A 18FDG PET/CT-based volume parameter is a predictor of overall survival in patients with local advanced gastric cancer
2019-09-10JinlingSongZhongwuLiPuyunChenJiangyuanYuFengWangZhiYangXuejuanWang
Jinling Song, Zhongwu Li, Puyun Chen, Jiangyuan Yu, Feng Wang, Zhi Yang, Xuejuan Wang
Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education/Beijing), 1Department of Nuclear Medicine; 2Department of Pathology, Peking University Cancer Hospital & Institute, Beijing 100142, China
Abstract Objective: The present study investigated the prognosis value of preoperative fluorodeoxyglucose (FDG)positron emission tomography/computed tomography (PET/CT) in patients with local advanced gastric cancer(LAGC).Methods: In total, 144 patients [median age 63 (range: 48-80) years old] with LAGC underwent 18F-FDG PET/CT prior to any treatment. The maximum standardized uptake values (SUVmax), mean standardized uptake values (SUVmean), metabolic tumor volume (MTV) and total lesion glycolysis (TLG) of the primary lesion were measured on PET/CT and correlated with clinicopathological features and survival.Results: Significant differences in SUVmean, SUVmax, MTV and TLG were found according to Lauren's classification, histologic grade and T category (P<0.05). During the 26.5-month follow-up, 51 (35.4%) patients died and 70 (48.6%) exhibited disease progression. The optimal thresholds of MTV and TLG were 15.1 cm3 and 47.3 cm3, respectively. The 3-year progression-free survival (PFS) and overall survival (OS) for patients with high TLG values were 30% and 38% compared to 38% and 47% for low TLG values, respectively (P<0.05). Univariate and multifactor analyses demonstrated that lymph node metastasis and T stage were independent prognostic factors for PFS; T stage, histologic grade and TLG were independent prognostic factors for OS (P<0.05). Molecular markers had no relationship with patient's outcomes.Conclusions: Metabolic activity of primary gastric tumors from 18F-FDG PET/CT is a prognostic factor in patients with LAGC.
Keywords: Gastric cancer; 18F-FDG PET/CT; metabolic parameters; prognosis
Introduction
Gastric cancer remains a leading cause of cancer-related deaths worldwide and causes approximately 700,000 deaths annually, especially in China (1). Given its aggressive nature, most gastric cancer cases are diagnosed in advanced stages in Chinese population, with overall survival (OS)being less than 12 months (2). Research has established the role of first-line palliative chemotherapy in advanced gastric cancer, particularly combination regimens, by improving survival and relieving symptoms compared to the best supportive care (3,4). However, patients do not benefit uniformly, and many patients experience considerable toxicity without being significantly benefitted(5). Therefore, information on whether patients achieve long-term survival through treatment is important to optimize treatment plans and improve risk-adapted treatment strategies.
Conventional radiologic technologies, including computed tomography (CT) and magnetic resonance imaging (MRI), have been more widely used to evaluate the response of patients to chemotherapy rather than to predict the prognosis of patients with gastric cancer. As a functional multimodality imaging system, several studies have reported fluorine-18 fluorodeoxyglucose (18F-FDG)positron emission tomography/computed tomography(PET/CT) to be a potentially effective noninvasive tool to evaluate therapeutic response and predict survival early in the treatment course for malignant tumors. However, the role of PET/CT in predicting the prognosis of patients with gastric cancer remains controversial. Some studies reported a longer survival in patients with negative PET compared to those with positive PET, whereas other studies have not identified any difference in the survival rate between patients with high and low FDG uptake.Furthermore, a comprehensive analysis of the relationship between semi-quantitative metabolic parameters at the primary tumor and inferior outcomes has not yet been conducted (6-9). Recent studies have revealed that volumebased parameters, such as metabolic tumor volume (MTV)and total lesion glycolysis (TLG), may provide both volume and metabolic information for prognosis and treatment response, making them better factors than maximum standardized uptake values (SUVmax).
Several reports have shown that gastric cancers have a high prevalence of genetic mutations and amplification of signalling proteins, including human epidermal growth factor receptor 2 (HER2), epidermal growth factor receptor (EGFR) and c-MET (10-12). These molecular indicators were correlated with targeting regimens and poor prognosis and may be related to metabolic changes of glucose (13). Moreover, Lauren's diffuse gastric cancers appear to have a different pattern of tumor glucose utilization and behaviour compare with intestinal gastric adenocarcinoma.
Thus, we aimed to evaluate the prognostic value of multiple metabolic parameters from preoperative18F-FDG PET/CT in patients with local advanced gastric cancer(LAGC) and investigate the prognostic values of HER2,EGFR and c-MET status for stratifying gastric patients and determining individualized treatment.
Materials and methods
Patients
This study was approved by an Investigational Review Board of the Peking University Cancer Hospital.Consecutive patients between January 2010 and December 2015 in Beijing Cancer Hospital were retrospectively chosen and enrolled based on the following inclusion criteria: 1) histologically proven GC or gastroesophageal junction cancer (GEJC) based on examination of surgical specimens; 2) underwent radical total or subtotal gastrectomy with D2 lymph node dissection; 3)18F-FDG PET/CT was performed prior to any therapy; and 4)availability of complete medical history and clinicopathological data. The exclusion criteria were as follows: 1)secondary malignant disease; 2) serious infection or inflammation [e.g. human immunodeficiency virus (HIV)];or 3) hepatic or renal dysfunction. Data from 144 patients constituted the final clinical database.
Pathologic criteria
Data regarding the following pathological parameters were collected from the surgical pathology report of cases that underwent resection: histologic diagnosis, Lauren classification, histologic grade, tumor stage, lymph node status, Ki-67 index and expression of HER2, c-MET and EGFR, if available. HER2 positivity was characterized as a score of 2+ or 3+ based on immunohistochemistry (IHC).EGFR or c-MET were scored as previously reported, and a score of 2+ or 3+ was defined as positive expression (14,15).
18F-FDG PET/CT acquisitions
Patients were instructed to fast for at least 6 h before the PET scan. Blood glucose level was measured to ensure that it was <200 mg/dL.18F-FDG was administered intravenously at a dosage of 3.7 MBq/kg. Approximately 60±10 min post-injection, a whole-body acquisition was initiated in 6-8 bed positions (1 min/bed) using a hybrid system (PHILIPS Gemini TF, Cleveland, Ohio, USA) that covered the area from the base of the skull to the upper thigh. This was followed by a CT component acquired in non-contrast phase (modulated 100 mAs, 120 kV, slice thickness: 3 mm) for attenuation correction and for anatomical localization purposes. Head acquisition was performed in one bed position (8-10 min/bed).
Imaging interpretation
Two experienced nuclear physicians were assigned to interpret each patient's PET imagines and data using a PHILIPS EBW workstation. A scan was considered to be positive for GC/GEJC lesions in the presence of a focal18F-FDG concentration and wall thickening in the areas of the stomach and gastroesophageal junction.
The SUVmax, SUVmean, MTV and TLG were assessed for the primary lesion, and these parameters were determined in a 3D-manner using the same vendorprovided software (PHILIPS). MTV was estimated by selecting the volume of interest (VOI) on the axial image and the size of the VOI was checked on the corresponding coronal and sagittal images to ensure that it included the entire active tumor in the VOI. To define the contouring margins around the target lesion, we used a SUVmaxof 2.5 as a central value and a margin threshold that could exactly cover the tumor lesion. SUVmaxwas calculated as (decaycorrected activity/tissue volume)/(injected dose/body weight), and TLG was calculated by multiplying SUVmeanand MTV (TLG=SUVmean× MTV).
Follow-up examinations and patient outcomes
The patients underwent clinical follow-up with serum biochemical tests, endoscopy and enhanced abdominopelvic CT every 3-6 months with or without follow-up18F-FDG PET/CT. When clinical assessment, serum tumor markers or imaging studies revealed any abnormality, additional diagnostic studies or pathological confirmation were performed to evaluate cancer progression. Progression-free survival (PFS) was defined as the time from the date of treatment to the time when tumor progression was first confirmed. OS was defined as the time from the treatment to the time of death by any cause.
Statistical analysis
Continuous variables were expressed as medians (range)and were compared using nonparametric test. The association between OS, PFS and SUVmax, SUVmean, MTV and TLG as continuous variables were analyzed.Continuous SUVmax, SUVmean, MTV and TLG value were dichotomized for ease of clinical utility. A maximally selected log-rank statistics approach was used to perform a cut-off point analysis. In the maximally selected log-rank statistics approach, selected values of SUVmax, SUVmean,MTV and TLG are examined as candidates for the cut-off point.
Univariate and multivariate analyses with clinicopathologic factors were performed to assess the association of PFS or OS and metabolic FDG PET parameters(SUVmax, SUVmean, MTV and TLG) using Kaplan-Meier method with log-rank test and Cox proportional hazards model, respectively. OS curves were generated using Kaplan-Meier estimates, and the significance of difference between survival curves was tested using log-rank tests.
All P-values were two-sided, and P<0.05 was considered to be statistically significant. Statistical analysis was performed using IBM SPSS Statistics (Version 23.0; IBM Corp., NewYork, USA) and R version 3.0.0 (http://www.rproject.org/).
Results
Patient characteristics
In total, 144 consecutive patients (114 males, 30 females)were included in this study. The patient pre-treatment characteristics are summarized in Table 1. The median age was 63 (range: 48-80) years old. The primary tumor was localized in the proximal third of the stomach in 62 patients(43.1%), in the middle third in 32 (22.2%) patients and in the distal third in 50 (34.7%) patients. The tumor was classified as stage T2 in 20 patients, stage T3 in 51 patients and stage T4 in 73 patients. Thirty-five patients had no lymph node metastases, whereas lymph node metastases were detected in 109 patients. According to the Lauren classification, 92 (72.4%) tumors were undiffused subtype and the remaining 35 (27.6%) were diffused subtype. Most of the tumors (97, 72.4%) showed aggressive histology(moderately-poor and poor). Of the 120 patients available,a high Ki-67 index was observed in 71 patients (59.2%).Positive HER2 expression was presented in 39 patients(30.2%), and most of the tumors (69.8%) lacked HER2 expression. Similar results were revealed in c-MET (18.0%positive) and EGFR expression (42.3% positive). All of patients underwent radical gastrectomy, with 52.8% of subtotal and 47.2% of total resection. Most of the patients(94.4%) underwent D2 lymphadenectomy.
Clinicopathologic characteristics and metabolic parameters
Of the 144 patients enrolled, the median of SUVmax,SUVmean, MTV and TLG values were 6.30 (range,2.50-58.10), 3.68 (range, 1.91-19.92), 15.52 (range,1.73-166.66) cm3and 60.10 (range, 4.90-1,721.10) cm3,respectively.
Statistical analysis revealed no significant differences in metabolic parameters in terms of age and lymph node metastasis (Table 1). However, there were significant differences in SUVmean, SUVmax, MTV and TLG valuesaccording to Lauren's classification, histologic grade and T category (P<0.05). The undiffused type and moderately differentiated tumors showed significantly higher18F-FDG uptakes than the diffused type and other differentiated tumors. A higher SUVmeanvalue was also observed in patients with GEJC and the HER2-positive group.Unfortunately, no statistical differences in metabolic parameters were found according to other molecular pathological markers, including Ki-67 index, c-MET or EGFR expression.
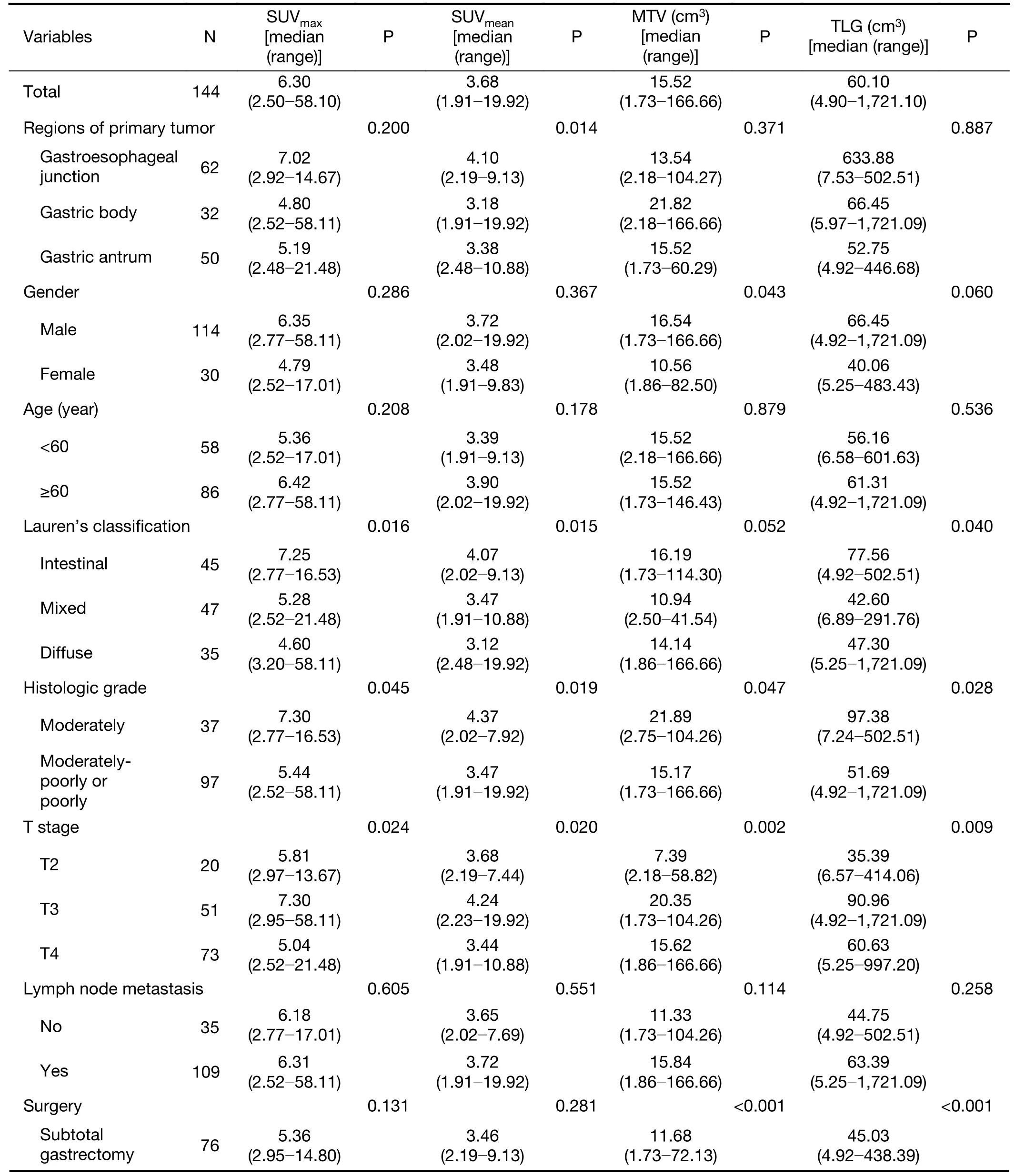
Table 1 Clinicopathological characteristics and metabolic parameters
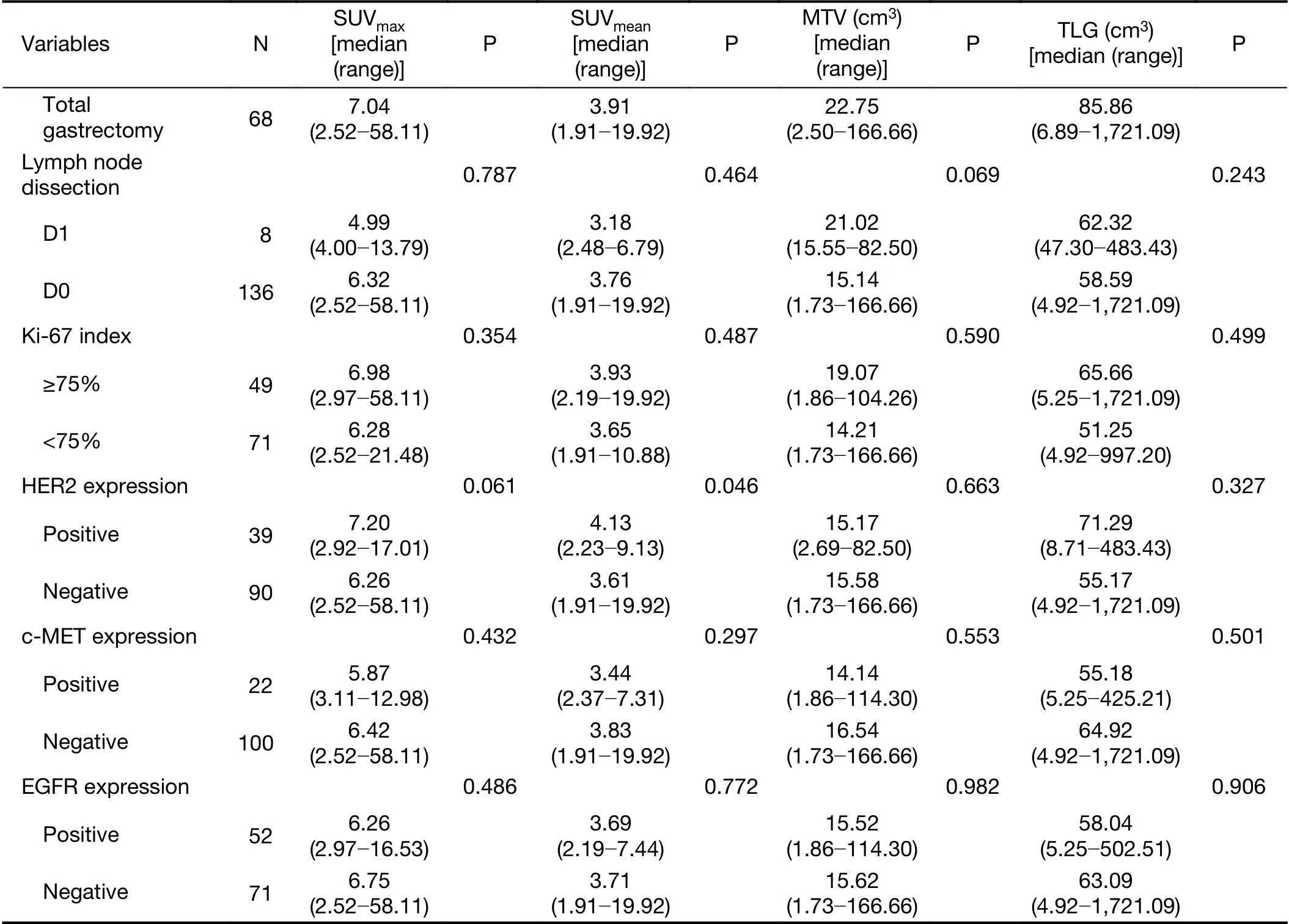
Table 1 (continued)
Survival prediction
The median follow-up time in 144 patients was 26.5 (range:6.0-81.0) months. In total, 51 patients (35.4%) died and 70 patients (48.6%) progressed as a result of related disease during the follow-up time; conversely, 74 patients remained in remission with no evidence of disease recurrence. Two-year OS and PFS of all patients were 61.8% and 48.6%, respectively.
Patient outcomes were compared according to the quantitative metabolic parameters of PET in Table 2. Of these four quantitative metabolic parameters, only MTV and TLG of the primary lesion were significantly correlated with the outcomes. SUVmaxand SUVmeanwere not significant predictors of outcome in this analysis. The optimal cut-off values of MTV and TLG were 15.1 cm3and 47.3 cm3, the sensitivities and specificities of MTV were 62.7% and 52.7%, and those of TLG were 68.6% and 47.3%. Based on the optimal threshold, PET parameters were dichotomized to generate Kaplan-Meier survival plots. Survival differed significantly between patients with high MTV or TLG values and low values based on the log-rank test. The 3-year PFS for patients with high TLG values was 30% compared to 38% for patients with low TLG values (P=0.032). The prognostic impact of pretreatment PET with regard to 3-year OS remained significant between the high TLG and low TLG groups:38% vs. 47%, respectively (P=0.028). Patients with low TLG values had median PFS of 26.0 (range: 7.0-71.0)months and an OS of 31.5 (range: 12.0-71.0) months;patients with high TLG values had mean PFS of 21.5(range: 2.0-81.0) months and an OS of 24.0 (range:6.0-81.0) months; this difference was statistically significant (Figure 1).
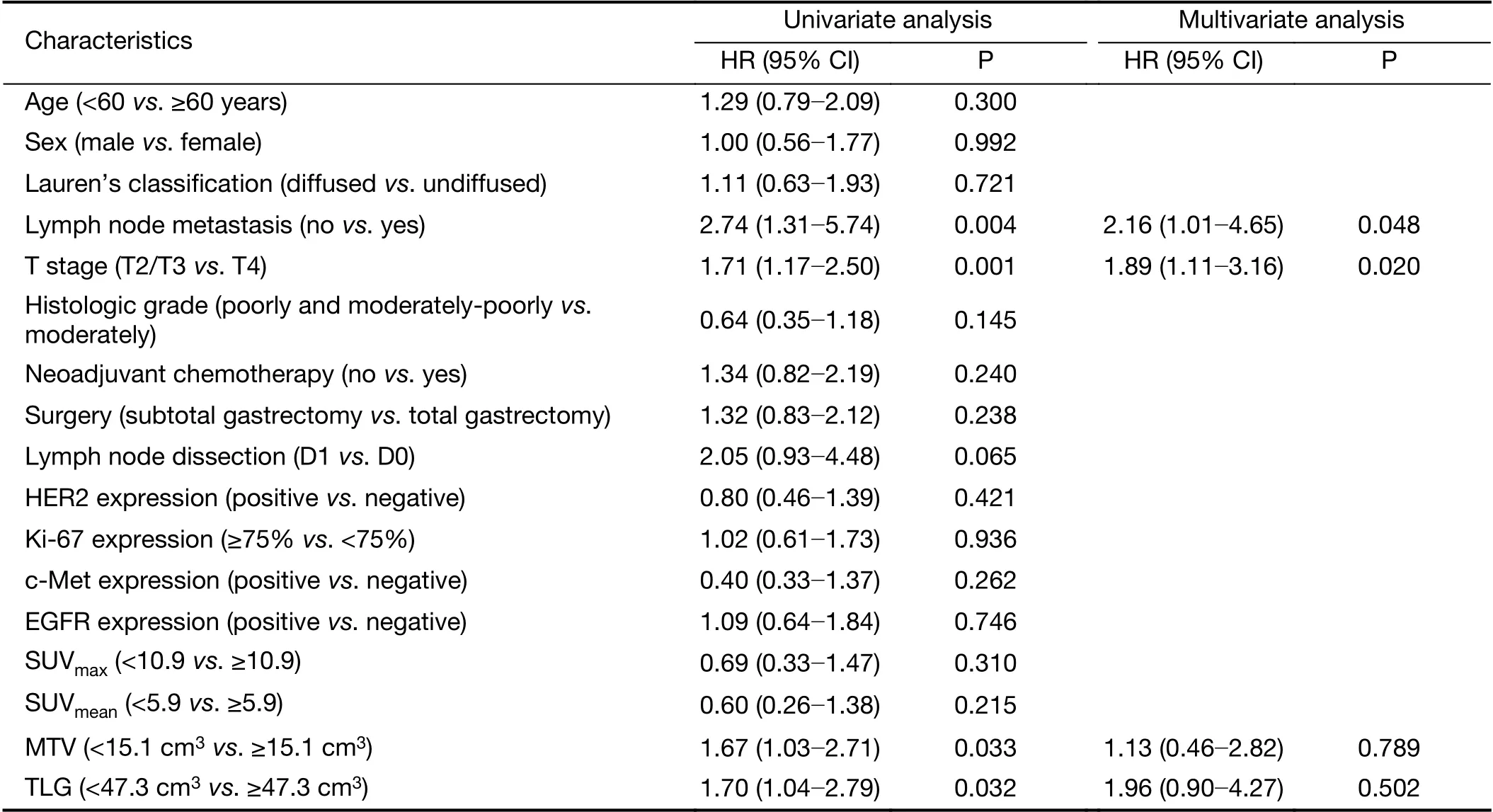
Table 2 Univariate and multivariate analyses of PFS
Univariate and multivariate analyses for PFS and OS
Univariate and multivariate analyses were conducted to compare the prognostic value of histologic prognostic factors and metabolic parameters (Table 2,3).
In univariate analysis, stage T4, metastatic lymph node status and high MTV and TLG were significant predictors of PFS (P<0.05), and stage T4, poor differentiation, and high MTV and TLG values were significant predictors of OS (P<0.05). A higher TLG was an important prognostic factor for PFS [hazard ratio (HR), 1.70; 95% confidence interval (95% CI), 1.04-2.79; P=0.032] and OS (HR, 1.90;95% CI, 1.05-3.45; P=0.028).
Multivariate Cox regression analysis was performed to further investigate the prognostic value of all factors described above. The results showed that lymph node metastasis (HR, 2.16; 95% CI, 1.01-4.65; P=0.048) and T stage (HR, 1.89; 95% CI, 1.11-3.16; P=0.020) were independent prognostic factors for PFS; whereas T stage(HR, 2.33; 95% CI, 1.21-4.51; P=0.012), histologic grade(HR, 0.39; 95% CI, 0.17-0.94; P=0.035) and TLG (HR,2.10; 95% CI, 1.13-3.89; P=0.019) were independent prognostic factors for OS.
Discussion
Our study showed that higher MTV and TLG values were associated with worse OS and PFS in the patients. TLG values from18F-FDG PET/CT could independently predict OS in this population of local advanced gastric cancers. We also found that metabolic18F-FDG PET parameters were associated with Lauren's classification,histologic grade and T grade in patients with gastric cancer.

Figure 1 Kaplan-Meier survival curves for 3-year progression-free survival (PFS) and 3-year overall survival (OS) in 144 patients with advanced gastric cancer. They were divided into four groups according to metabolic tumor volume (MTV) and total lesion glycolysis (TLG) cut-off values. OS (A) and PFS (C) were longer in the low MTV group than the high MTV group (30.0 vs. 24.5 months, P=0.037, and 23.5 vs. 22.5 months, P=0.033,respectively). OS (B) and PFS (D) were longer in the low TLG group than the high TLG group (31.5 vs. 24.0 months, P=0.028,and 26.0 vs. 21.5 months, P=0.032, respectively).
Gastric cancer remains a disease with a poor prognosis.Current prognostication is deficient, and many patients undergo treatments that they may not benefit from.18FFDG PET/CT has been recognized as a useful diagnostic technique in clinical oncology. An association between FDG uptake intensity at the gastric primary tumor and poor outcomes has previously been documented. The SUVmaxrepresents the metabolic activity of the most aggressive cells in malignant lesions. Park et al. performed pre-treatment FDG PET scans in 82 patients with metastatic gastric cancer (7). They found that a SUVmaxof less than 6.0 at the primary tumor independently predicted superior PFS and OS. Similar conclusions have also been reached by Kim et al. and Chung et al. (8,16). However,our study failed to find the SUVmaxof gastric primary tumors to be predictive of OS, despite the use of several thresholds. This result is consistent with those reported by Coupe et al (17). Our inclusion of a homogeneous population comprising operable and advanced gastric cancers may explain this result.
Volume-based parameters, such as MTV or TLG, may provide both volume and metabolic information for prognosis and treatment response, thus making them better factors than SUVmax. Many studies have suggested that volume-based parameters are independent factors of prognosis in several types of malignancies (18-20). Kim et al. measured the MTV of primary lesions in 50 patients with gastric cancer and found that a high MTV of gastric lesions is an independent factor for disease progression (8).Grabinska et al. reported that TLG and MTV were prognostic factors for OS and TLG was the only significant prognostic variable for PFS (21). In our cohort, high MTV or TLG values were associated with adverse prognosis, as indicated by Kaplan-Meier survival plots. Patients with high TLG values had a mean PFS of 21.5 months and an OS of 24.0 months compared to a PFS of 26.0 months and an OS of 31.5 months in the low TLG group. MTV or TLG may help identify patients at the onset of treatment who are at increased risk for relapse and death.
The well-established prognostic factors for a lower survival rate in patients with gastric cancer include the depth of tumor invasion and the extent of lymph node metastasis (22). In our study, univariate and multivariate analysis of survival demonstrated that stage T4 and metastatic lymph nodes had significant predictive values for PFS, and the T stage and histologic grade were independent prognostic factors for OS (P<0.05).
Recent studies have reported that molecular pathological markers of gastric cancer could provide patient prognostic information; these studies have stratified patients for trials of targeted therapies (23,24). The overexpression of HER2,EGFR or c-MET, which was detected in 20%-40% of gastric cancer patients via an IHC assay, was associated with poor prognosis. Unfortunately, none of these molecular markers appear capable of being prognostic factors in LAGC patients.
Our study also identified18F-FDG-avid predictors in gastric cancer and enabled the use of18F-FDG PET/CT to assess the extent of disease before planned surgical resection and treatment planning. Results demonstrated that the undiffused type and moderately differentiated tumors showed significantly higher18F-FDG uptakes than the diffused type and other differentiated tumors. T4 tumors were related to higher metabolic parameters. A higher SUVmeanvalue was also observed in HER2-positive gastric cancer.
Our study supports the use of FDG PET in the staging of gastric cancer by providing additional prognostic information. The identification of patients with poor risk disease could assist in the upfront selection of additional treatments such as radiotherapy for patients. However, this study is a retrospective study that may have biasedprognostication. Therefore, a prospective validation study in a large cohort is necessary in patients with LAGC.
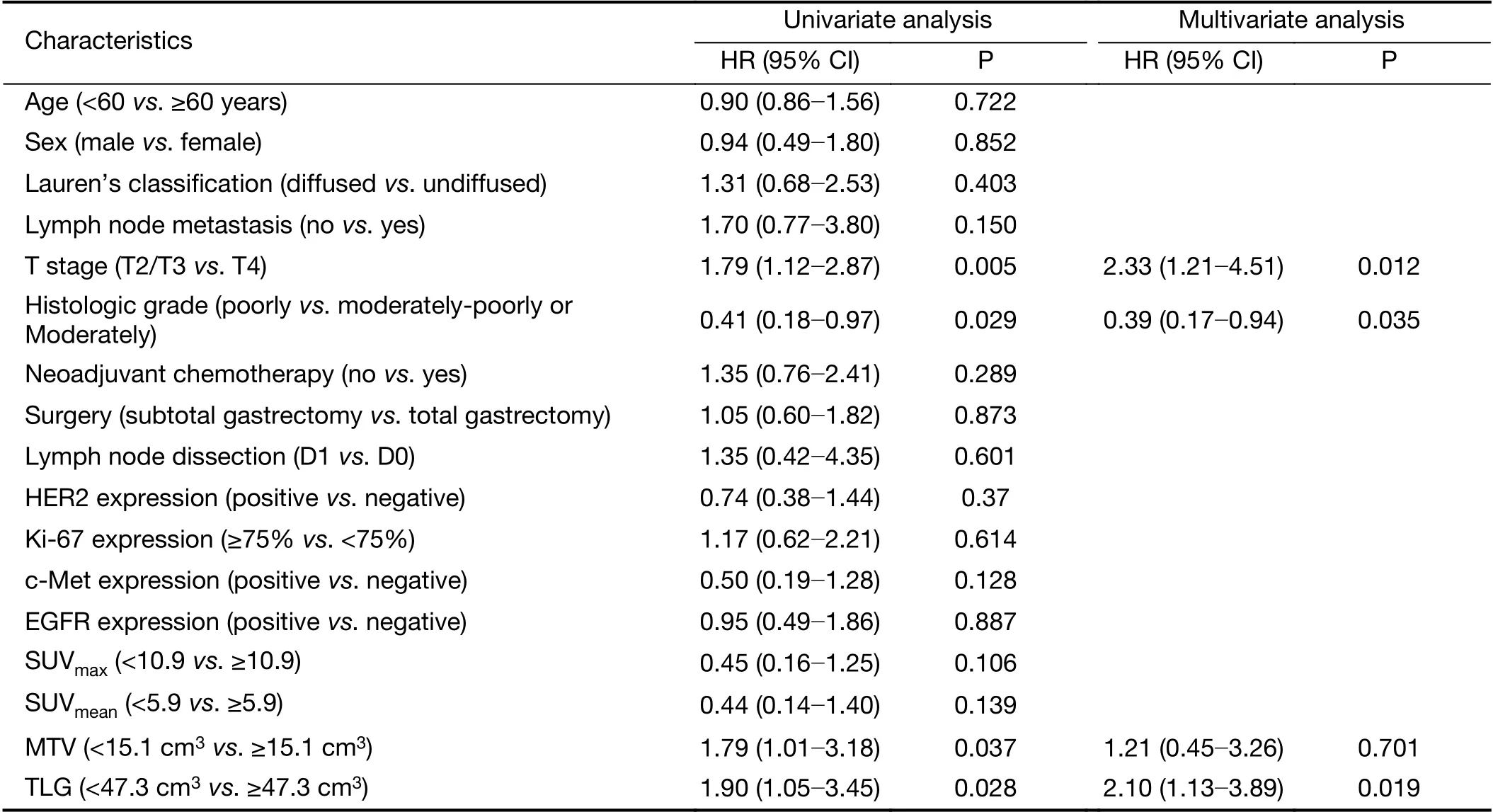
Table 3 Univariate and multivariate analyses of OS
Conclusions
The present study suggests that TLG, a metabolic volume parameter, provided via FDG PET, is a prognostic factor in the staging of patients with LAGC. Undiffused type and moderately differentiated tumors had high FDG uptake in these patients. Further prospective studies should be performed to establish the role of18F-FDG PET/CT in gastric cancer.
Acknowledgements
The study was supported by a grant from Beijing Municipal Natural Science Foundation (No. 7172043).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
杂志排行
Chinese Journal of Cancer Research的其它文章
- Chinese guidelines for diagnosis and treatment of melanoma 2018 (English version)
- An update on biomarkers of potential benefit with bevacizumab for breast cancer treatment: Do we make progress?
- Association of cancer prevention awareness with esophageal cancer screening participation rates: Results from a populationbased cancer screening program in rural China
- FAT1, a direct transcriptional target of E2F1, suppresses cell proliferation, migration and invasion in esophageal squamous cell carcinoma
- Clinical significance of MET gene amplification in metastatic or locally advanced gastric cancer treated with first-line fluoropyrimidine and platinum combination chemotherapy
- Radiomics-based predictive risk score: A scoring system for preoperatively predicting risk of lymph node metastasis in patients with resectable non-small cell lung cancer
