbs). Avoid direct enlarged resection, biopsies should also be planed so as to not interfere of sentinel lymph node biopsy. Partial biopsy is not conducive to histological diagnosis and thickness measurement, and increases the risk of misdiagnosis and staging. Generally, incisional biopsy and punch biopsy are only used for diagnostic biopsy of large-scale lesions or special parts, such as lesions in the face, palm, sole, ear,finger, toe or subnail, or huge lesions, when complete excisional biopsy is not available, incision biopsy and punch biopsy can be considered.
2.3 Pathological diagnosis of melanoma
2.3.1 Criteria for pathological diagnosis
The most important method for the diagnosis of melanoma is histopathology, and immunohistochemistry staining is a main assistant method for differential diagnosis. Histopathological diagnosis is required for both primary superficial melanoma and a metastatic lesion, either by biopsy or surgical excision of tissue samples. Pathological diagnosis should be combined with clinical evidence, and the patient's medical history, imaging examination and other information should be fully evaluated.
2.3.2 Standard pathological diagnosis of melanoma
The standard of pathological diagnosis of melanoma consists of specimen handling, specimen sampling,pathological examination and pathological report.
(1) Key points of specimen handling 1) The surgeon should provide some important information of the lesion (ulcer,nodule, or colored patches), and surgical margins and important lesions can be marked with dye or suture; 2)Larger specimens must be cut and fixed at intervals of 3 mm or so; 3) The specimens should be fixed in 10% neutral buffer formalin for 6-48 h.
(2) Key points of specimen sampling: Ink the margins first,then cut the tissue at 2-3 mm intervals vertically to the skin surface in order to identify the thickness and invasion depth of the tumor. Choose an appropriate sampling method according to clinical requirements, specimen type and size,as well as the distance between the lesion and the cutting edge. Make sure that the thickest or deepest regions and the ulcers, if there are, are sampled. The skin between the main tumor and the satellite must be evaluated to find out the relationship between them. For tumors less than 2 cm in diameter, all tissues should be collected, and for tumors over 3 cm, 1 piece per 5 mm is recommended. There are two methods for margin sampling, that is, radial sampling perpendicular to actual margins and lateral cutting parallel to actual margins. Since lateral cutting cannot measure the distance between negative margin and the tumor, the radial sampling method is suggested as far as possible (Figure 1).Never place two pieces or more of skin tissue in an embedding box. And in order to precisely evaluate T staging by histology, make sure that the embedded sections have shown the structural layers of skin or mucous membrane at the site of tumor.
(3) Essential components of pathological examination
A. Gross evaluation: Orientate a specimen in anatomical position, then examine and descript the size, shape and color of the tumor. For a skin melanoma, inspection of superficial ulceration and transferred satellite focuses is essential, accompanied with the number, size and its distance to the main tumor if any satellite exists.
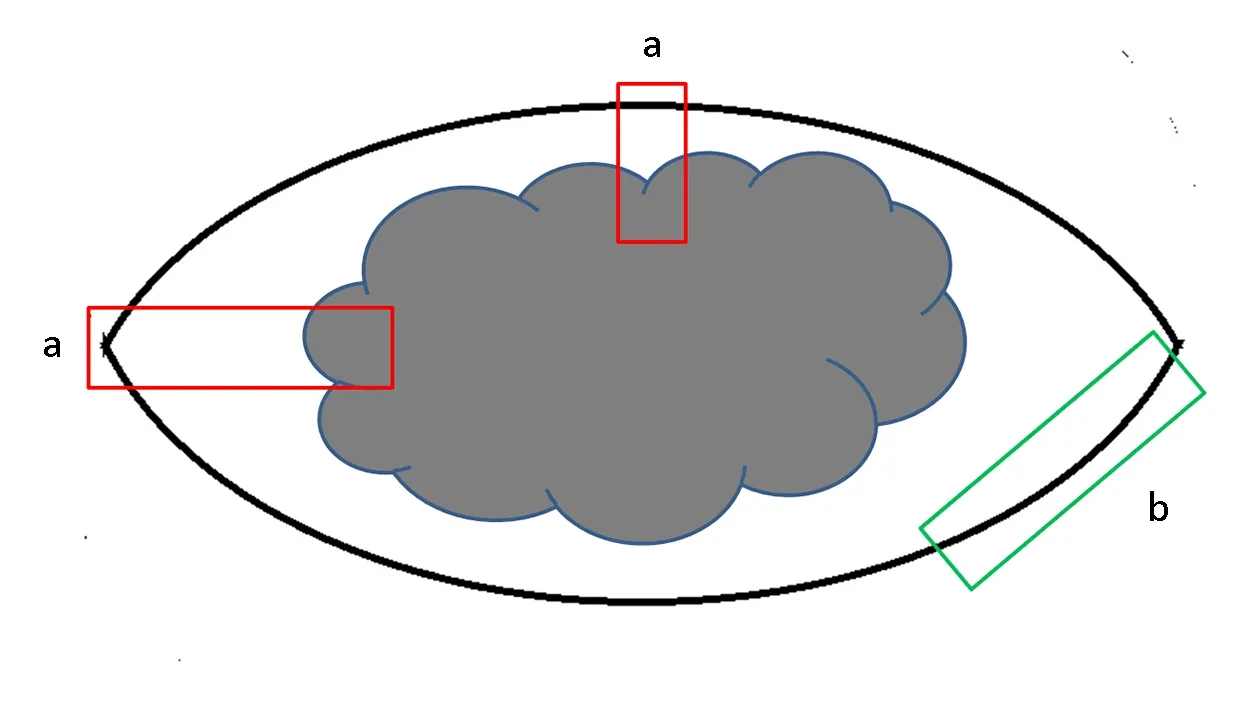
Figure 1 Sample of margin in skin melanoma. (a) Radial sampling perpendicular to actual margins; (b) Lateral cutting parallel to actual margins.
B. Microscopic evaluation: Diagnosis of melanoma refers to the WHO classification of skin tumors 2010 edition, and essential description contents should contain the following indexes: (a) Origin of melanoma, is it from skin or mucosa;(b) Histology subtypes of melanoma, of which the major four subtypes are superficial spreading, lentigo maligna,acral lentigine and nodular type. Rare subtypes include desmoplastic melanoma, melanoma arising from blue nevus, melanoma arising in giant congenital naevus,childhood melanoma and nevoid melanoma; (c) Invasion depth of melanoma. Breslow thickness is a quantitative index of depth in millimeters, and Clark level is a qualitative indicator indicating which level of the skin is infiltrated by melanoma; (d) Other prognostic indexes including ulceration, lymphovascular invasion, satellite or microsatellite nodules, mitotic rate and neurotropism.
Breslow thickness, defined as tumor thickness of melanoma of skin, is a fundamental index for T staging. It is a vertical dimension measured from the top of the granular layer of the epidermis to the deepest invasive cell for non-ulcerated lesions, while for the ulcerated lesions it is measured from the base of the ulcer to the deepest invasive part. Clark levels are defined as five levels according to the invasion depth: Level 1, tumor cells are confined to the epidermis (melanoma in situ). Level 2,tumor cells invade into but do not fill the papillary dermis.Level 3, tumor cells fill the papillary dermis and infiltrate into the papillary-reticular dermal interface. Level 4, tumor cells infiltrate into the reticular dermis. Level 5, tumor cells infiltrate into the subcutaneous tissues.
C. Immunohistochemistry: Because of the diversity morphologies of tumor cells, melanomas, especially the amelanotic lesions, need to be distinguished from different kinds of tumors such as carcinoma, sarcoma and lymphoma. Common characteristic markers of melanocytes include S100, Sox-10, Melan-A, HMB45, Tyrosinase and MITF. S100 is the most sensitive and is used as a marker to rule out melanoma if it is negatively expressed, but its specificity is relatively low and generally cannot be considered a definite diagnostic marker for melanoma.Melan-A, HMB45 and Tyrosinase are more specific, but their sensitivities are various. So we recommend that more than 2-3 of the former markers as well as S100 should be used if a differential diagnosis is necessary, in order to improve the detection rate of melanoma.
D. Special melanoma subtypes: Mucosal melanomas: usually they are invasive lesions, and a pagetoid dissemination of melanocytes in mucosal epithelium can be seen sometimes.The tumor cells manifest as epithelioid, spindle,plasmacytoid or balloon-like, with melanin or not.Commonly, immunohistochemistry is needed to make a diagnosis of melanoma. Uveal melanomas: Uveal melanomas are classified as spindle cell type, epithelioid cell type and mixed type based on the morphology of melanocytes. Cell type is an independent prognostic factor of uveal melanoma in predicting metastatic risks, with the best prognosis for spindle cell type and the worst outcome for epithelioid cell type.
2.3.3 Pathological report of melanoma
A histopathological report of primary skin melanoma should contain all the essential factors associated with the treatment and prognosis predicting of the tumor,including: tumor site, specimen type, tumor size,histological subtype, Breslow thickness, with or without ulceration, invasion depth (Clark level), mitotic rate,surgical margin status (including the distance between the neoplasm and resection margin by the unit of millimeter under the microscope and the melanoma subtype in the margin if it is positive), satellite or microsatellite,lymphovascular invasion and neurotropism (Table A2 in Appendix 1). Besides, auxiliary diagnosis such as immunohistochemistry or fluorescence in situ hybridization (FISH)test as well as genetic tests associated with targeted therapy(BRAF, c-KIT mutation et al.) should also be reported.Another component in pathology report is status of sentinel or regional lymph node, information such as the sum of the nodes, the number of metastatic nodes, and with or without extracapsular invasion needs to be reported. For those cases difficult to make a diagnosis, a multi-center consultation is recommended.
2.4 Clinical diagnostic criteria and treatment modalities of melanoma
The diagnosis of melanoma mainly depends on clinical symptom and pathological diagnosis, and gets complete information on the stage combined with the examination of systemic imaging (Figure 2).
3. Staging
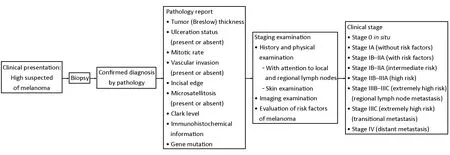
Figure 2 Clinical stage of melanoma and treatment modalities.
The staging of melanoma is crucial for prognostic assessment and treatment decision-making. Melanomas in different sites have different pTNM staging parameters.The pTNM staging of cutaneous melanomas is given in Appendix 1 which can be applied to melanomas in lip,eyelid, external ear, other parts of face, skin of scalp and neck, trunk, upper limb, shoulder, lower limb, buttock, skin spanning lesions, labium majus, labium minus, clitoris,transvaginal lesions, vulva, prepuce, glans, corpus penis,transvaginal lesions of penis, penis and scrotum. The pTNM staging of head and neck mucosal melanoma can be seen in Appendix 2. The scope of application includes nasal cavity, sinus, oral cavity, oropharynx, nasopharynx, larynx and hypopharynx. Ocular melanoma includes iris melanoma, ciliary choroidal melanoma and conjunctival melanoma. They all have different pTNM staging systems respectively. Details can be found in the relevant chapters of the eighth edition (2016) American Joint Committee on Cancer (AJCC) staging system. However, there is no pTNM staging system for melanoma arising from the digestive tract (esophagus, small intestine and large intestine). According to the guidelines on clinical diagnosis and treatment of melanomas in China, it is suggested to describe the depth of invasion of the digestive tract.Vaginal melanomas don't have pTNM staging either. We can determine the pTNM staging of cervical melanoma by reference to the staging of cervical cancer. The pTNM staging system of meningeal melanoma is same as that of other meningeal tumors.
4. Treatment
Since a variety of methods and disciplines are involved in the treatment of melanoma, we should attach importance to multidisciplinary team-based diagnosis and treatment,avoiding the limitations of single-discipline treatment.Thus, we can provide one-stop medical services for patients, promote disciplinary communications, and promote developing principles and guidelines of treatments based on multi-disciplinary consensus. The proper treatment decisions should be made by the support of highlevel evidence. The regional and economic differences should also be taken into account.
4.1 Surgical treatment
Most patients who diagnosed as early melanomas can be cured by surgical treatment.
4.1.1 Wide excision
Wide excision is recommended for patients who were preliminary diagnosed as melanoma without distant metastasis. The safe margin/optimal surgical margin of complete resection is determined by the depth of tumor invasion (Breslow thickness) according to the pathological report: 1) Thickness ≤1.0 mm, margin 1 cm; 2) Thickness 1.01-2 mm, margin 1.01-2 cm; 3) Thickness >2 mm,margin 2 cm ; 4) Thickness > 4 mm, margin 2 cm. From the perspective of surgery, acral lentiginous melanoma should not only consider complete tumor removal, but also fully consider the functions as much as possible, especially finger function.
Amputation is not recommended for the treatment of most acral lentiginous melanomas, except melanomas on fingers or toes as it does not cause too much loss of function, and can remove the tumor more thoroughly.
4.1.2 Sentinel lymph node biopsy (SLNB)
Sentinel lymph node is defined as the first lymph node for cutaneous melanoma metastasis. SLNB is a surgical procedure developed to accurately stage patients with cutaneous melanoma. It is generally recommended for patients with tumor thickness greater than 0.8 mm or primary lesions with ulceration. SLNB can be performed at the time of wide resection or subsequently.
4.1.3 Lymph node dissection
Indications: patients with positive sentinel lymph nodes,and patients with stage III disease diagnosed by physical examination, imaging and pathology.
Principles of surgery: An anatomically complete dissection of involved nodal basin is required. As a measurement of the completeness of a regional lymph node dissection, the number of lymph nodes dissected is required at least 10 on the groin, and 15 for cervical or axillary lymph node dissection. Prophylactic lymph node dissection is not recommended.
Inguinal lymph node dissection: Superficial group and deep group dissection was required for patients with pelvic lymph nodes metastasis diagnosed by imaging; it is also required for patients with 3 or more suspected lymph nodes metastasis in the superficial group or metastasis of Cloquet's node (lymph node is black or swollen) during operation.
Axillary lymph node dissection: A complete dissection of LEVEL I-III lymph nodes is recommended when subclavicular lymph node metastasis is confirmed before or during operation. While dissection of LEVEL I-II lymph nodes is recommended when there is no evidence of subclavicular lymph node metastasis or the sentinel lymph node is confirmed as micrometastasis.
Cervical lymph node dissection: An extensive or total cervical lymph node dissection should be avoided. For patients in clinical stage III disease, the range of dissection should be determined according to the region of swollen lymph nodes and primary lesion.
4.1.4 Treatment of local recurrence or local metastasis
Local recurrence or limb metastasis may be treated by surgical excision, intratumoral injection of oncolytic virus,isolated limb infusion chemotherapy (ILI), and isolated limb perfusion chemotherapy (ILP). Surgery remains the primary treatment for local recurrence. Intratumoral injection can be recommended when local recurrence or multiple transitional lesions cannot be treated by ILI or ILP due to complications.
4.1.5 Postoperative adjuvant therapy (prevention and treatment for metastasis and recurrence)
Adjuvant therapy generally refers to a variety of supplementary treatments except surgery, the main purpose of which is to reduce the risk of recurrence and metastasis.At present, the recommended strategy with most evidence is high-dose interferon α-2b. Besides, the latest adjuvant therapy methods include the combination therapy of BRAF and MEK inhibitors (for patients with BRAF mutation) and anti PD-1 monoclonal antibody. But the two regimens are lack of evidence-based medical evidence and application experience among Chinese people.
(1) Applicable population of adjuvant therapy: patients with high-risk (stage IIB-IIIA) and very high-risk (stage IIIB-IV) melanoma are recommended to receive postoperative adjuvant therapy to improve the survival. So far interferon α-2b is approved to be the adjuvant therapy for patients with high-risk melanoma.
(2) Main adverse reactions of interferon: flu-like symptoms,myelosuppression, hepatotoxicity, fatigue, mental and neurological symptoms, autoimmune reaction and so on.
(3) Contraindications of interferon: pregnancy or planned pregnancy, severe hepatic or renal organ dysfunction,decompensation of liver cirrhosis, recent immunosuppressive therapy for hepatitis, active or previous autoimmune diseases requiring immunosuppressive therapy, organ transplants, uncontrolled thyroid diseases after routine treatment, epilepsy or other central nervous system diseases, past serious heart disease includes unstable or uncontrolled heart disease in past 6 months, severe mental disorders, and psoriasis.
4.2 Radiotherapy
In general, melanoma was considered to be insensitive to radiotherapy, but in some special situations, radiotherapy is still an important treatment. Radiotherapy included radical radiotherapy for primary lesions of patients who could not tolerate operation or who had positive surgical margins but was not suited for reoperation; adjuvant radiotherapy for patients whose surgery margin of the primary lesion resection was insufficient and who could not receive extensive surgical excision, or who has received lymph node dissection; and palliative radiotherapy for brain and bone metastases.
4.2.1 Adjuvant radiotherapy
Adjuvant radiotherapy was recommended for patients with melanoma of head and neck (especially for nasal cavity) or who has received lymph node dissection, in order to obtain local control.
Principle of adjuvant radiotherapy was as follows:
(1) LDH <1.5 times normal upper limits
(2) Lymph node metastasis status
1) Lymph node extracapsular extension
2) Parotid: ≥1 involved node, any size of involvement
3) Cervical: ≥2 involved nodes and/or ≥3 cm tumor within a node
4) Axillary: ≥2 involved nodes and/or ≥4 cm tumor within a node
5) Inguinal: ≥3 involved nodes and/or ≥4 cm tumor within a node
(3) Should meet both (1) and any subline of (2). Adjuvant radiotherapy can improve local disease control, but not for progression free survival (PFS) or overall survival (OS). It might aggravate the complications such as edema, pain,fibrosis of skin and subcutaneous tissue. Therefore,adjuvant radiotherapy is only recommended to prevent regional recurrence, or as a backup strategy when system adjuvant therapy cannot be accepted.
4.2.2 Plaque radiotherapy
Plaque radiotherapy was recommended for small or medium-sized choroid melanoma. As one kind of brachytherapy, plaque radiotherapy places a metal plate with 125I or 106Ru radioactive particles on the surface of local sclera.
4.3 Systemic therapy
For advanced melanoma patients without contraindications, systemic therapy can reduce tumor burden,improve tumor-related symptoms, improve quality of life,and prolong survival.
4.3.1 Systemic therapy and response evaluation criteria(1) Targeted therapies
To date, Vemurafenib is the only molecular-targeted drug approved by the China Food and Drug Administration(FDA) for the treatment of patients with BRAF-V600E mutation melanoma. A number of multi-center phase III clinical trials have fully demonstrated the significant survival benefits of Vemurafenib. Recommended dose: 960 mg orally twice daily. Patients should pay attention to the damage of liver function. The most common toxicities are photo-allergy, musculoskeletal complaints, diarrhea, handfoot syndrome, rash, and high blood pressure.
(2) Chemotherapy
Traditional cytotoxic drugs including dacarbazine,temozolomide, fluoxetine, paclitaxel, albumin paclitaxel,cisplatin and carboplatin, are not effective in advanced melanomas with an overall response rate (ORR) of 10%-15%. Dacarbazine is the gold standard among chemotherapy drugs with the best survival benefit than any other drugs. Multiple combinations of chemotherapy and mono-chemotherapy did not bring significant survival benefits in advanced melanoma patients.
(3) Immunotherapy
Anti-PD-1, anti-CTLA-4 agents and IL-2 are the immunotherapies approved by the US FDA currently.These drugs showed significantly survival benefits in advanced cutaneous melanoma patients. It should be noted that the value of the above treatment needs further studies in Chinese acral and mucosal melanomas.
(4) Response evaluation criteria of systemic therapy
Response Evaluation Criteria in Solid Tumors 1.1(RECIST 1.1) is usually used to evaluate efficacy in chemotherapy and targeted therapy, the changes of LDH and tumor necrosis also help to evaluate efficacy. Imaging evaluation is usually performed every 6-8 weeks during treatment. At the same time, comprehensive assessment of the patient's symptoms, signs and treatment-related adverse reactions through dynamic observation showed be considered. Immunotherapy can be evaluated by RECIST 1.1 or iRECIST criteria.
4.3.2 Symptomatic supportive therapy
Moderate rehabilitation exercises can enhance immune function. In addition, symptomatic supportive therapy should be strengthened in patients with advanced melanoma, including active analgesia, correction of anemia,correction of hypoalbuminemia, enhancement of nutritional support, control of blood glucose in patients with diabetes, and treatment of accompanying symptoms such as hydrothorax, ascitic fluid and jaundice.
For patients with poor performance status, understanding the mentality of patients and their families can be considered. We should take measures to adjust their corresponding state into positive psychology, and reduce their depression and anxiety through soothing treatment to make them feel safe and comfortable.
4.3.3 Therapy of special lesions
(1) Therapy of liver metastases
Liver metastases occur in about 50%-80% of patients with advanced melanoma, especially mucosal melanomas from choroid, nasal cavity and rectum, which are more prone to liver metastases. The prognosis of melanoma with liver metastases is extremely poor due to poor efficacy of systemic chemotherapy and limited opportunities for treatment. It is estimated that the median survival time is 2-6 months, and the 1-year survival rate is 13% under active treatment. The extent of progression of liver metastases often determines the patient's survival, which is even more important for survival than the primary or other organ metastases. Compared with systemic chemotherapy,hepatic arterial chemotherapy and hepatic arterial chemoembolization, the response rate of systemic chemotherapy is less than 1%, but the hepatic arterial chemoembolization is 36%. The platinum-based hepatic arterial chemoembolization as the only approach that can benefit and improve survival is obviously superior to the other two treatments.
(2) Therapy of brain metastases
Melanoma is the malignancy with the high rate of dissemination to the brain. It is reported that the incidence of brain metastasis of malignant melanoma (BMM) is 8%-46%, and about 2/3 melanoma patients have BMM in the autopsy studies. BMM is the end phase of melanoma,confering a poor prognosis with high mortality rate and rapidly progressing. Surgery is still an important treatment approach for BMM nowadays in China. There is evidence that the median survival time of BMM after surgery is 5.0-6.7 months.
Indications for surgery: isolated metastasis, increased intracranial pressure and obstructive hydrocephalus caused by mass tumor occupation, uncontrollable epilepsy. Local radiotherapy is recommended for patients after surgery to eliminate residual lesions and subclinical lesions that may have spread. SRS is recommended at first by National comprehensive cancer network (NCCN) for radiotherapy of BMM and post-operation patients. Patients with symptomatic brain metastases and clinical or pathological meningeal metastases who cannot receive SRS can be provided with whole brain radiotherapy (WBRT).However, the value of WBRT for patients with poor performance score or excessive brain metastases may not necessary.
(3) Therapy of bone metastases
The therapy of melanoma bone metastases, similar to the other tumor bone metastases, aims to reduce the occurrence of bone-related events and relieve pain. It is mainly treated according to the site of metastasis (whether or not load-bearing bone) and symptoms. For isolated bone metastasis, surgery and local radiotherapy after surgery will be optimal therapies. For patients with multiple bone metastases, systemic treatment combined with local treatment can be considered, and local treatment includes surgery, bone cement filling and local radiotherapy.Patients with bone metastases should use bisphosphonate regularly to reduce the occurrence of bone-related events,while painkillers should be used regularly to relieve pain.Strategic decision for patients with spinal cord compression should be made according to different individual situations.Surgery and postoperative radiotherapy are preferred for patients who were diagnosed with better prognosis and light tumor burden. However, radiotherapy alone may be considered for patients who were diagnosed with poor prognosis. Radiotherapy could be performed after internal fixation to relieve bone pain.
杂志排行
Chinese Journal of Cancer Research的其它文章
- An update on biomarkers of potential benefit with bevacizumab for breast cancer treatment: Do we make progress?
- Association of cancer prevention awareness with esophageal cancer screening participation rates: Results from a populationbased cancer screening program in rural China
- FAT1, a direct transcriptional target of E2F1, suppresses cell proliferation, migration and invasion in esophageal squamous cell carcinoma
- Clinical significance of MET gene amplification in metastatic or locally advanced gastric cancer treated with first-line fluoropyrimidine and platinum combination chemotherapy
- A 18FDG PET/CT-based volume parameter is a predictor of overall survival in patients with local advanced gastric cancer
- Radiomics-based predictive risk score: A scoring system for preoperatively predicting risk of lymph node metastasis in patients with resectable non-small cell lung cancer
