Genetic testing vs microforceps biopsy in pancreatic cysts:Systematic review and meta-analysis
2019-07-24SandraFaiasLuisaPereirangeloLuPaulaChavesMarliaCravo
Sandra Faias, Luisa Pereira, Ângelo Luís, Paula Chaves, Marília Cravo
Sandra Faias, Department of Gastroenterology, Instituto Português de Oncologia de Lisboa de Francisco Gentil, EPE, Lisboa 1099-023, Portugal
Sandra Faias, Paula Chaves, Faculdade de Ciências da Saúde, Universidade da Beira Interior,Covilhã 6200-506, Portugal
Sandra Faias, Luisa Pereira, Ângelo Luís, GRUBI-Grupo de Revisões Sistemáticas,Universidade da Beira Interior, Covilhã 6200-506, Portugal
Luisa Pereira, Centro de Matemática e Aplicações (CMA-UBI), Universidade da Beira Interior,Covilhã 6200-506, Portugal
Ângelo Luís, Centro de Investigação em Ciências da Saúde (CICS-UBI), Universidade da Beira Interior, Covilhã 6200-506, Portugal
Paula Chaves, Department of Pathology, Instituto Português de Oncologia de Lisboa de Francisco Gentil, EPE, Lisboa 1099-023, Portugal
Marília Cravo, Department of Gastroenterology, Hospital Beatriz Ângelo, Loures 2674-514,Portugal
Marília Cravo, Faculdade de Medicina, Universidade de Lisboa, Lisboa 1099-023, Portugal
Abstract BACKGROUND AIM To compare the accuracy of genetic testing and microforceps biopsy in pancreatic cysts referred for surgery.METHODS We performed a literature search in Medline, Scopus, and Web of Science for studies evaluating genetic testing of cystic fluid and microforceps biopsy of Carcinoembryonic antigen (CEA) and cytology in pancreatic cystic fluid are suboptimal for evaluation of pancreatic cystic neoplasms. Genetic testing and microforceps biopsy are promising tools for pre-operative diagnostic improvement but comparative performance of both methods is unknown.Manuscript source: Invited manuscript Received: February 18, 2019 Peer-review started: February 18,2019 First decision: April 8, 2019 Revised: April 17, 2019 Accepted: May 18, 2019 Article in press: May 18, 2019 Published online: July 14, 2019 P-Reviewer: Kamimura K,Karstensen JG, Lee HC, Luo HS S-Editor: Gong ZM L-Editor: A E-Editor: Zhang YL pancreatic cysts, with endoscopic ultrasound with fine-needle aspiration (EUSFNA) prior to surgery and surgical pathology as reference standard for diagnosis.We evaluated the diagnostic accuracy for: 1- benign cysts; 2- mucinous low-risk cysts; 3- high-risk cysts, and the diagnostic yield and rate of correctly identified cysts with microforceps biopsy and molecular analysis. We also assessed publication bias, heterogeneity, and study quality.RESULTS Eight studies, including 1206 patients, of which 203 (17%) referred for surgery who met the inclusion criteria were analyzed in the systematic review, and seven studies were included in the meta-analysis. Genetic testing and microforceps biopsies were identical for diagnosis of benign cysts. Molecular analysis was superior for diagnosis of both low and high-risk mucinous cysts, with sensitivities of 0.89 (95%CI: 0.79-0.95) and 0.57 (95%CI: 0.42-0.71), specificities of 0.88 (95%CI: 0.75-0.95) and 0.88 (95%CI: 0.80-0.93) and AUC of 0.9555 and 0.92,respectively. The diagnostic yield was higher in microforceps biopsies than in genetic analysis (0.73 vs 0.54, respectively) but the rates of correctly identified cysts were identical (0.73 with 95%CI: 0.62-0.82 vs 0.71 with 95%CI: 0.49-0.86,respectively).CONCLUSION Genetic testing and microforceps biopsies are useful second tests, with identical results in benign pancreatic cysts. Genetic analysis performs better for low- and high-risk cysts but has lower diagnostic yield.
Key words: Pancreatic cysts; Endoscopic ultrasound; Endoscopic ultrasound with fineneedle aspiration; Genetic testing; Microforceps biopsy; Molecular analysis; KRAS;Carcinoembryonic antigen; Cytology
INTRODUCTION
Pancreatic cystic neoplasms (PCNs) are on the rise in clinics due to an ageing population and the increase in routine use of high-quality abdominal imaging[1]. PCNs are generally classified into two main groups: mucinous cystic neoplasms (MCNs)and non-mucinous cystic neoplasms (NMCN). MCNs include intraductal papillary mucinous neoplasms (IPMNs) and mucinous cystadenomas, which are precursor lesions of pancreatic carcinoma, and may be low-risk (pre-malignant with low or intermediate-grade atypia) or high-risk: pre-malignant with high-grade atypia (HGA)or malignant, including adenocarcinomas secondarily cystic. NMCNs include serous cystadenomas and inflammatory cysts (pseudocysts), mostly benign cysts, but may include some rare lesions, considered high-risk as cystic neuroendocrine tumors(cNETs), and acinar cell cystadenomas (ACCs). The heterogeneity in malignant potential, increased frequency, and significant morbidity and mortality of surgical treatment, makes pre-operative diagnosis of PCNs essential for management. The treatment options for PCNs encompass surgery or conservative surveillance for MCNs, according to malignancy risk, or no further evaluation for most NMCNs.
The differentiation between MCNs and NMCNs is critical, because a misdiagnosis of a MCN can lead to a missed opportunity to treat pancreatic cancer in an early stage and a misdiagnosis of NMCN can result in unnecessary surgery or surveillance with associated morbidity, costs, and negative impact on quality of life.
Currently, morphologic characterization of PCNs and pancreatic cystic fluid (PCF)analysis for carcinoembryonic Antigen (CEA) and cytology are central in diagnosis. A CEA level ≥ 192 ng/mL is the most accurate diagnostic test for MCNs and cytology is highly specific for malignancy[2], but with suboptimal results in large studies with surgical pathology as the gold standard[3]. In fact, a significant part of these lesions remains indeterminate and incorrect pre-operative diagnosis occurs in one third of patients[4,5], making new reliable diagnostic tools urgently needed.
In the last decade numerous studies have shown that genetic analysis of aspirates obtained by EUS-FNA provided a better characterization of PCNs than CEA and cytology[6-14]. Next-generation sequencing (NGS) is a very sensitive technique for detection of genetic mutations that allows the rapid detection of mutations in predefined panels of cancer genes, even in samples with limited DNA content, such as PCF. NGS requires storage, infrastructure, data processing, and expert personnel.Moreover, to be cost-effective, large numbers of samples need to be processed,making it applicable only in large centralized laboratories. These reasons make the implementation of NGS in clinical practice still a matter of debate.
The clinical need of better diagnostic tests in PCNs has recently led to the development of a through-the-needle miniature biopsy device for use during EUSFNA[15,16]. The Moray micro forceps biopsy (MFB) device (US Endoscopy, Mentor,Ohio) is disposable and can pass through a standard 19-gauge EUS-FNA needle that is already used routinely. It allows tissue sampling from the cyst wall, septa or mural nodules and the obtention of a histological evaluation of the epithelial architecture and subepithelial stroma[17]. Adding to the high technical success and excellent safety profile[18,19], the new device has shown to improve the diagnostic accuracy of specific cyst subtypes[20,21]. Another major advantage of MFB is the simultaneous tissue sampling and PCF acquisition, with just an additional histologic analysis that follows standard definitions and is already routine in clinics.
The aim of this systematic review and meta-analysis is to evaluate the diagnostic performance of molecular analysis (MA) and MFB and find the most robust additional diagnostic technique in PCNs, in the pre-operative setting.
MATERIALS AND METHODS
This systematic review and meta-analysis is conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis of Diagnostic Test Accuracy Studies, the PRISMA-DTA Statement[22], and the protocol is registered at PROSPERO (CRD42018111910).
Literature search and study selection
A comprehensive search of databases, including Medline, Scopus, and Web of Science, for the past 8 years (January 1st, 2010 to July 31st, 2018) and restricted to human studies was performed. No language restrictions were applied. The following search terms were used in two independent searches: “pancreas”, “cyst”, “molecular”,“analysis”; and “micro”, “forceps”, “microforceps”, “biopsy”. A search of related articles was performed, adding additional studies. Duplicate articles, reviews, trials including other kinds of neoplasms, and trials with molecular markers not compliant with the defined inclusion criteria were removed. The references of all selected studies were hand-searched for additional articles.
Inclusion criteria:Published studies were included in the meta-analysis if they analyzed: (1) Patients with symptomatic or incidental pancreatic cysts with a definitive surgical pathology diagnosis; (2) Genetic mutations performed with high sensitive techniques, such as NGS in PCF obtained by EUS-FNA prior to surgery; (3)At least four genetic mutations, including KRAS, GNAS, VHL, and at least another genetic mutation representative of aggressive neoplasms (PIK3CA, TP53, SMAD4,PTEN, CDKN2A); (4) PCNs evaluated by EUS-FNA with MFB for diagnosis; and (5)Surgical pathology specimens with available data.
Exclusion criteria:(1) Studies of MA with fewer than the four genetic mutations previously defined; (2) Studies involving solid pancreatic lesions; (3) Studies using PCF not obtained by EUS-FNA; (4) Reviews, case reports, case series with fewer than five patients, letters to editor, exploratory studies, and papers published only in abstract form; (5) Studies with cytology and clinical surveillance as standard of diagnosis. Two authors (SF and AL) independently judged study eligibility and disagreements were resolved by consensus.
Histological criteria:We classified the PCNs of the included studies into three main groups: (1) High-risk cysts (adenocarcinoma or high grade dysplasia in IPMNs and MCNs, secondarily cystic adenocarcinomas, cNETs, and ACCs; (2) Low-risk mucinous cysts (IPMNs and MCNs with intermediate or low-grade dysplasia); and (3)Benign cysts (SCAs, pseudocysts, and other rare cysts (RCs) included in some articles,as retention cysts, lymphoepithelial cysts, epidermoid cysts, squamoid cysts).
Tests under investigation:The index tests were: (1) MA of PCF; and (2) MFB of PCNs, including cyst wall, septs, and nodules. A diagnosis of cNET or ACC does not warrant a malignancy diagnosis, but surgery is recommended in surgically fit patients. Due to a recommendation of identical treatment to malignant and mucinous high-risk cysts, for the purpose of analysis in this study, each one of these diagnoses was classified as a high-risk cyst.
Data extraction
After study selection, two authors (SF and AL) extracted and registered the data from each study onto a standardized worksheet. Disagreements were discussed and reviewed by a third author (LP). The data retrieved were: first author, publication year, study period and design (prospective or retrospective), reference for diagnosis,sample size (all patients included in the study), technical success, adverse events,diagnostic yield, surgical cohort (number of patients with a surgical pathology specimen), cyst size, cyst location, specific cyst types, number of high-risk cysts,mucinous low-risk and benign cysts diagnosed by MA and MFB comparing to surgical pathology specimens. In the MFB studies, technical success was defined as the ability to puncture the cysts and perform the biopsies; and the diagnostic yield was defined as the ratio between the number of patients included in the study and the patients in whom enough material allowed the acquisition of a histopathologic diagnosis. In the MA group, diagnostic yield was defined as a ratio between the number of patients included in the study and the number of patients with DNA available to perform molecular analysis in PCF.
Outcomes
The primary outcomes of this study were the data to obtain the accuracies of MA and MFB for the diagnosis of PCNs, including high-risk cysts, mucinous low-risk cysts,and benign cysts. Secondary outcomes were the diagnostic yield of genetic testing and MFB and the number of cysts correctly identified for each of the tests studied.
Quality analysis
Methodological quality of included primary studies was assessed by two authors (SF and AL) using the modified QUADAS-2 tool[23]. The PRISMA-DTA Statement recommendations were used for reporting this systematic review[22,24].
Statistical analysis and data synthesis
The reference standard was a surgical pathology specimen that allowed the classification of PCNs into three defined groups of diagnosis: high-risk cysts,mucinous low-risk cysts, and benign cysts. This resulted in a two-by-three table with correct and incorrect test results in each of the three referenced groups, for each of the tests analyzed, MA and histology were obtained by MFB.
To calculate tests' accuracy and to reflect on the categories that are useful in clinical practice and that guide management, we constructed two-by-two tables, considering three definitions of “relevant” cysts: (1) High-risk cysts - proven malignant cysts,IPMNs, and MCNs with HGA, cNETs, ACCs; Non-High-risk cysts - all cysts except those proven to be high-risk. (2) Low-risk mucinous cysts - proven mucinous low-risk cysts; High-risk cysts - all except those proven to be mucinous low-risk or benign.And (3) Non-benign cysts - all cysts except those proven to be benign; Benign cysts -proven benign cysts.
The ability of the tests to discriminate “relevant” and “non-relevant” cysts using the three definitions of “relevant cysts” was evaluated and the accuracy of the two tests was compared.
The data of the two-by-two tables were used to calculate sensitivity and specificity for each study. We present individual study results graphically by plotting the estimates of sensitivity and specificity (and their 95% confidence intervals (CI)) in both forest plots and on the summary receiver operating characteristic (sROC) curve plots. The area under the curve (AUC) is equal to 1 for a perfect test and 0.5 for a completely uninformative test. The AUC is equal to the probability that if a pair of relevant and non-relevant cysts is selected at random, the relevant cyst will have a higher test result than the non-relevant cyst. Pooled estimates of the sensitivity and specificity were obtained by the DerSimonian-Laird method (random effect model) to incorporate variation among studies, when data are heterogeneous. Otherwise, we used the Mantel-Haenszel method (fixed effect model).
Heterogeneity was investigated in the first instance through visual examination of forest plots of sensitivities and specificities and through visual examination of the ROC plot of the raw data. Last, we used statistical tests, including chi-square and Cochran-Q to evaluate if the differences across the studies were greater than expected by chance alone. A low P value suggests presence of heterogeneity. In addition to these statistics we used the statistic I2of Higgins, which has been proposed as a measure to quantify the amount of heterogeneity[25,26]. The scale of I2has a range of 0 to 100% and values on the order of 25%, 50% and 75% are considered low, moderate,and high heterogeneity, respectively.
Another goal of this work was to obtain, for each of the tests, the correctly identified cyst rate and the diagnostic yield in predicting a histopathologic diagnosis.
We used Comprehensive Meta-Analysis software (Version 2.0) for assessment of diagnostic yield of the tests and Meta-DiSc (version 1.4 - Meta-Analysis of Diagnostic and screening tests[27]) to obtain the accuracy of each of the tests.
RESULTS
Systematic Review
Our search revealed 16 study titles and abstracts for MFB and 264 titles for MA. In Figure 1A and B are described the selection process of the articles included in this study. After all steps, eight studies were considered suitable for qualitative and seven for quantitative analysis. We excluded 20 full-text articles after review, because they were case series of two patients[16](n = 1), exploratory or pilot studies[28,29](n = 2), no information of mutation status was available[30](n = 1), pancreatic cystic fluid was obtained during surgery[31](n = 1), insufficient or absent data of cysts with surgical pathology diagnoses[12,32,33](n = 3), and mutations only of KRAS and/or GNAS[14,34-44](n= 12).
Of the eight studies that met the inclusion criteria, design was retrospective in six and prospective in two, all were published from 2015 to 2018. These eight studies included a total of 1206 patients, of which 203 (17%) underwent surgical resection and a surgical pathology specimen was available as reference standard and included in the analysis. We excluded all patients with cytology and clinical follow-up data, but for whom a surgical pathology specimen was not available. The characteristics of the studies, surgical pathology diagnoses, and MA and MFB results are presented in Tables 1[32,45-47]and 2[18-21].
Quality assessment and publication bias:Methodological quality of primary studies included was assessed by two authors (SF and AL) using the modified QUADAS-2 tool[23], which evaluates the quality of articles for systematic reviews of diagnostic accuracy studies in four domains, including patient selection, index test, reference standard, and flow and timing, for risk of bias and applicability concerns. Results are presented in Figure 2, which was sketched with templates available at www.quadas.org. The studies included in this review all showed a “low-risk”classification as the index tests (MA and MFB) and the reference standard (surgical pathology specimen) were reliable and mentioned in all studies. However, a “highrisk” of selection bias was demonstrated in patient selection (neither random nor sequential patients included in several studies) and in flow and timing because only a small proportion of the patients evaluated in all studies, except one, were included in the analysis. In fact, most patients were excluded in all studies as the inclusion criteria requiring surgical pathology as diagnostic reference were not met. Applicability concerns in patient selection were also significant in all studies, because the subgroup of PCNs referred for surgery is more often malignant than PCNs on surveillance,which would also be targeted with this review. Because of this bias, there may be an overestimation of both the sensitivity of the index tests, due to a more severe spectrum of PCNs that are referred for surgery, and the positive predictive value(PPV) for diagnosis of high-risk cysts, due to an increased prevalence of malignant cysts in a surgical cohort of PCNs.
Meta-analysis
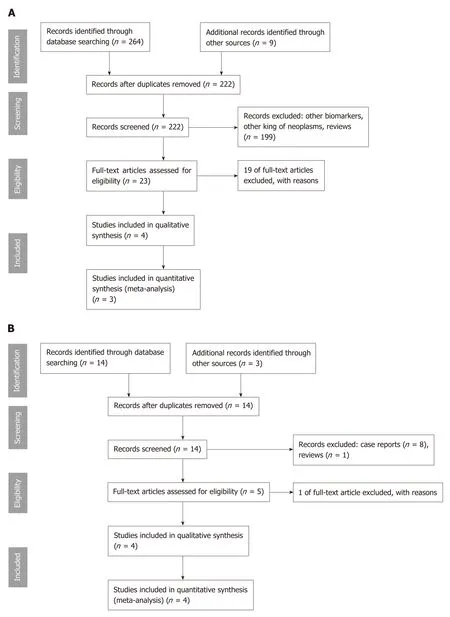
Figure 1 Flowchart with identification of eligible studies. A: Molecular analysis; B: Microforceps biopsy.
Molecular analysis:Four articles were included in the meta-analysis for diagnostic accuracy of MA. For each of the three definitions of relevant cyst, forest plots of sensitivity and specificity with heterogeneous denoted are shown in Figure 3.
The three criteria to define “relevant cysts” resulted in a different range of the specificity and sensitivity of the studies included as shown in Figure 3. For diagnosis of the subgroup with high-risk and low-risk mucinous cysts that require intervention(either surgery or surveillance) comparing to benign cysts the pooled sensitivity was 0.75 (95%CI: 0.66-0.83) and the pooled specificity was 0.72 (95%CI: 0.56-0.85) for MA.In the subgroup of high-risk cysts that require surgery, comparing to other cysts requiring conservative management, the sensitivity was 0.57 (95%CI: 0.42-0.71) with a specificity of 0.88 (95%CI: 0.80-0.93). In the subgroup of low-risk mucinous cysts comparing to high-risk, the pooled sensitivity was 0.89 (95%CI: 0.79-0.95) and the pooled specificity was 0.88 (95%CI: 0.75-0.95).
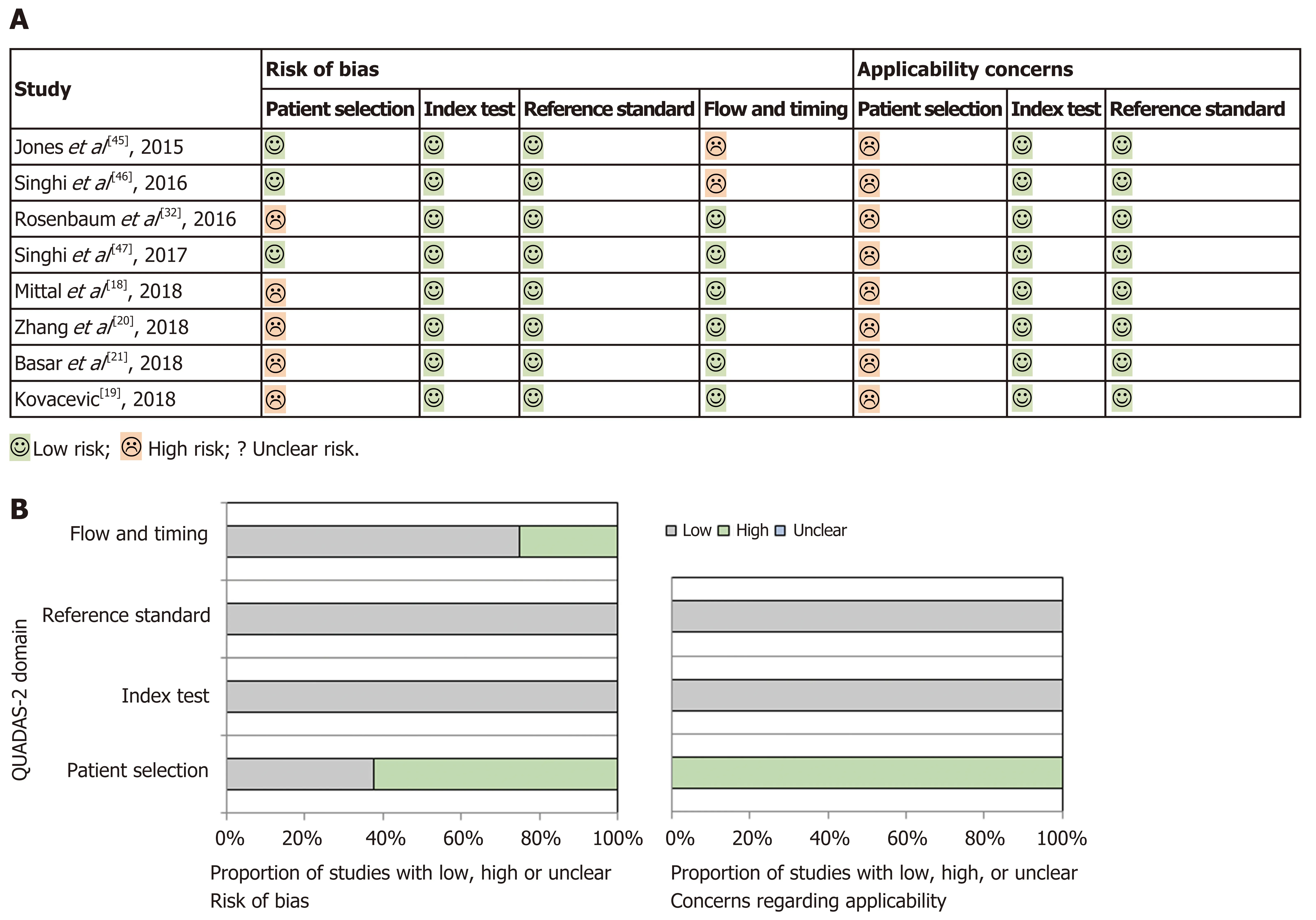
Figure 2 Quality assessment of the studies using QUADAS-2. A: Tabular presentation of risk bias for each study; B: Graphical display of bias.
Figure 4 displays the sROC curves of MA, showing the sensitivity of the individual articles mapped on the vertical scale, 1-specificity on the horizontal scale, with the summary (sensitivity, 1-specificity) point marked, as well as the summary ROC curve and the confidence region for the summary (sensitivity, 1-specificity) points. The area under the sROC curve was 0.7706 (SE: 0.0927) in non-benign cysts, 0.9248 (SE: 0.0691)in high-risk cysts, and 0.9555 (SE: 0.0293) in mucinous low-risk cysts. The results of the studies had greater variation in non-benign cysts as shown by the wide confidence region.
In the four studies, 566 patients had DNA available to perform MA in PCF. Pooled analysis (Figure 5) showed a diagnostic yield of 54.3% (95%CI: 49.8%-58.7%; I2=39.605%; test for heterogeneity P = 0.174).
By considering the classification of cysts by specific type (IPMNs, MCNs, cNETs,SCAs, pseudocysts, ACCs, and other RCs), MA identified correctly 73.1% of cysts(95%CI: 61.6%-82.2%; I2= 37.381%; test for heterogeneity P = 0.203) (Figure 5).
Micro forceps biopsy: Four articles were included in the meta-analysis for diagnostic accuracy of histology obtained using MFB. Figure 6 shows the forest plots of sensitivity and specificity for the three subgroups of relevant cysts. The forest plots for MFB show variable specificities within the papers, from 0 to 1, which can be due to the small numbers of patients with the target condition in some studies.
For each of the three subgroups there exists a low heterogeneity in sensitivity (I2=0%, I2= 21.4%, I2= 0%) and specificity (I2= 0%, I2= 0%, I2= 21.4%), therefore fixed effect models were used. As presented in Figure 6, in the first subgroup the pooled sensitivity was 0.73 (95%CI: 0.50-0.89) and the pooled specificity was 0.88 (95%CI:0.28-1.00). In the second subgroup sensitivity was 0.81 (95%CI: 0.46-0.98) with a specificity of 0.77 (95%CI: 0.50-0.94) and in the last subgroup the pooled sensitivity was 0.64 (95%CI: 0.33-0.88) and the pooled specificity was 0.81 (95%CI: 0.46-0.98).
The results were plotted as a symmetrical sROC curve (Figure 4). The area under the sROC curve was 0.7640 (SE: 0.1261) in the first subgroup, 0.8154 (SE: 0.098) in the second subgroup, and 0.7509 (SE: 0.1277) in the last subgroup.
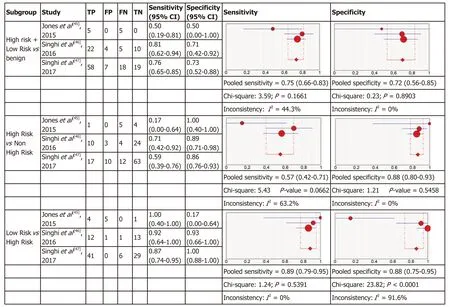
Figure 3 Forest plots of the studies included for molecular analysis. In parentheses are the 95% confidence intervals (CI) of the sensitivity and specificity. The figure shows the estimated sensitivity and specificity of the study (red circle) and its 95% CI (blue horizontal line). The area of the circle reflects the weight that the study contributes to the meta-analysis.
By pooling the data of the four studies that investigated the use of MFB to obtain a histopathologic diagnosis, we obtained a diagnostic yield of 73.1% (95%CI: 61.4%-82.2%; I2= 47.774%; test for heterogeneity P = 0.125) (Figure 5).
By considering the outcome “specific cyst type” diagnosis, MFB correctly identified 70.7% of the cysts (95%CI: 49.4%-85.6%; I2= 0%; test for heterogeneity P = 0.056)(Figure 5).
DISCUSSION
In this meta-analysis we analyzed two different but promising tests to diagnose PCNs- molecular analysis and microforceps biopsy. To our knowledge this is the first study of this nature, and it included 1206 patients with PCNs of which 1058 underwent MA and 148 MFB. All patients had the index tests performed in PCF obtained preoperatively, exclusively with NGS for MA and the Moray micro forceps biopsy device(US Endoscopy, Mentor, Ohio) used for MFB. We analyzed 203 cysts, 178 evaluated with MA and 25 with MFB, all referred for surgery, and with a surgical pathology specimen used as reference standard for diagnosis.
In this comparative analysis we included all studies, without restriction to simultaneous evaluation of both tests, because only one of such studies has been published[20]. This study, which includes 48 patients but only 10 surgical pathology specimens, showed identical results for MA and MFB in low-risk and high-risk cyst diagnosis, but higher specific cyst type diagnosis for MFB.
The data from the seven studies included in the meta-analysis, although with limited number of patients, particularly for MFB, suggests that MA is more accurate than MFB for diagnosis of PCNs, including high-risk and low-risk lesions. MA has superior accuracy to discriminate high-risk cysts from other PCNs and low-risk from high-risk neoplastic cysts. MA performance was considered excellent with AUC values of 0.92 and of 0.96 for high-risk and low-risk neoplastic lesions, respectively, as compared to MFB, which showed a fair or good performance, with an AUC of 0.81 and 0.75, respectively for the same lesions (Figure 4). The specificity of MA is good(0.88) but it has a low sensitivity (only 0.57) for high-risk cysts. This may be explained by technical issues, by low prevalence of relevant genetic mutations in malignant PCNs, or by mutations not included in the current NGS panels. The sensitivity and specificity are high (0.89 and 0.88, respectively) for MA when comparing low-risk to high-risk cysts, which reflects the genetic nature of pancreatic carcinogenesis with cumulative mutations from benign to malignant cysts[48].
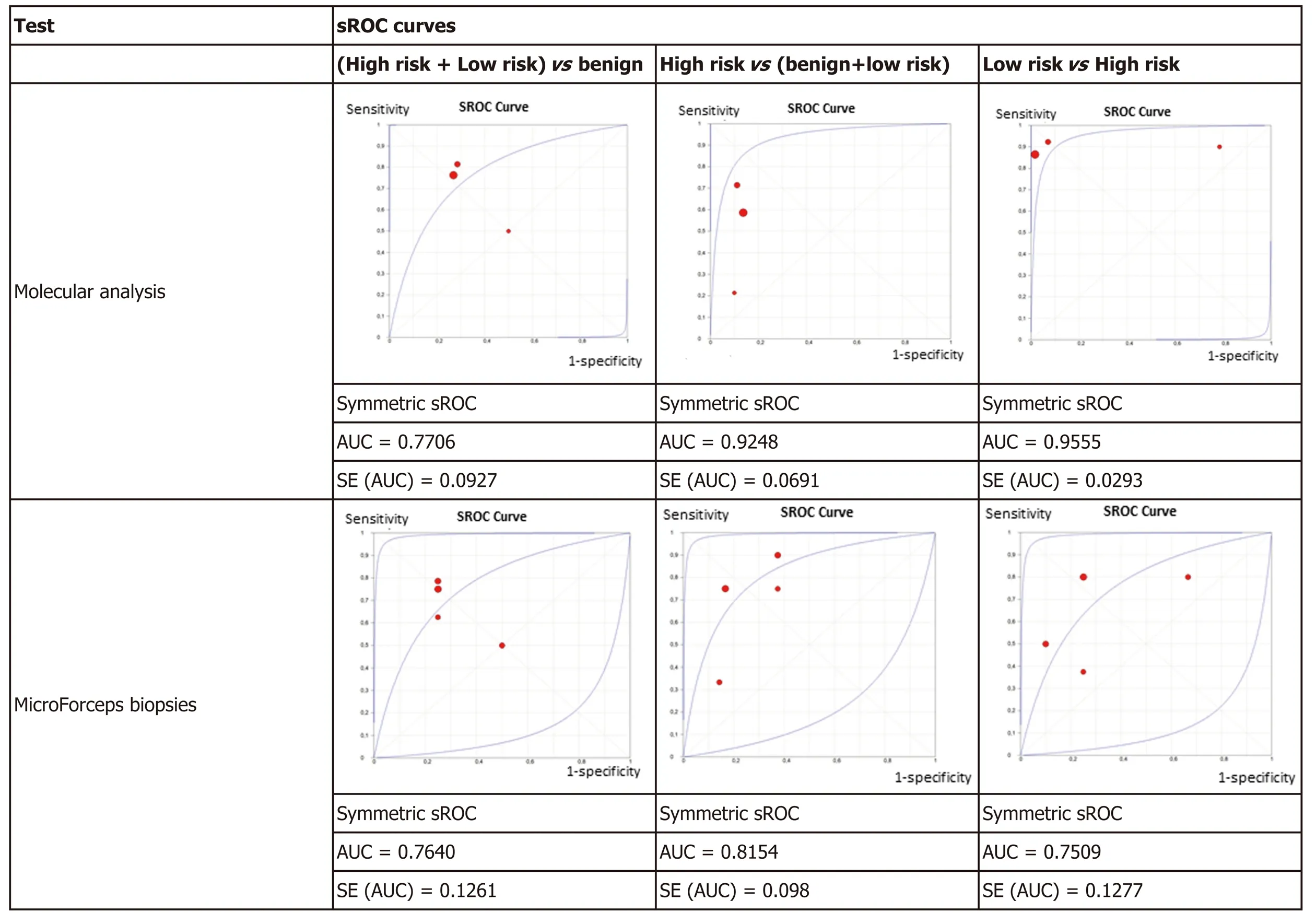
Figure 4 Summary receiver operating characteristics plots. ROC: Receiver operating characteristic curve; AUC: Area under the curve; SE: Standard error.
For discriminating benign cysts from both low-risk and high-risk cysts, the performance of MA and MFB was identical and fair according to AUC values of 0.77 and 0.76, respectively. This non-superiority of MA in the diagnosis of benign cysts in this meta-analysis may be due to technique-inherent issues and/or underrepresentation of benign cysts in surgical series. In fact, “no genetic mutation” is considered a false negative result in most benign rare cysts, but some of these lesions(retention cysts, etc.) have no diagnostic genetic mutations. On the contrary, the most frequent benign cysts, SCAs, harbor a VHL mutation, exclusively present in these benign lesions and allowing for discarding a malignant lesion. In the MA studies, one third of rare benign cysts were classified as false negative results, due to absence of characteristic mutations (Table 1). Another example of PCN that is not amenable to a MA diagnosis with current genetic panels is cNET, also reducing the accuracy of MA for diagnosis of high-risk cysts. The sensitivities were identical for MA and MFB (0.75 and 0.72), but the latter had higher specificity (0.73 and 0.88, respectively). Limited tissue sampling with MFB can explain the reduced sensitivity with robust specificity.As MA depends on denuded DNA in suspension in PCF, no sampling error is expected, which may explain its greater accuracy in neoplastic cysts, comparing to MFB.
Concerning secondary outcomes, even with the limitations of tissue sampling inherent to MFB, this meta-analysis showed that the diagnostic yield of MFB was superior to MA with rates of correctly identified cyst identical with MA and MFB(Tables 1 and 2). In fact, the definition of diagnostic yield, which for MA was“detection of genetic mutations”, may have led to a falsely low value due to the presence of some rare types of benign cysts (retention cysts, lymphoepithelial cysts,epidermoid cysts, squamous cysts in two studies[46,47]) that have no characteristic diagnostic genetic mutations.
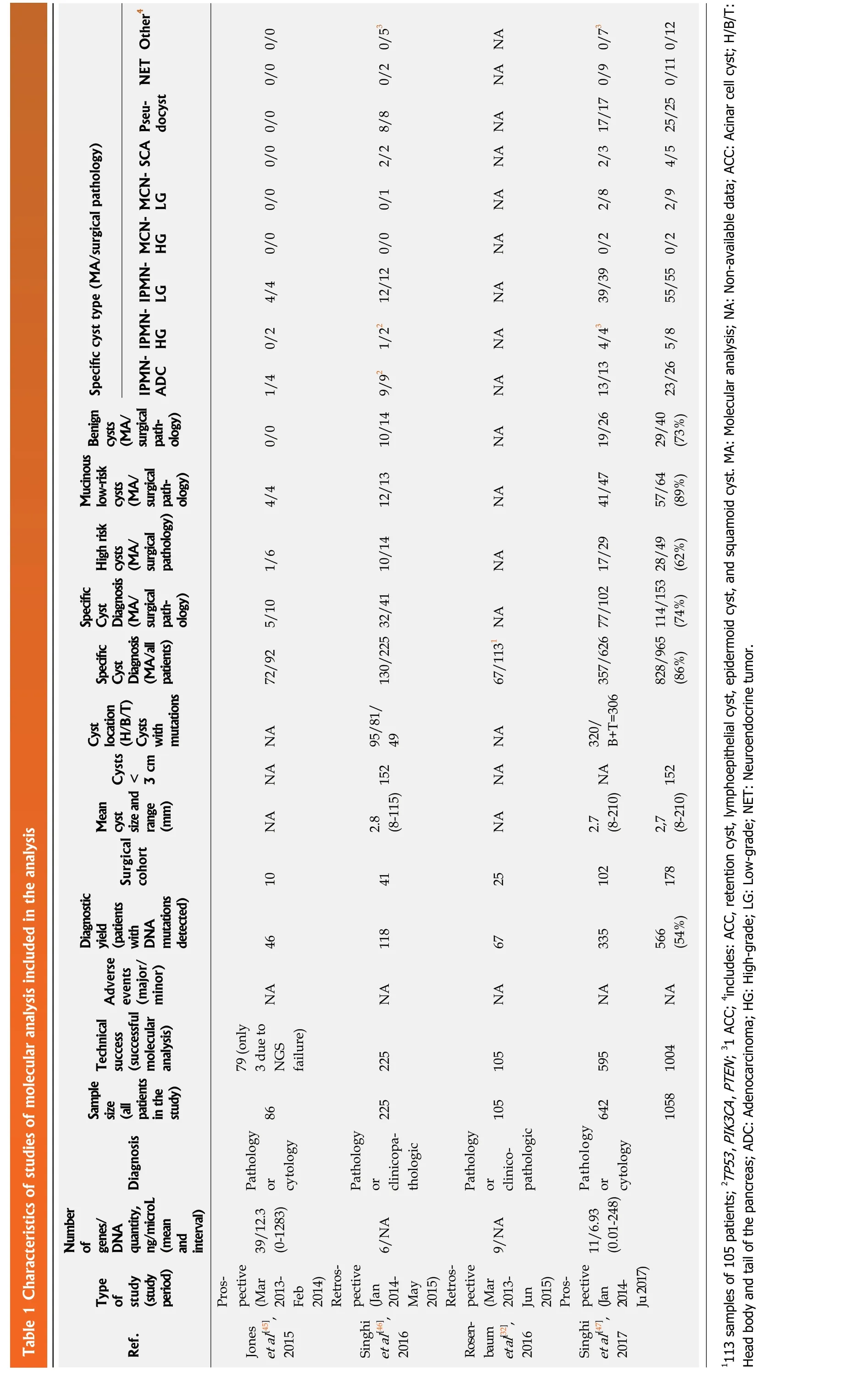
Table 1 Characteristics of studies of molecular analysis included in the analysis NET Other4 0/0 0/0 0/2 0/53 NA NA docyst Specific cyst type (MA/surgical pathology)SCA Pseu-MCN-0/0 0/0 2/2 8/8 NA NA 2/3 17/17 0/9 0/73 4/5 25/25 0/11 0/12 MCNLG 0/0 0/1 NA 2/8 2/9 IPMNHG 0/0 NA IPMNLG 4/4 12/12 0/0 NA 39/39 0/2 55/55 0/2 IPMNHG 0/2 1/22 NA ADC 1/4 NA Benign cysts(MA/surgical pathology)High risk cysts (MA/surgical pathology)Mucinous low-risk cysts (MA/surgical pathology)0/0 10/14 9/92 NA 19/26 13/13 4/43 29/40(73%) 23/26 5/8 4/4 12/13 NA 41/47 57/64(89%)Specific Cyst Diagnosis (MA/all patients)Specific Cyst Diagnosis (MA/surgical pathology)1/6 10/14 NA 28/49(62%)5/10 NA 114/153(74%)72/92 130/225 32/41 67/1131 828/965(86%)Cyst location(H/B/T)Cysts with Cysts <3 cm mutations NA NA 49 NA NA B+T=306 357/626 77/102 17/29 Mean cyst size and range (mm)Surgical NA (8-115) 152 95/81/2.8 NA (8-210) NA 320/2.7 2,7(8-210) 152 cohort Diagnostic yield (patients with DNA mutations detected)10 41 25 102 178 46 118 67 335 566(54%)Adverse events(major/minor)Technical success (successful molecular analysis)NA NA NA NA NA 3 due to 79 (only NGS failure)225 105 595 1004 Sample size(all patients in the study)86 225 105 642 1058 Diagnosis Pathology or cytology Pathology or clinicopathologic Pathology or clinicopathologic Pathology or cytology Ref.Type of study (study period)Number of genes/DNA quantity, ng/microL (mean and interval)39/12.3(0-1283)6/NA 9/NA 11/6.93(0.01-248)Prospective(Mar 2013-Feb 2014)Retrospective(Jan 2014-May 2015)Retrospective(Mar 2013-Jun 2015)Prospective(Jan 2014-Ju 2017)Jones],et al[45],],2015 Singhi et al[46 2016 Rosenbaum],et al[32 2016 Singhi et al[47 2017 1113 samples of 105 patients; 2TP53, PIK3CA, PTEN; 31 ACC; 4includes: ACC, retention cyst, lymphoepithelial cyst, epidermoid cyst, and squamoid cyst. MA: Molecular analysis; NA: Non-available data; ACC: Acinar cell cyst; H/B/T:Head body and tail of the pancreas; ADC: Adenocarcinoma; HG: High-grade; LG: Low-grade; NET: Neuroendocrine tumor.
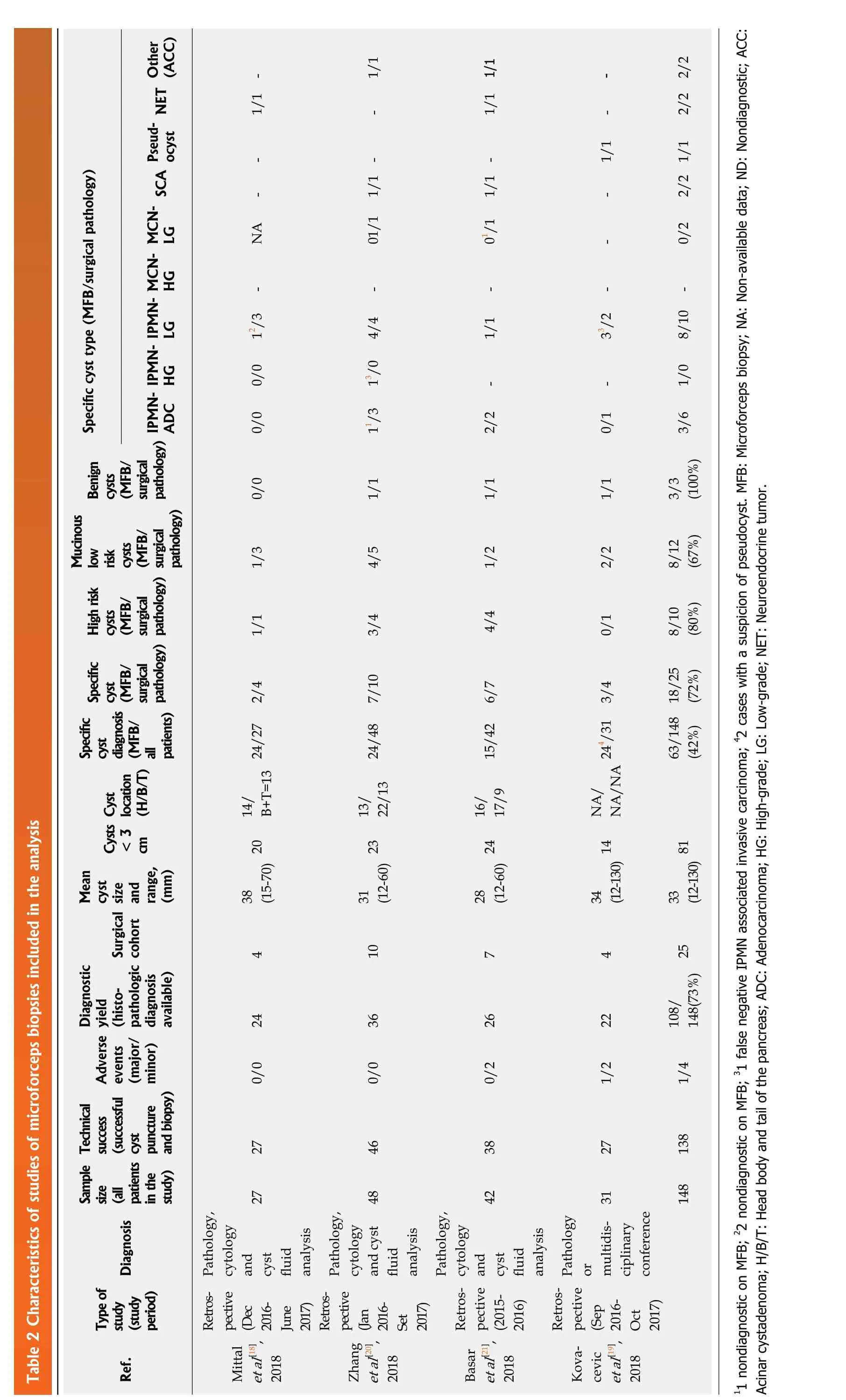
Table 2 Characteristics of studies of microforceps biopsies included in the analysis NET Other (ACC)1/1-SCA Pseudocyst 1/1 --1/1 1/1-2/2 2/2 Benign cysts (MFB/surgical pathology)Specific cyst type (MFB/surgical pathology)-1/1-1/1 --2/2 1/1 MCNLG NA 01/1 1/1 -/1 01-0/2 MCNHG IPMNLG----/3/2 IPMNHG 12 4/4 1/1 33 8/10 -/0 IPMNADC 0/0 13--1/0 0/0/3 11 2/2 0/1 3/6 0/0 1/1 1/1 1/1 3/3(100%)High risk cysts(MFB/surgical pathology)Mucinous low risk cysts (MFB/surgical pathology)1/3 4/5 1/2 2/2 8/12(67%)1/1 3/4 4/4 0/1 8/10(80%)Specific cyst (MFB/surgical pathology)2/4 7/10 6/7 18/25(72%)cyst diagnosis(MFB/all patients)Cyst location(H/B/T)Specific 24/48 15/42/31 3/4 63/148(42%)Cysts < 3 cm 14/B+T=13 24/27 13/22/13 16/17/9 NA/NA/NA 244 Mean cyst size and range,(mm)38 Surgical (15-70) 20 31(12-60) 23 28(12-60) 24 34 (12-130) 14 33 (12-130) 81 Diagnostic cohort 4 10 7 4 yield(histopathologic diagnosis available)24 36 26 22 108/148(73%) 25 Adverse events(major/minor)0/0 0/0 0/2 1/2 1/4 Technical success(successful cyst puncture and biopsy)27 46 38 27 138 Sample size(all patients in the study)27 48 42 31 148 Diagnosis Pathology,cytology and cyst fluid analysis Pathology,cytology and cyst fluid analysis Pathology,cytology and cyst fluid analysis Pathology or multidisciplinary conference Type of study(study period)Retrospective(Dec 2016-],June 2017)Retrospective(Jan 2016-Set 2017)Retrospective(2015-],2016)Retrospective(Sep 2016-],Oct 2017)et al[18 Mittal Zhang],Ref.2018 et al[20 2018 Basar et al[21 2018 Kovacevic et al[19 2018 11 nondiagnostic on MFB; 22 nondiagnostic on MFB; 31 false negative IPMN associated invasive carcinoma; 42 cases with a suspicion of pseudocyst. MFB: Microforceps biopsy; NA: Non-available data; ND: Nondiagnostic; ACC:Acinar cystadenoma; H/B/T: Head body and tail of the pancreas; ADC: Adenocarcinoma; HG: High-grade; LG: Low-grade; NET: Neuroendocrine tumor.
In clinical practice, patient symptoms, cyst imaging features, CEA, and cytology of PCF are required for diagnosis and decision for either treatment or surveillance according to cyst types[49]. PCF analysis, including CEA to distinguish mucinous from non-mucinous cysts and cytology to select those that harbor HGA or early pancreatic carcinoma and require surgical treatment, have suboptimal accuracies[3], due to scant cellularity and limited PCF volume. In this context, additional diagnostic tests are necessary to improve cyst classification and refine clinical decision. DNA markers require limited amounts of PCF, increasing the diagnostic yield[32,45,50,51], but with considerable technical complexity and costs. In fact, in routine clinical practice a major pitfall for PCNs diagnosis is the limited volume of PCF obtained, precluding routine pre-operative testing. As DNA analysis requires less volume of PCF, it may become an alternative test in these circumstances. This major advantage of molecular analysis was not possible to evaluate in this meta-analysis, because the volume of cystic fluid obtained in pancreatic cysts was not available in most studies analyzed.
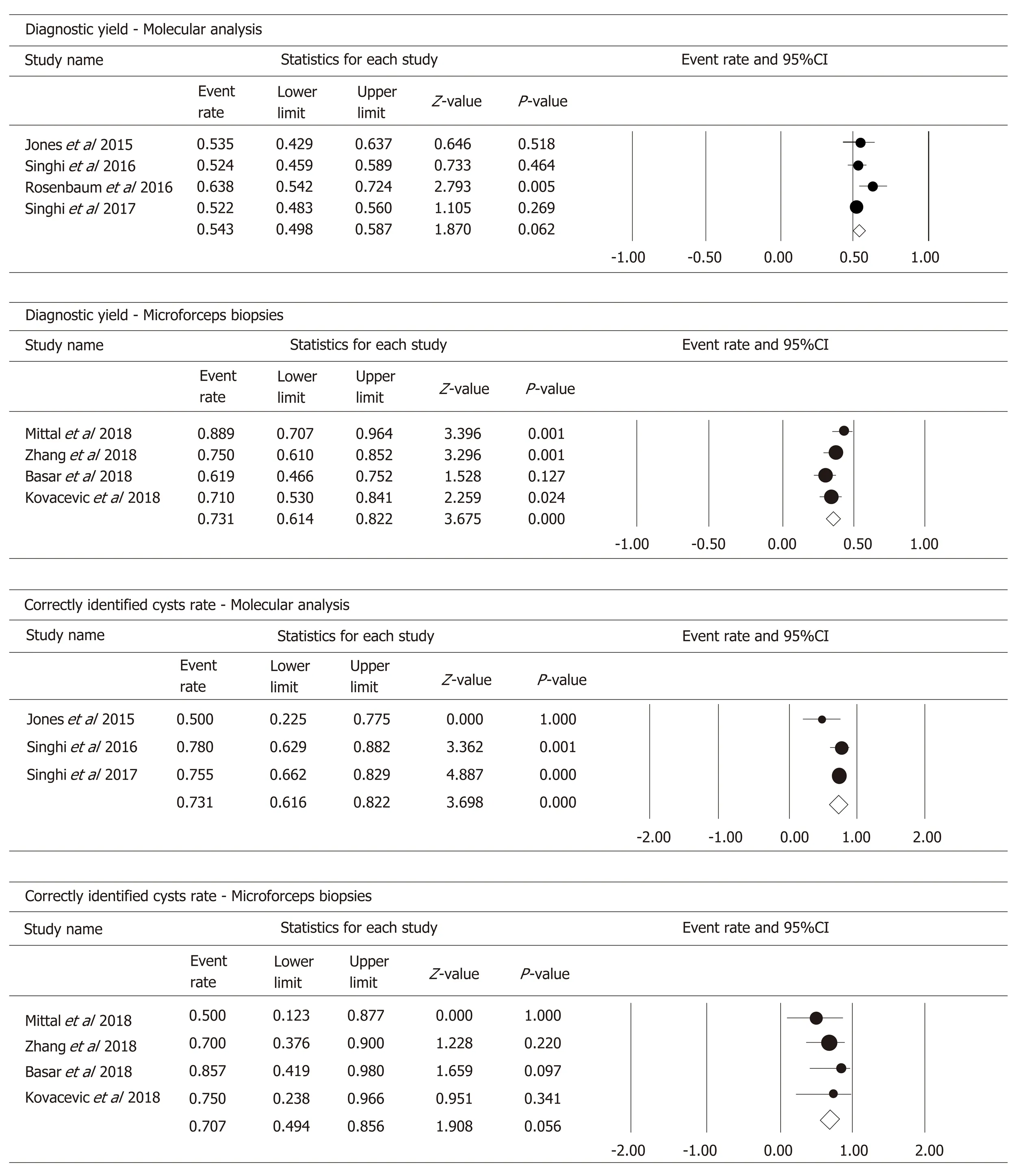
Figure 5 Forest plots of molecular analysis and microforceps biopsies on the secondary outcomes of this meta-analysis.
As MA continues to evolve, questions remain about its accuracy, how it influences patient management, and in what order the analysis should be performed to better support clinical decisions. Previous studies[49]have shown that DNA testing combined with clinical features increased PCNs diagnosis compared to either alone. With multiple recent advances in biomarkers, molecular genetics will probably prove to be useful in the management of PCNs[52]. In a previous meta-analysis, pre-operative cytology of PCNs has shown low sensitivity for diagnosis[53], endorsing additional tests to improve diagnosis. Another meta-analysis of diagnostic accuracy of EUS-FNA with CEA and cytology analysis in differentiating mucinous cysts has demonstrated to be accurate to confirm the diagnosis but performed poorly in excluding it[54]. The role of KRAS as individual screening test has been analyzed before[55]with poor accuracy and added benefit coming from a combined approach with cytology. A recently published meta-analysis supporting KRAS, GNAS, and RNF43 mutations as diagnostic markers of IPMNs[56]used different methods for mutation detection,different tumor materials, and clinicopathologic data as reference standard for diagnosis, which may limit its clinical application in evaluation of PCNs with mutational analysis performed only in PCF.
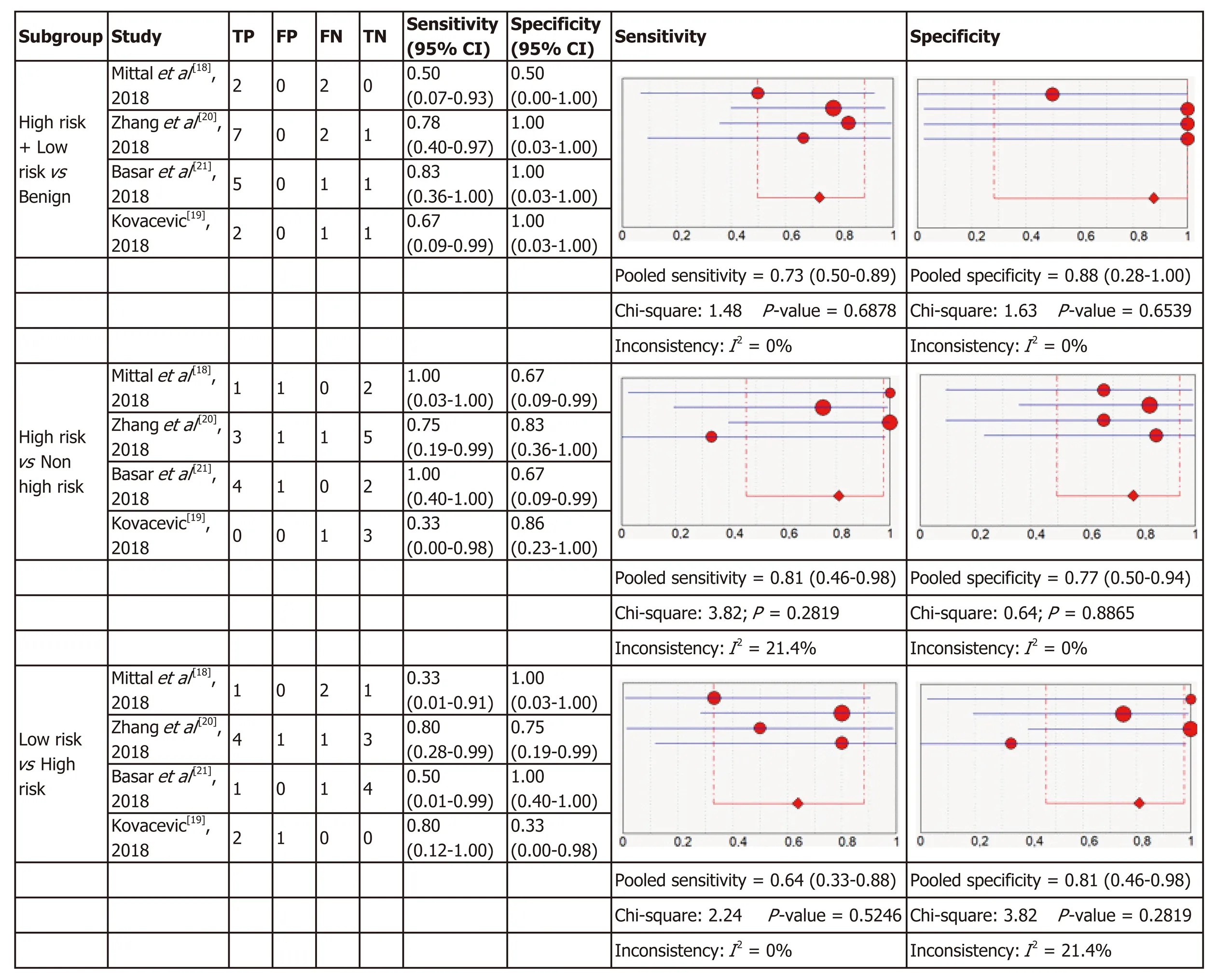
Figure 6 Forest plots of the included studies for microforceps biopsies. In parentheses are the 95% confidence intervals (CI) of the sensitivity and specificity.The figure shows the estimated sensitivity and specificity of the study (red circle) and its 95%CI (blue horizontal line). The area of the circle reflects the weight that the study contributes to the meta-analysis.
In this scenario, new markers are needed for PCNs stratification, and in our metaanalysis both MA and MFB have acceptable diagnostic accuracies. The two largest studies of MA[46,47]showed higher accuracy for diagnosis, which underscores the role of technical aspects of PCF collection, storage, and laboratory analysis for improved accuracy with this technique.
On the other hand, MFB provides tissue fragments for routine histological evaluation, without additional PCF required other than for standard analysis. The technical feasibility of through-the-needle microforceps biopsies revealed to be excellent, even in cysts located in the pancreatic head, despite the required 19-gauge caliber of the EUS-FNA needle. Another potential advantage of MFB is to allow the diagnosis of histologic subtypes of IPMNs, which can potentially be used for risk stratification[57], but still requires further validation.
Strengths and limitations
We applied strict exclusion criteria, with all analyzed patients having a surgical pathology specimen as the reference standard for diagnosis, because histopathology is the gold standard for diagnosis of neoplasia. Another major strength of this metaanalysis is having identical lesions (size and location) analyzed in both groups. These important strengths provide a more realistic accuracy estimate of the tests evaluated.In previous studies of cytology including both surgical pathology and clinical followup[54]as reference standard, pooled sensitivities were 12% higher than in studies with exclusive surgical pathology[55]as reference standard in the diagnosis of mucinous cysts, with test accuracy overestimation. Finally, the pooled results have low heterogeneity.
The quality of a systematic review depends on the quality of studies included, and our quality assessment of patient selection regarding the risk of bias and applicability was high. As sensitivity and specificity are sensitive to study design and influenced by the spectrum of disease, sample collection, and processing, there may be a risk of bias and the results, although correct, their interpretation may be inaccurate.Moreover, there was incomplete reporting in one primary study, having no separate information on specific cyst type, mucinous or malignant cyst diagnosis[32], and the study was excluded from quantitative analysis. Although one study was excluded from the meta-analysis, MA with three studies included more patients (953, of whom only 153 in the surgical cohort) than the group of MFB with four studies but fewer patients (148, with only 25 in the surgical cohort). This can represent a surgical selection bias for both tests studied. Moreover, MFB studies were all retrospective,with small sample size, without pathology diagnosis for most benign and premalignant cysts, and non-consecutive patients that were selected on endoscopist discretion, which may have led to bias. Another limitation is the time between the index tests and the reference standard, because the final diagnosis could have been made at different time intervals from the tests. If the time between index tests and reference standard is too long, the true disease status of the patient may have changed by the time the reference standard was assessed. Aditionally, the different number of malignant cysts per study, particularly in the MA group, may have led to part of the heterogeneity in sensitivity and specificity. Finally, as MA does not increase the risks of standard EUS-FNA (the analysis is performed in remnant cystic fluid after standard diagnosis) we did not perform a safety analysis of MFB, but the four studies analyzed described only rare non-severe adverse events.
Future perspectives
With the increasing diagnosis of asymptomatic PCNs, most with potential for malignancy, there is a growing need to find accurate and affordable tests for diagnosis. The goal of management of patients with pancreatic cysts is to detect and resect cysts before progression of malignancy, while avoiding unnecessary follow-up procedures in benign cysts and surgery in low-risk PCNs.
Biomarkers of malignancy are promising, but clinicians should be aware of their current diagnostic performance limitations and type of lesions identified. In addition to significant costs, logistic difficulties in preserving material for future molecular analysis in busy general hospitals, and the technical complexity of the test, the generalized use of MA seems difficult in clinical practice. On the other hand, if MFB proves in larger studies to be safe and to allow tissue acquisition and gives the histological criteria needed for a correct diagnosis of PCNs, it may be immediately implemented in clinics, because the endoscopic procedure is standard, and histology is already a widespread procedure in clinics. MFB may be especially useful for benign lesions, for which both surgery and surveillance are unnecessary, representing a considerable burden in pancreas clinics due to current diagnostic limitations[58].
For MA to become relevant in routine clinical care in the future, its role in early cancer diagnosis and its prognostic value in PCNs requiring periodic surveillance must be confirmed. Also, for successful massive implementation, it is required to develop as an universal, highly accurate, first line test with clinical impact in cyst diagnosis, prognosis, and patient management. MA, both in PCF and peripheral blood, for standard analysis of multiple simultaneous biomarkers, allowing noninvasive diagnosis and risk stratification of these lesions[59]would be valuable. For the present time, MA and MFB can only be recommended as complementary or as second line tests in case CEA and cytology of PCF are non-diagnostic. For both tests, large multicenter validation studies are still missing.
CONCLUSION
Our study confirms the diagnostic value of both MA and MFB, with higher diagnostic accuracy of MA than MFB for both low-risk and high-risk mucinous cysts. Genetic analysis should not be replaced by MFB in this context. Clinicians should be aware of the higher accuracy of MA for the diagnosis of malignant and high-risk cysts.
ARTICLE HIGHLIGHTS
Research background
Carcinoembryonic antigen (CEA) and cytology of pancreatic cystic fluid (PCF) obtained preoperatively with endoscopic ultrasound with fine-needle aspiration (EUS-FNA) are suboptimal for diagnostic evaluation of pancreatic cystic neoplasms. Genetic testing of PCF and microforceps biopsy obtained by EUS-FNA are promising tools for pre-operative diagnostic improvement. The comparative performance of both methods has not been previously studied.
Research motivation
In the last decade numerous studies have shown that genetic analysis of aspirates obtained by EUS-FNA provided a better characterization of pancreatic cysts than standard CEA and cytology. Next-generation sequencing (NGS) is a very sensitive technique for detection of genetic mutations in pre-defined panels of cancer genes, even in samples with limited DNA content,such as PCF. NGS requires storage, infrastructure, data processing, expert personnel, and large numbers of samples need to be cost-effective. These reasons make the implementation of NGS in clinical practice still a matter of debate. The clinical need of better diagnostic tests in pancreatic cysts led to the development of a through-the-needle miniature biopsy device for use during EUS-FNA. The Moray micro forceps biopsy device (US Endoscopy, Mentor, Ohio) is disposable and can pass through a standard 19-gauge EUS-FNA needle that is already used routinely. It allows tissue sampling from the cyst wall, septa or mural nodules and the obtention of a histological evaluation of the epithelial architecture and subepithelial stroma, with improved pancreatic cyst diagnosis.
Research objectives
To compare the diagnostic accuracy of genetic testing and microforceps in the diagnosis of pancreatic cystic neoplasms referred for surgery.
Research methods
We performed a literature search in Medline, Scopus, and Web of Science for studies evaluating genetic testing of cystic fluid and microforceps biopsy of pancreatic cysts, with EUS-FNA prior to surgery. We used surgical pathology as reference standard for diagnosis. We evaluated the diagnostic accuracy for: benign cysts; mucinous low-risk cysts; high-risk cysts; the diagnostic yield; and rate of correctly identified cysts with microforceps biopsy and molecular analysis.
Research results
Eight studies, including 1206 patients, of which 203 (17%) referred for surgery who met the inclusion criteria were analyzed in the systematic review, and seven studies were included in the meta-analysis. Genetic testing and microforceps biopsies were identical for diagnosis of benign cysts. Molecular analysis was superior for diagnosis of both low and high-risk mucinous cysts.The diagnostic yield was higher in microforceps biopsies than in genetic analysis, but the rates of correctly identified cyst types were identical.
Research conclusions
This study underlines the diagnostic value of both MA and MFB, with higher diagnostic accuracy of MA than MFB for both low-risk and high-risk mucinous cysts. Genetic analysis should not be replaced by MFB in this context. However, MA has higher accuracy in the diagnosis of malignant and high-risk cysts.
Research perspectives
For the present time, MA and MFB can only be recommended as complementary or as second line tests in case CEA and cytology of PCF are non-diagnostic. In the future, for MA to become relevant in routine clinical care, its role must be confirmed, in order to become a first line test with clinical impact in cyst diagnosis, prognosis, and patient management. MA, both in PCF and peripheral blood, for multiple simultaneous biomarkers and non-invasive diagnosis and risk stratification would be valuable. If MFB proves in larger studies to be safe and to allow a correct diagnosis of pancreatic cysts, it may be immediately implemented in clinics. MFB may be especially useful for benign lesions, for which both surgery and surveillance are unnecessary,with uncertain diagnosis due to current diagnostic limitations. For both tests, larger validation studies are missing.
杂志排行
World Journal of Gastroenterology的其它文章
- Diuretic window hypothesis in cirrhosis: Changing the point of view
- Fluoroquinolones for the treatment of latent Mycobacterium tuberculosis infection in liver transplantation
- Reactivation of hepatitis B virus infection in patients with hemolymphoproliferative diseases, and its prevention
- Current status of endoscopic retrograde cholangiopancreatography in patients with surgically altered anatomy
- Choledochal cysts: Similarities and differences between Asian and Western countries
- Gastro-duodenal disease in Africa: Literature review and clinical data from Accra, Ghana
