Fluoroquinolones for the treatment of latent Mycobacterium tuberculosis infection in liver transplantation
2019-07-24JoseTiagoSilvaRafaelSanJuanMarioFernndezRuizJosMarAguado
Jose Tiago Silva, Rafael San-Juan, Mario Fernández-Ruiz, José María Aguado
Abstract Solid organ transplantation (SOT) is the best treatment option for end-stage organ disease. Newer immunosuppressive agents have reduced the incidence of graft rejection but have increased the risk of infection, particularly due to the reactivation of latent infections due to opportunistic agents such as Mycobacterium tuberculosis. Active tuberculosis (TB) after SOT is a significant cause of morbidity and mortality. Most cases of posttransplant TB are secondary to reactivation of latent tuberculosis infection (LTBI) due to the effects of long-term immunosuppressive therapy. Risk minimization strategies have been developed to diagnose LTBI and initiate treatment prior to transplantation. Isoniazid with vitamin B6 supplementation is the treatment of choice. However, liver transplantation (LT) candidates and recipients have an increased risk of isoniazid-induced liver toxicity, leading to lower treatment completion rates than in other SOT populations. Fluoroquinolones (FQs) exhibit good in vitro antimycobacterial activity and a lower risk of drug-induced liver injury than isoniazid. In the present review, we highlight the disease burden posed by posttransplant TB and summarize the emerging clinical evidence supporting the use of FQs for the treatment of LTBI in LT recipients and candidates.
Key words: Fluoroquinolones; Mycobacterium tuberculosis; Latent tuberculosis infection;Liver transplantation; Drug-induced liver graft injury
INTRODUCTION
Solid organ transplantation (SOT) is the best treatment option for end-stage organ disease. New immunosuppressive agents have reduced the incidence of acute graft rejection but have increased the risk of infection and the development of de novo malignancies in the long term[1-4]. Prophylactic and preemptive treatment strategies have been especially effective in diminishing the incidence of donor and recipientderived infections as well as the frequency of most opportunistic infections (OI) in the first six months after transplantation. However, the incidence of active tuberculosis(TB), one of the most concerning OIs after SOT, is still significant. A study that prospectively analyzed the online database of the Spanish Network of Infection in Transplantation (RESITRA) found that Mycobacterium tuberculosis (M. tuberculosis) was responsible for almost a quarter of the cases of OI diagnosed after the sixth month posttransplantation[5]. Cases of disseminated TB were not infrequent.
In this review, we focus on the importance of TB after liver transplantation (LT).We also discuss the most important strategies for the prevention of M. tuberculosis reactivation in LT recipients, particularly the role that fluoroquinolones (FQs) could play in the treatment of latent tuberculosis infection (LTBI) in LT candidates and recipients.
THE BURDEN OF M. TUBERCULOSIS AFTER LT
Active TB poses important challenges to clinicians who attend SOT recipients[6], with an incidence that can vary significantly-from 0.35% to 15%-depending on the prevalence of TB in a specific region or country and the type of SOT performed. A prospective study that included 16 transplant centers of the Spanish Network for Research in Infectious Diseases (REIPI) reported that LT recipients were at particular risk and had the third highest incidence rate, with 541 cases per 100000 transplant recipients per year, much higher than the rates in recipients of similar SOTs, such as kidney transplant (KT) and heart transplant (HT) recipients, which were 358 and 255/100000 patients per year, respectively[7].
Pulmonary TB (PTB) is the most frequently presenting type of M. tuberculosis infection after LT[8,9]. The risk for extrapulmonary (EPTB) or disseminated TB (DTB) is also higher than that observed in the general population; these conditions have an incidence rate of up to 33% among all patients[10,11]. In these cases, patients tend to complain only of fever or unspecific constitutional symptoms, without any other accompanying signs[6,12]; thus, clinicians should always consider this disease and have a high index of suspicion for active TB[13]. Active TB treatment after LT is also problematic. The incidence of isoniazid-induced hepatotoxicity after LT is increased when isoniazid is combined with rifampicin[14], and accordant histological changes can be found in up to 83% of treated LT recipients[15]. Interactions with immunosuppressive drugs can also favor allograft rejection; rifampicin, a potent inducer of the cytochrome P450 enzyme system, can reduce the blood levels of most immunosuppressants used in daily practice[15,16].
A number of studies have exhaustively addressed active TB in the LT setting. Abad et al[17], who reviewed 187 MEDLINE, EMBASE, and OVID publications, thoroughly described a total of 2082 cases of TB in SOT recipients, including 253 cases after LT. In this specific population, the relative frequency and median interval from transplantation to presentation of PTB, EPTB and DTB were 36.51%, 36.51% and 26.91%and 4, 9, and 48 mo, respectively. The liver allograft was involved in 30.0% of recipients in whom M. tuberculosis was isolated outside the pulmonary system, and 3.51% experienced allograft dysfunction or loss. Notably, 27.5% of LT recipients developed anti-TB drug-induced hepatoxicity, which was a higher percentage than in the overall cohort (19.56%). A systematic review and meta-analysis of TB after LT identified 139 cases of active TB. Most patients were diagnosed with EPTB (67%), and in 26, there was allograft involvement[18]. TB drug therapy was stopped or changed in 35% of the patients, mostly due to hepatoxicity (80%)[18]. Póvoas et al[19]described 8 cases of active TB after LT diagnosed in a transplant unit located in Lisbon, Portugal,over a five-year span. The incidence rates of PTB, EPTB and DTB were 37.5%, 37.5%and 25.0%, respectively. Liver allograft involvement was identified in 1 patient. Most cases were diagnosed more than 12 mo after transplantation. All patients received a four-drug treatment regimen, and one patient developed mild to moderate cholestasis that did not require therapy discontinuation[19].
The mortality rate of TB after LT can be as high as 50%, 20.31%, and 37.5%,respectively of the published studies[9,17,19].
TB can be secondary to reactivation of a latent infection or due to a new infection after transplantation, which can be acquired in the community or the healthcare environment or can be donor-derived[12,17]. However, the most common way of developing active TB disease after LT is by reactivation of a latent infection upon the initiation of immunosuppressive therapy[17]. For this reason, in recent years, an important effort has been made to diagnose and treat LTBI in all SOT candidates,including LT candidates.
TREATMENT OF LTBI IN LT
LTBI can be defined as an infection by viable M. tuberculosis microorganisms that are resting in a dormant state[20]. The LT candidate or recipient must have no signs,symptoms or radiographic findings of active TB disease[20].
Although a discussion concerning the controversies about the best method of diagnosing LTBI is beyond the scope of this article, all LT candidates should be screened for LTBI (Table 1). Screening requires collection of an exhaustive clinical history, a careful physical examination, a tuberculin skin test (TST) and/or tuberculosis interferon-γ release assay (IGRA), and a chest radiograph[21]. Isoniazid(2.5 to 5 mg/kg per day, without exceeding 300 mg/day) plus vitamin B6 (25 to 50 mg/day) for 9 mo is the traditional treatment of choice for LTBI in the SOT population[20,22,23](Table 1). Treatment should ideally be started before LT, although in candidates with advanced end-stage liver disease, current clinical guidelines recommend different LTBI treatments until liver function is stable after LT[22,23].Another alternative to isoniazid is rifampicin for 4 months, although such a regimen should be restricted to the pretransplant period due to the risk of drug interactions with immunosuppressive agents. Combination treatment with isoniazid/rifampicin for 3 to 4 months carries an important cumulative risk of fulminant hepatitis and should not be prescribed to patients with liver disease[22]. Two recent studies describing the experience with a 12-wk course of once-weekly directly observed therapy (DOT) with isoniazid plus rifapentine included 8 and 7 LT candidates[24,25]and reported adequate tolerance and a relatively high completion rate. However, data are still scarce, and future multicenter prospective studies are warranted to confirm the safety of this treatment regimen and to identify the most suitable LT candidates.
Thus, LTBI treatment in LT candidates or recipients is especially difficult. Isoniazid is usually poorly tolerated, and it has been suggested that drug-induced hepatitis can occur in up to 6%-20% of LT recipients[26]. In the RESITRA study, only 29.6% of LT patients with a TST positivity actually received treatment, a rate substantially lower than that observed in the overall cohort of SOT recipients (mean of 43.5%)[7]. A recent European survey performed in 55 transplant programs from 19 countries reported that the rate of LTBI treatment prescription was significantly lower for LT candidates than for other SOT candidates (38% vs 73%, respectively)[27]. A retrospective Canadian study that compared the tolerability of LTBI treatment in 23 LT candidates and 24 LT recipients against 130 non-liver SOT candidates plus 23 non-LT SOT recipients concluded that the completion rate of LTBI therapy with isoniazid and/or rifampicin was significantly lower in LT candidates or recipients than in non-LT candidates and recipients [36.1% vs 73.9%, respectively; odds ratio (OR) = 0.20; P < 0.001][28]. Therapy discontinuation due to liver enzyme elevation was also more frequent in LT candidates and recipients than in non-LT candidates and recipients (28.3% vs 3.5%;OR = 10.48; P value < 0.001)[28]. A study that included 15 LT recipients diagnosed with LTBI and treated with 300 mg of daily isoniazid plus 50 mg of pyridoxine for six months reported that 53% of patients required premature discontinuation of the therapy[29]. The most important side effects were thrombocytopenia, hepatotoxicity,rejection, and tacrolimus toxicity. All patients had started LTBI treatment within the first six months after transplantation. A similar South Korean study reported that the incidence rate of isoniazid-induced hepatotoxicity was significantly lower in LT recipients with an aspartate aminotransferase (AST) level lower than 50 than in those with an AST level higher than 50[30]. The authors of both studies suggested deferring LTBI treatment until liver allograft function was stable, avoiding its prescription in the early post-LT period[29,30]. However, this delay could lead to an increased risk of TB reactivation, as most cases of active TB are diagnosed within the first months after transplantation[6,8,12]. Moreover, in candidates already receiving isoniazid-based LTBI treatment regimen, therapy must be stopped at the time of LT and resumed only when the allograft is stabilized[22], delaying the end of treatment.

Table 1 Risk factors and indications of latent tuberculosis infection treatment in the liver transplant setting (adapted from Meije et al[20]and Bosh et al[22])
EXPERIENCE WITH FQs FOR THE TREATMENT OF LTBI IN LT
Most data for LTBI treated with FQ-based regimens are derived from preventive therapy of multidrug resistant TB cases. Levofloxacin and moxifloxacin have been shown to exhibit good in vitro activity against TB[31,32]. In addition, in these series,levofloxacin and moxifloxacin appeared to be well tolerated and cost-effective[33,34],with treatment completion rates as high as 83% and 100%[35]. FQs, such as levofloxacin, also have a lower hepatotoxic potential than other anti-TB drugs[36].However, it must be remembered that serious side effects are associated with FQs,such as QT interval prolongation[37], tenosynovitis[38]and an increased risk of aortic aneurysm and dissection[39], which is a theoretical concern in long-term therapeutic regimens. Data regarding the risk of rhegmatogenous retinal detachment are still under debate due to contradictions in the published data[40,41]. Furthermore, the European Medicines Agency (EMA) recently issued a warning regarding the risk of hepatotoxicity associated with moxifloxacin, recommending close follow-up during treatment.
In the LT setting, FQs have been used particularly as part of isoniazid- or rifampinsparing regimens prescribed to recipients diagnosed with active TB. Lee et al[42]described nine LT recipients diagnosed with active TB and treated with a therapeutic regimen that principally included ethambutol, cycloserine and a FQ (levofloxacin in 6,moxifloxacin in 1 and ofloxacin in 2 recipients). All nine patients successfully completed treatment and did not experience any apparent FQ-associated side effects.Several case reports of LT recipients treated with a therapeutic regimen that included levofloxacin[43,44]or moxifloxacin[45]have also been published. FQs were administered for periods ranging from 2 mo to 18 mo. No cases of FQ-induced adverse effects were reported.
Regrettably, data concerning the use of FQs in LT for LTBI treatment are still scarce and mostly based on retrospective single-center studies with a small series of patients(Table 2). To date, only one prospective multicenter randomized study has compared the efficacy and safety of levofloxacin (500 mg daily for 9 mo) initiated before transplantation to isoniazid (300 mg every day for 9 mo) initiated between 3 and 6 months after transplantation[46]. Isoniazid was started only when liver function was considered stable, i.e., when the transaminase, alkaline phosphatase, and bilirubin levels did not exceed twice the upper limit of normal. The primary efficacy endpoint was the number of patients who developed tuberculosis at 18 mo after transplantation, whereas the secondary aim was to determine whether adverse events limited the efficacy of levofloxacin. The study was active between January 2012 and February 2014, when a safety analysis resulted in suspension of the trial. Notably, 6 of the 33 candidates (18.2%) in the levofloxacin arm developed tenosynovitis, which required stopping the drug, and 6.1% were diagnosed with severe hepatotoxicity,which resolved after discontinuing the antibiotic. Tenosynovitis occurred from 14 to 133 days after the start of prophylaxis. The authors concluded that levofloxacin was associated with an unacceptably high incidence of tenosynovitis in LT candidates.However, it should be mentioned that only 18 of the 31 LT recipients in the isoniazid arm (58.1%) actually began prophylaxis, and only 10 (32.2%) completed the 9-month treatment course[46]. Hepatotoxicity, which was observed in 38.9% of patients receiving isoniazid, was the most common adverse event associated with this drug.No cases of TB were diagnosed during follow-up.
Since the publication of the RESITRA cohort study, several small single-center reports have been published (Table 2). Tien et al[47]described an experience with 44 LT candidates treated for LTBI, including 25 patients who received FQ (17, levofloxacin;and 8, moxifloxacin). Adverse events were described in 14 of the 25 (56%) treated patients, including 4 (16%) with musculoskeletal symptoms that ranged from diffuse joint pain to unilateral tendinopathy. One patient required permanent FQ discontinuation, whereas the remaining 3 patients were able to complete the 9-mo treatment after temporary suspension of the drug. The incidence of adverse events leading to permanent discontinuation significantly differed among the drugs, From 8% for FQ to 33% and 40% for rifampicin and isoniazid, respectively[47]. The same authors also described 6 LT recipients who received FQs after transplantation for LTBI. Although one recipient presented joint pain, he was able to complete treatment.No cases of FQ-induced hepatotoxicity prior to or after transplantation were reported,and none of these patients developed TB during follow-up[47]. Grim et al[48]published a retrospective study that focused on the tolerability and completion of LTBI treatment in SOT candidates. Of the 129 patients included, 30 were LT and 8 were LT/kidney transplant candidates, and 8 (21.0%) received moxifloxacin. Although much data are lacking, in this particular group, there were no early discontinuations of the drug, and at least 2 patients (25.0%) completed treatment (data for the other 6 candidates concerning the end of treatment was not retrieved). Sgarabotto et al[49]reported an experience with 35 LT recipients who received a combination course of levofloxacin and ethambutol from posttransplant day 1 until hospital discharge and three times weekly for 1 year thereafter. Four patients (11.4%) developed side events that required treatment cessation. Interestingly, during follow-up, TB was diagnosed in 2 patients (5.7%); one had discontinued LTBI treatment due to drug-induced side effects[49]. Anecdotally, a combination of levofloxacin and pyrazinamide for MDR TB prophylaxis prescribed to 57 SOT recipients, including 3 LT patients, resulted in a high incidence of side effects (approximately 1.4 events/patient) with very limited tolerability[50].
CONCLUSION
Active TB is a serious OI after SOT, particularly after LT. Treatment is difficult due to the increased risk of drug-induced liver allograft injury, fulminant hepatic failure and rejection. Most centers either do not prescribe or delay the start of LTBI treatment, as it seems reasonable to consider therapy with isoniazid only in patients with compensated cirrhosis or good liver allograft function and in whom hepatotoxicity can be closely monitored. FQs, which have good anti-TB activity and appear to be less hepatotoxic than isoniazid, could be an alternative for LTBI treatment in the LT setting. Data in some points are small and conflicting, with the rate of permanent withdrawal of the FQ due to side effects ranging from 6% to 33% of all treated patients. More studies are necessary to determine which patients could have the greatest benefit from this therapeutic regimen as well as to compare the tolerability and side effects of levofloxacin vs moxifloxacin and the risk of multidrug-resistant gram-negative bacterial selection. These studies would also allow us to determine the real efficacy of FQs in preventing LTBI in the LT setting.
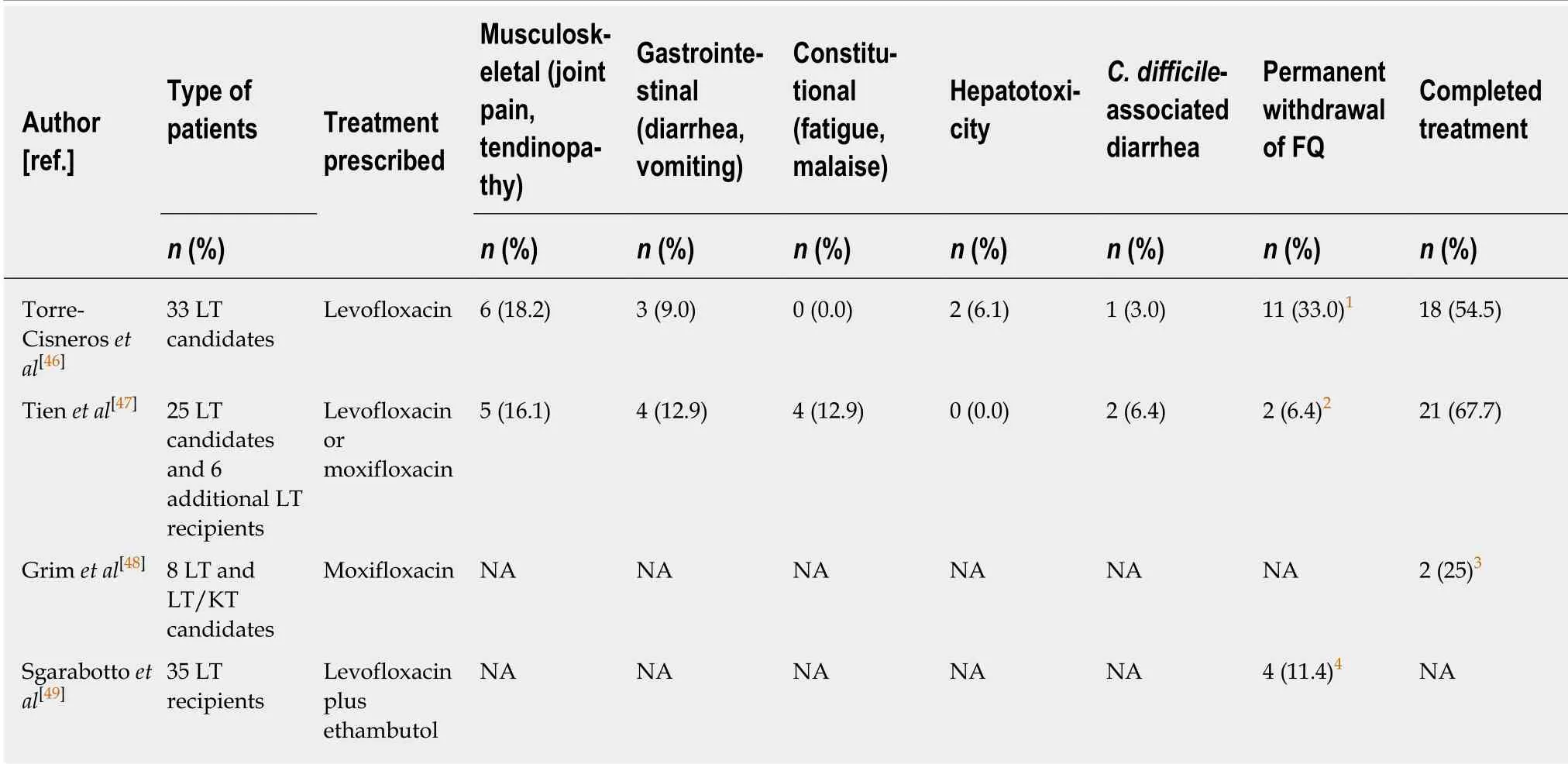
Table 2 Reported fluoroquinolone-induced adverse events and outcomes associated with treatment of latent mycobacterium tuberculosis infection in liver transplantation
杂志排行
World Journal of Gastroenterology的其它文章
- Diuretic window hypothesis in cirrhosis: Changing the point of view
- Reactivation of hepatitis B virus infection in patients with hemolymphoproliferative diseases, and its prevention
- Current status of endoscopic retrograde cholangiopancreatography in patients with surgically altered anatomy
- Choledochal cysts: Similarities and differences between Asian and Western countries
- Gastro-duodenal disease in Africa: Literature review and clinical data from Accra, Ghana
- Screening of aptamers and their potential application in targeted diagnosis and therapy of liver cancer
