Diuretic window hypothesis in cirrhosis: Changing the point of view
2019-07-24AndersonBritoAzevedo
Anderson Brito-Azevedo
Abstract Since the 1970s, non-selective beta-blockers (NSBB) have been used to prevent variceal upper bleeding in advanced cirrhotic patients. However, several recent studies have raised the doubt about the benefit of NSBB in end-stage cirrhotic patients. In fact, they suggested a detrimental effect in these patients that even reduced survival. All of these studies have been assembled to compose the“window therapy hypothesis”, in which NSBB would have traditional indication to be initiated to prevent variceal upper bleeding; however, treatment should be stopped (or not be initiated) in patients with end-stage cirrhosis. NSBB would reduce the cardiac reserve of these patients, worsening systemic perfusion and prognosis. However, it should be emphasized that these studies present important bias issues, and their results also suggested that diuretic treatment may also be behind the effects observed. In this opinion review, we changed the point of view from NSBB to diuretic treatment, based on a physiopathogenic approach of circulatory parameters of cirrhotic patients studied, and based on diuretic effect in blood pressure lowering and in other hypervolemic disease, as heart failure. We suggest a “diuretic window hypothesis”, composed by an open window in hypervolemic phase, an attention window when patient present in a normal plasma volume phase, and a closed window during the plasma hypovolemic phase.
Key words: Cirrhosis; Non-selective beta-blockers; Diuretic; Window hypothesis;Baveno; Cardiac output
INTRODUCTION
Cirrhosis exhibits a complex hemodynamic behavior that changes through different stages. Hyperdynamic circulation characterized by increased cardiac output (CO) and decreased systemic vascular resistance is well described in advanced cirrhosis and recently, a systemic inflammatory pattern seems to assume a central role in the pathogenesis of circulatory dysfunction[1,2].
Recent studies correlated changes in cardiac function as detrimental in systemic hemodynamics, leading to poor prognosis in cirrhosis[3-5]. Therefore, non-selective beta-blockers (NSBB) have been suggested to have probable deleterious effects in endstage cirrhosis. Krag et al[6]suggested the existence of a “therapeutic window” for the use of NSBB in cirrhosis. They postulated that NSBB treatment in end-stage cirrhosis promotes an important decrease in cardiac index (CI), reducing the cardiac compensatory reserve to maintain blood pressure, compromising organ perfusion. It should be emphasized, however, that studies supporting the “window hypothesis”did not explore a possible and important bias in results, the diuretic effect in hemodynamics (Table 1).
EVIDENCE ANALYSIS
In 2003, Ruiz-del-Arbol et al[3]published the first important study that supported“window hypothesis”. The aim was to investigate circulatory dysfunction in patients with spontaneous bacterial peritonitis. The authors concluded that the incidence of renal failure in these patients was caused by a decrease in CO. Although the authors excluded patients in “excessive diuretic treatment”, baseline characteristics should be analyzed carefully. The group that developed renal injury presented higher baseline blood urea nitrogen (BUN) levels, with a BUN/creatinine ratio of 28:1, suggesting the presence of prerenal injury by hypovolemia a priori[7]. Also noteworthy, the group that developed renal injury was primarily composed by more advanced patients, reflected by higher Bb levels (> 4 mg/dL). Regarding the suggestion made in the study that renal failure is a consequence of decreased CO, some aspects should be mentioned:the decrease in CO was to values that were still in the normal range, and therefore, it is improbable that this change would be implicated in renal injury per se. Moreover, if CO decreases, there would be an expected increase in pulmonary pressures.However, the authors noted that pulmonary pressures were not affected despite the decrease in CO, suggesting a decrease in venous return. This is an important observation because baseline characteristics suggest the presence of prerenal injury by hypovolemia in these patients. Therefore, we should examine whether a decrease in CO could be a consequence of central hypovolemia in these patients. Would a diuretic effect present?
The second study that contributed to the “therapeutic window” hypothesis aimed to evaluate hemodynamic status before and after hepatorenal syndrome (HRS). They concluded that HRS results from decreased CO in the setting of arterial vasodilation[4].However, it should be emphasized the worst basal renal function of group that evolved with HRS, adding an important bias to conclusions. In addition, even though the authors discontinued diuretics prior to the study, the hemodynamic values of the HRS group are characteristic of hypovolemia since they presented not only lower CO,but also lower pulmonary pressures and lower stroke volume. Indeed, according to parameters explaining different shock patterns, central hypovolemia must be strongly suggested when pulmonary pressures and stroke volume are low[8]. Would a diuretic effect still present?
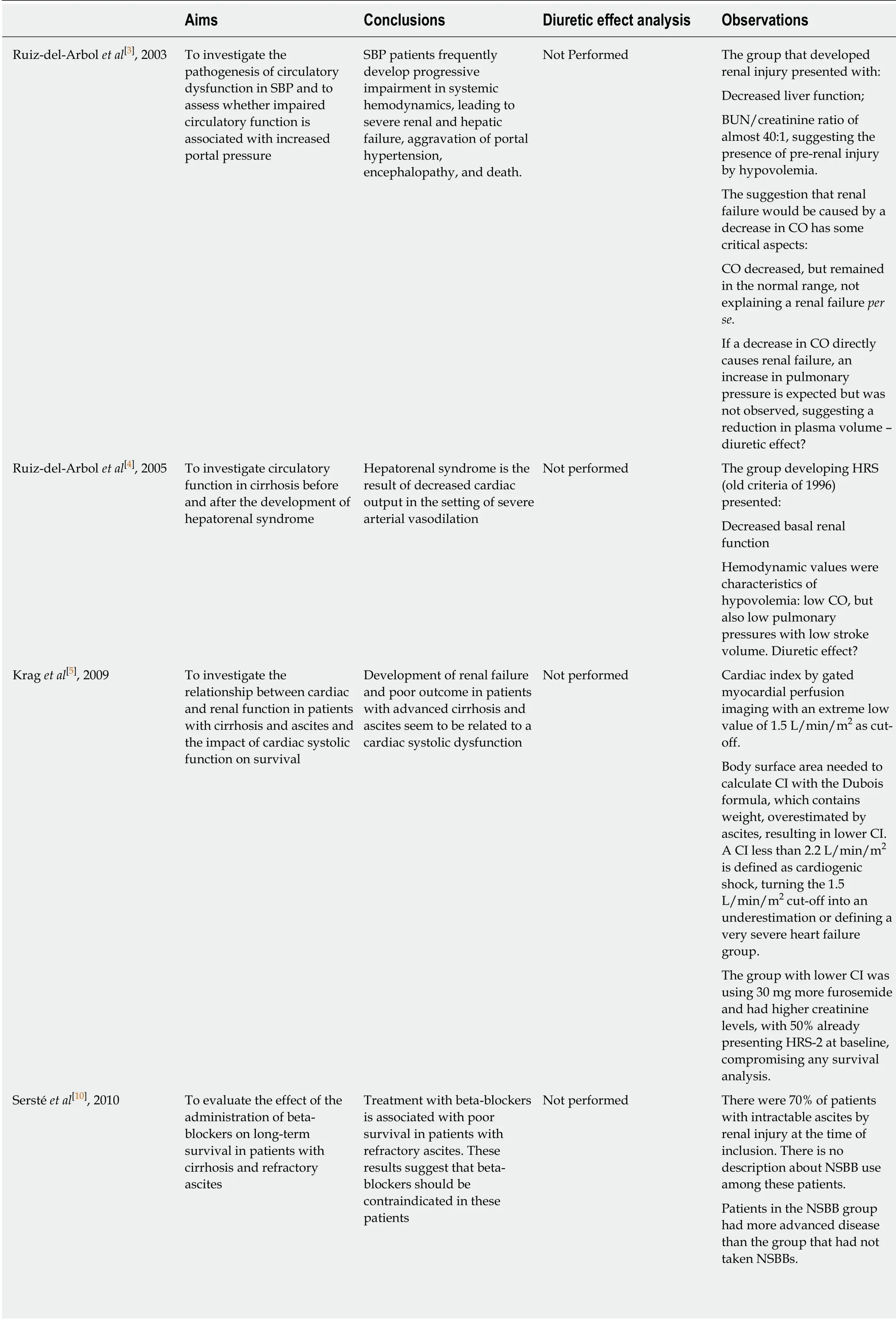
Table 1 Non-selective beta-blockers “window therapy” hypothesis-studies analyses
BUN: Blood urea nitrogen; CI: Cardiac index; CO: Cardiac output; HR: Hazard ratio; HRS: Hepatorenal syndrome; NSBB: Non-selective beta-blockers;PICD: Paracentesis-induced circulatory dysfunction; SBP: Spontaneous bacterial peritonitis.
The third study included in the “therapeutic window” hypothesis aimed to investigate the relationship between cardiac and renal function in patients with cirrhosis and ascites and the impact of cardiac systolic function on survival. They concluded that development of renal failure and poor outcome in patients with advanced cirrhosis and ascites are related to cardiac systolic dysfunction[5]. To perform the study, authors estimated CI by gated myocardial perfusion imaging. The first concern is regarding the body surface area (BSA) formula used to calculate CI.Authors adopted the DuBois formula, described as follows: BSA = (Weight0.425×Height0.725) × 0.007184, and CI was calculated as follows: CI = CO/Body surface.However, all patients included in the study had ascites, which increased their weight,therefore overestimating BSA, leading to an underestimation of CI (BSA is inversely correlated to CI). Based on this methodology, high volume ascites results in low CI,already compromising any conclusion since patients with refractory ascites (high volume ascites) present a well-known worse prognosis. Nevertheless, authors pragmatically adopted a value of 1.5 L/min/m2as a cut-off. However, this value is below the cut-off value used to diagnosis cardiogenic shock (2.2 L/min/m2)[9],reflecting the influence of “ascites weight” in the results. Actually, considering these aspects, a more accurate conclusion for this study is that patients with more ascites develop more renal failure and decreased survival, and these are already well-known outcomes. Notably, patients with CI < 1.5 L/min/m2were using 30 mg more furosemide[5].
Sersté et al[10]performed an important study that significantly contributed to the“therapeutic window”. They evaluated the effect of NSBB administration on longterm survival in patients with cirrhosis and refractory ascites. The authors concluded that use of beta-blockers is associated with poor survival in patients with refractory ascites. They suggest that beta-blockers should be contraindicated in these patients.However, there were 14% more Child-Pugh C patients in the NSBB group, presenting a lower sodium value and higher bilirubin level. Moreover, all patients had esophageal varices in the NSBB group, compared to only 4% in the group without NSBB. These aspects conferred an important bias, since the NSBB group was evidently more severe than the other group. It must also be emphasized that 70% of total patients included in the study had intractable ascites, i.e., with probable diureticinduced renal injury, and there was no information about NSBB use among these patients. Regression analysis demonstrated that the most important independent variables that predicted death were hyponatremia and renal injury, and both could be related to diuretic treatment, with higher hazard ratios (HR 7.07 and 3.07,respectively) than NSBB use (HR 2.61). Therefore, we should consider the presence of a potential harmful diuretic treatment in these patients[10].
Another study aimed to evaluate the incidence of paracentesis-induced circulatory dysfunction (PICD) before and after discontinuation of beta-blockers in patients with cirrhosis and refractory ascites. It was a small study that included only ten patients,and six had diuretic-resistant ascites. They observed a higher incidence of PICD in paracentesis performed during NSBB treatment. However, there is no information about diuretic dosage in each assessment, compromising the interpretation of results since renin activation also occurs by hypovolemia[11].
In contrast to the “therapeutic window” hypothesis, there were important studies that demonstrated a beneficial role of BBNS in advanced cirrhosis. Leithead et al[12]aimed to determine whether NSBB use was a risk factor for mortality in patients with end-stage cirrhosis and ascites awaiting liver transplantation. They evaluated 322 patients with ascites awaiting liver transplantation. To reduce the probability of selection bias, they repeated the analysis in a cohort of NSBB and non-NSBB propensity risk score-matched patients. Matching patients by propensity risk score is a recognized method of controlling for selection bias. They concluded that NSBB in patients with ascites and refractory ascites listed for liver transplantation are not detrimental, but instead are associated with reduced waitlist death[12].
Another important study included patients from the CANONIC study. It was a prospective and observational study that aimed to evaluate whether ongoing use of NSBBs reduced 28-d mortality in acute-on-chronic liver failure patients. Among the patients included, 164 (47%) received NSBB treatment. The results demonstrated a reduction in 28-d mortality among patients receiving NSBBs (24.4%) compared to patients without NSBB treatment (34.1%)[13].
There is also “real-life” evidence about the importance of NSBB in advanced cirrhosis, as demonstrated in the study by Bossen et al[14]that included 1198 cirrhotic patients with ascites. NSBB treatment did not increase mortality [HR 0.92 (0.72-1.18)].Among 559 patients undergoing NSBB treatment, 29% discontinued treatment. It is noteworthy that the discontinuation correlated to increased mortality, with an HR of 5.13 (2.28-11.55).
In contrast to the study by Ferrarese et al[15]that suggested increased incidence of PICD in patients with refractory ascites and NSBB, recent evidence has suggested that NSBB introduction does not increase PICD incidence. It should be also considered that NSBB has been implicated in beneficial non-hemodynamic effects in cirrhosis. An anti-inflammatory role was observed in outpatients using propranolol, which also improved the endothelial dysfunction that occurs during end-stage cirrhosis[16,17].There is evidence that propranolol reduces intestinal bacterial translocation, reducing the incidence of spontaneous bacterial peritonitis[18-20].
Taken together, it is clear that these studies do not support the hypothesis that NSBB is detrimental to organ perfusion and prognosis in advanced cirrhosis.Moreover, studies on the “window hypothesis” that included hemodynamic measurements support a possible diuretic effect, as parameters are consistent with hypovolemia. However, since the study by Krag et al[6]suggested the existence of a“window” for NSBB treatment in cirrhosis, clinical practices have been changed,mostly due to new recommendations by the Baveno VI consensus regarding the use of NSBBs in advanced cirrhosis. The consensus recommended that NSBB be reduced/discontinued if patients with refractory ascites develops any of following events: (1) Systolic blood pressure < 90 mmHg; (2) Hyponatremia (< 130 mEq/L); or(3) Acute kidney injury[21]. Considering the studies previously discussed, evidence for a deleterious effect of NSBB in patients with refractory ascites is not well defined;instead, the hemodynamic parameters seem to describe a deleterious diuretic effect,not only in these patients with refractory ascites, but also in other end-stage cirrhotic patients.
Systolic blood pressure is highly influenced by furosemide treatment, as demonstrated in a systematic review aimed to evaluate the blood pressure-lowering efficacy of loop diuretics for primary hypertension. The total number of patients included 262 patients in loop diuretic use versus 198 in the placebo group. They observed a significant mean reduction in systolic blood pressure, -7.92 (95%confidence interval: -10.40, -5.44) mmHg, with I2= 0.0% and test for overall effect: Z =6.26 (P < 0.00001)[22].
Another interesting study evaluated the effect of furosemide on elderly patients with heart failure (HF). One outcome was postprandial hypotension that occurs in the context of blood flow deviation to splanchnic circulation, a similar condition to portal hypertension in cirrhotic patients. The authors observed a significant reduction in systolic blood pressure in the postprandial period after furosemide administration[23].While we often ignore the prognostic effect size of diuretic treatment in hepatology,the diuretic effect is well described in HF studies, another hypervolemic disease such as cirrhosis, but with obviously marked reduced cardiac reserve.
Dini et al[24]evaluated the effect of furosemide on patients with compensated and decompensated HF, totaling 400 outpatients. The authors observed that a normalized furosemide dose was a major determinant of prognosis in patients with chronic HF but without ongoing signs and symptoms, suggesting a possible negative interaction of this drug in clinically stable patients. The authors further suggest that it is more difficult to identify the hypervolemic state in stable patients, putting them at higher risk of a deleterious diuretic effect.
Because results suggest a deleterious effect, it would be interesting to consider a“diuretic window” treatment hypothesis (Figure 1), maintaining NSBB during all disease stages until more clear evidence emerges about a harmful effect. Patients should have indicated diuretics during ascites formation. At this stage, cirrhotic patients present a hypervolemic status, and diuretics should be administered to reduce ascites and edema. During the hypervolemic phase, cirrhotic patients usually have an inflammatory profile with peripheral vasodilation, leading to reninangiotensin-aldosterone system hyperactivation with sodium reabsorption. At this initial stage, there is no reduction in plasma volume. The attention window should be open when central volemia reverts to initial stages during diuretic treatment, since loss of plasma volume could lead to an increasing risk of hypoperfusion, despite a global hypervolemic status, in which part of the plasma volume is “trapped” inside splanchnic vessels. The window to diuretic treatment must be closed when central volemia is reduced due to an increased risk of hypoperfusion and worsening prognosis. The studies that evaluated hemodynamic status and prognosis in cirrhosis demonstrated parameters compatible with reduced central volemia in patients with the worst outcomes[3,4]. Indeed, in stable HF patients, this evidence is more clearly demonstrated.
CONCLUSION
Easy and reliable tools should be developed to accurately assess central volemia in cirrhosis. Currently, echocardiographic parameters could be used at the time of diuretic dosage adjustment associated with clinical evaluation to identify the attention window. Prospective studies must be performed to evaluate the role of diuretic treatment in cirrhosis, considering the complex hemodynamic behavior during disease course. It should be reinforced that strong evidence is generated by randomized controlled trials, but also by detailed reanalysis of previous studies.
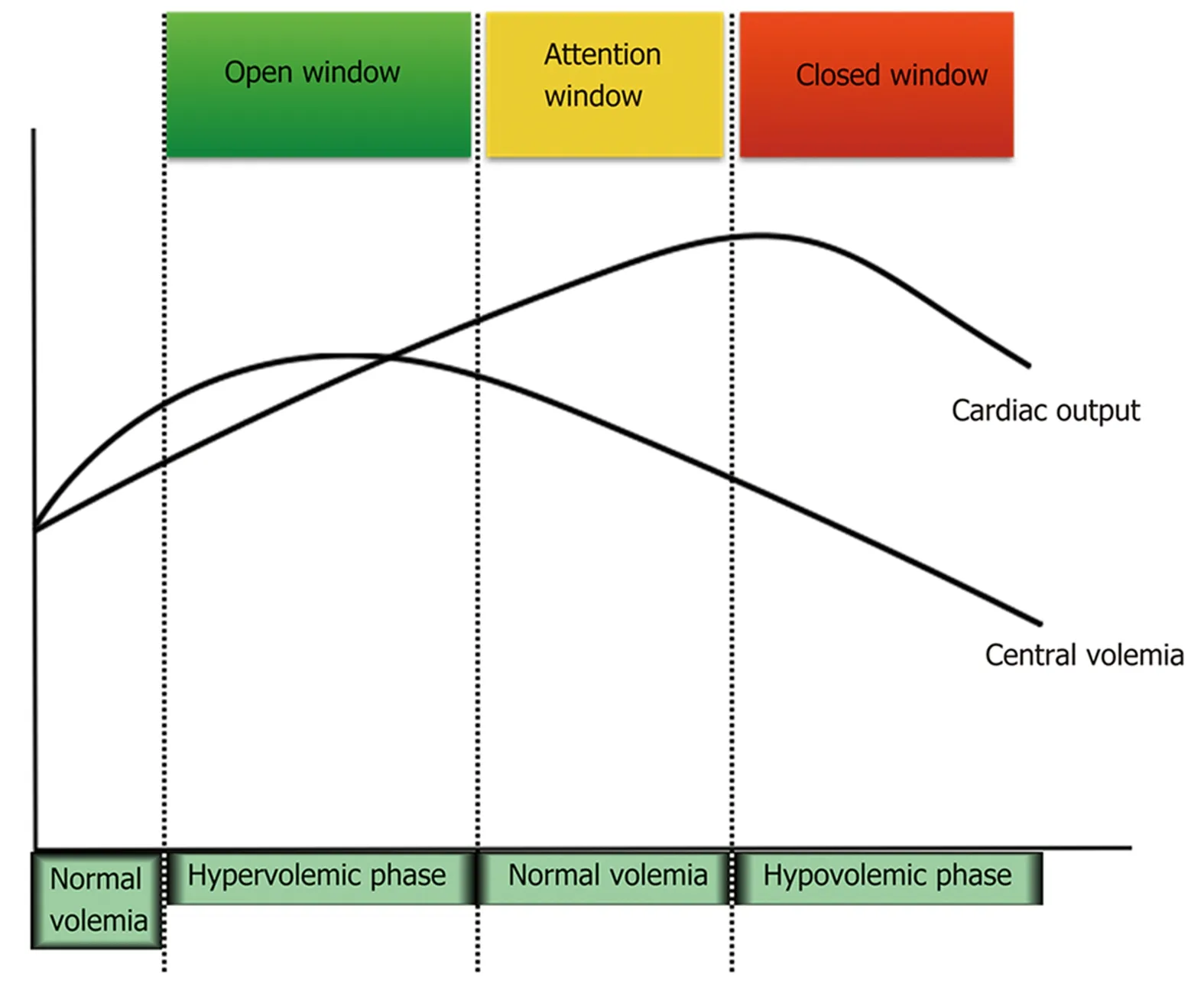
Figure 1 Proposed diuretic window hypothesis.
杂志排行
World Journal of Gastroenterology的其它文章
- Fluoroquinolones for the treatment of latent Mycobacterium tuberculosis infection in liver transplantation
- Reactivation of hepatitis B virus infection in patients with hemolymphoproliferative diseases, and its prevention
- Current status of endoscopic retrograde cholangiopancreatography in patients with surgically altered anatomy
- Choledochal cysts: Similarities and differences between Asian and Western countries
- Gastro-duodenal disease in Africa: Literature review and clinical data from Accra, Ghana
- Screening of aptamers and their potential application in targeted diagnosis and therapy of liver cancer
