Raddeanin A promotes apoptosis and ameliorates 5-fluorouracil resistance in cholangiocarcinoma cells
2019-07-24ShuangShuangGuoYingWangQingXiaFan
Shuang-Shuang Guo, Ying Wang, Qing-Xia Fan
Shuang-Shuang Guo, Qing-Xia Fan, Department of Oncology, the First Affiliated Hospital of Zhengzhou University, Zhengzhou 450052, Henan Province, China
Shuang-Shuang Guo, Ying Wang, Department of Oncology, First Affiliated Hospital, College of Clinical Medicine, Henan University of Science and Technology, Luoyang 471000, Henan Province, China
Abstract BACKGROUND Bile duct cancer is characterized by fast metastasis and invasion and has been regarded as one of the most aggressive tumors due to the absence of effective diagnosis at an early stage. Therefore, it is in the urgent demand to explore novel diagnostic approaches and therapeutic strategies for bile duct cancer to improve patient survival. Raddeanin A (RA) is extracted from the anemone raddeana regel and has been demonstrated to play antitumor roles in various cancers.AIM To investigate the effects of RA treatment on bile duct cancer cells.METHODS In this study, four cholangiocarcinoma cell lines (RBE, LIPF155C, LIPF178C, and LICCF) treated with RA were used to test the cell viability. The RA-associated cell functional analysis, 5-fluorouracil (5-Fu) effectiveness as well as cell cycle- and apoptosis-related protein expression were investigated.RESULTS RA reduced cell viability in a dose-dependent pattern in four cell lines, and the migration and colony formation abilities were also impaired by RA in RBE and LIPF155C cell lines. RA sensitized cell lines to 5-Fu treatment and enhanced the effects of 5-Fu in cholangiocarcinoma. Also, RA decreased protein expression of Wee1, while the combinational effect of RA and 5-Fu decreased protein expressions of cyclooxygenase-2, B cell lymphoma 2, and Wee1 but increased protein levels of Bax, cyclin D1, and cyclin E.CONCLUSIONTaken together, the results suggest that RA acts as an anti-cancer agent and enhancer of 5-Fu in bile duct cancer cells via regulating multiple cell cycle and apoptosis-related proteins. This finding provides novel clues to exploring a novel antitumor drug for bile duct cancer.
Key words: Bile duct cancer; Raddeanin A; 5-fluorouracil;
INTRODUCTION
Bile duct cancer (cholangiocarcinoma) is characterized by fast metastasis and invasion and, more importantly, the absence of effective diagnosis at an early stage[1], and it thus has been regarded as one of the most aggressive tumors that occur in bile duct epithelial cells[2]. To date, although surgical resection is one of the few curative treatments for bile duct cancer, the patients are often diagnosed at the advanced stage and have lost the chance of surgical resection at diagnosis[3]. In addition, the 5-year survival rate and effective therapeutic level for bile duct cancer have not greatly improved in recent years[3]. Therefore, it is in the urgent need to explore novel diagnostic approaches and therapeutic strategies for bile duct cancer to improve patient survival.
Raddeanin A (RA), an active triterpenoid saponin, is extracted from the anemone raddeana regel that is a traditional medicinal herb in Chinese medicine[4]. An increasing amount of evidence has demonstrated that RA plays a cytotoxic role in facilitating apoptosis of tumor cells and suppressing their proliferation, migration,and invasion[5], thus exhibiting anticancer effects on various cancer types. In gastric cancer, RA results in tumor cell apoptosis via regulating the molecules involved in the caspase-cascade pathway, such as B cell lymphoma 2 (Bcl-2) family[4]. Also, RA has been demonstrated to cause apoptosis in a concentration-dependent pattern in colorectal cancer cells via mechanisms associated with suppressing NF-κB and activated Wnt/β-catenin signaling in colorectal cancer cells[6]. Furthermore, the PI3K/AKT/mTOR pathway is involved in the RA-induced apoptosis in breast cancer cells[7]. Collectively, RA-associated apoptosis in various types of cancer cells display a cancer type-dependent pattern, suggesting the necessity of proapoptotic effect of RA in cholangiocarcinoma cells.
Malignant tumor formation is a highly complicated process, involving a series of carcinogens, tumor promotion, apoptosis, and metastatic process[8]. Cyclooxygenase-2(Cox-2), a cyclooxygenase enzyme, catalyzes the first step of prostanoids synthesis[9].Cox-2 is highly expressed in cancer and stroma cells during tumor progression and displays anti-apoptosis roles in tumor cells[10]. In addition, a group of factors that are essential for the regulation of the cell cycle have been demonstrated to be closely related to malignancies. For example, aberrant overexpression of cyclins D1 and E,two members of the cyclin family[11], contributes to carcinogenesis and malignant progression due to the dysfunction of the cell cycle[12,13]. Wee1 is essential for G2 cell cycle checkpoint in response to DNA damage and repair[14]and overexpressed Wee1 has been linked to a number of cancers, such as carcinoma[15]and glioblastoma[16].Furthermore, apoptosis has been shown to be closely related to a series of cancers[17],in which the mitochondrial-related proteins bcl-2 and Bax are highly involved in the regulation of mitochondrial membrane properties, leading to activation of subsequent pathways of apoptosis[18,19].
Currently, 5-fluorouracil (5-Fu) is one of the most common chemotherapeutic compounds for cancer treatment[20]. The resistance to chemotherapy is a primary challenge for cancer treatment[21]. Thus, any approach or compound that can sensitize 5-Fu-resistant cells would be beneficial to cancer patients. Given its potential anticancer effect, the objective of this study was to investigate the effects of RA treatment on bile duct cancer cells including related chemotherapy resistance as well as the underlying mechanisms. This study also aimed to provide clues to the application of RA for the therapy of bile duct cancer.
MATERIALS AND METHODS
Cell lines
Cholangiocarcinoma cell lines (RBE, LIPF155C, LIPF178C, and LICCF) were purchased from the cell back of China Center for Type Culture Collection (CCTCC at Wuhan University). The normal intrahepatic biliary epithelial cell line HIBEpiC was obtained from the ATCC (Manassas, VA, United States).
Viability assay
The ATPlite assay (Perkin Elmer, United States) was applied to evaluate cell viability according to the manufacturer's instructions (n = 3 independent experiments). Cells were seeded in 96-well plates (Corning-Costar, United States) and cultured for 24h.RA at doses ranging from 0 to 160 μg/mL was applied to treat the cells for 24 h. Cell viability was evaluated using a microplate reader (BioRad, United States).
Migration and invasion assays
Wound-healing migration and Transwell invasion assays were used to test migratory cell ability in RBE and LIPF155C cell lines (n = 3 independent experiments). The assays were performed as previously reported[22].
Clonogenic assay
The clonogenic assay was performed to test colony formation ability in RBE and LIPF155C cell lines (n = 3 independent experiments). RBE and LIPF155C cells (70%confluence) were treated transiently with metformin (0.5mM). Cells were then detached and seeded in 6-well plates (600 cells/well) (Corning-Costar, United States)in drug-free media. Fresh media (25%) were added every three days. Cell colony was stained with cristal violet and the number of colonies was counted ten days later.
Hoechst staining assay
Hoechst staining assay was performed to test cell apoptosis (n = 3 independent experiments). The assay was performed as previously reported[23]. Hoechst (Sigma,United States) stained cells were visualized by fluorescence microscopy (Olympus,Japan).
Western blot analysis
Cell protein was isolated by using the cell lysis buffer (Beyotime Institute of Biotechnology, China). Western blot analysis was performed as previously reported[22].The primary antibodies for Cox-2, Bcl-2, Bax, GAPDH, cyclin D1, cyclin E, and Wee1 were obtained from Santa Cruz Biotechnology (United States). Quantification of optical density was evaluated using Uvitec Alliance software (Eppendorf, Germany)(n = 3 independent experiments).
Statistical analysis
Data are expressed as the mean ± standard error of the mean and analyzed via SPSS 19.0 (SPSS Inc, United States). The two-group comparison was performed by twotailed t-tests and multiple comparisons were analyzed by ANOVA and the post-hoc Tukey test. In this study, statistical significance was set at P < 0.05.
RESULTS
Viability of cholangiocarcinoma cell lines treated with RA
RA at doses ranging from 0-160 μg/mL was applied for 24 h to test the cell viability of four cholangiocarcinoma cell lines (RBE, LIPF155C, LIPF178C, and LICCF) and one normal intrahepatic biliary epithelial cell line (HIBEpiC) via the ATPlite assay. RA was found to reduce cell viability in a dose-dependent pattern (Figure 1A). The half-maximal effective concentration (EC50) and the half-maximal lethal concentration(LC50) for each line were also evaluated (Figure 1B). EC50 and LC50 ranges in the four tumoral cell lines were 50.95-64.76 μg/mL and 34.65-49.47 μg/mL, respectively,while HIBEpiC showed higher values of EC50 (79.52 μg/mL) and LC50 (63.17 μg/mL) compared with those of tumoral cell lines, indicating that the RA does that reduced cell viability of tumoral cell lines were not toxic for the normal cell line.
Functional analysis for cholangiocarcinoma cell lines treated with RA
Based on the results from the cell viability assay, RA at a dose of 13 μg/mL that reduced about 25% cell viability was chosen to use in subsequent experiments. The wound healing (Figure 2A-D) and Transwell migration (Figure 3A) assays were conducted to test the migration ability of the two cholangiocarcinoma cell lines RBE and LIPF155C. The results revealed that the migration ability of both lines was impaired by 13 μg/mL RA. In addition, clonogenic assay results indicated that RA reduced colony formation capability of the two cholangiocarcinoma cell lines (Figure 3B).
Effects of RA on 5-Fu effectiveness in cholangiocarcinoma cell lines
Chemoresistance is currently one of the major issues for therapy of bile duct cancer.Thus, the effects of RA on 5-Fu effectiveness were evaluated by using the combination of RA (13 μg/mL) and different increasing doses of 5-Fu, a common proapoptotic compound, in RBE and LIPF155C lines. RA was found to sensitize both tumoral cell lines (Figure 4A and B), namely, greatly reduced the EC50 (about 2-fold) and LC50(more than 3-fold) (Figure 4C). Clonogenic assays revealed that 5-Fu displayed more potent roles to reduce cell colony formation compared with RA; however, RA was able to enhance such inhibitory effect of 5-Fu in both cell lines compared with the effect of using 5-FU alone (Figure 4D and E).
Effects of RA on 5-Fu-resistant cell line RBE/5-Fu
Hoechst staining assay was performed to evaluate the effects of RA on 5-Fu-resistant cell line RBE/5-Fu (Figure 5A and B). RBE/5-Fu (50 μmol/L) cells were treated with 13 μg/mL RA and the results suggested that either RA or 5-Fu promoted cell death.Furthermore, the effects of the combination of both were more potent than those of each one.
Effects of RA on cell cycle- and apoptosis-related protein expression
The RBE cell line was treated with 13 μg/mL RA for 48 h and cell cycle- and apoptosis-related protein expression was detected by Western blot (Figure 6A-D).Compared with the control group, RA treatment only decreased Wee1 protein level while 5-Fu treatment alone reduced Cox-2 and Bcl-2 expression and increased Bax and cyclin D1 protein expression. RA treatment combined with 5-Fu decreased protein expression of Cox-2, Bcl-2, and Wee1 whereas increased protein levels of Bax,cyclin D1, and cyclin E. 5-Fu treatment was associated with a lower protein level of Cox-2 and higher Wee1 expression compared with the effect of RA.
DISCUSSION
As a malignant cancer of the biliary tract, there are still limited precise diagnosis and effective therapy for bile duct cancer[24], although the incidence and mortality rates of bile duct cancer are increasing in recent years[25]. Therefore, there is an urgent demand to seek a novel strategy to improve diagnostic efficiency and therapeutic effect. RA has been demonstrated to play cytotoxic roles in various tumor cell lines via initiating apoptosis and impairing the cell cycle[6]. In our study, the cell viability, migration, and colony formation abilities in four cholangiocarcinoma cell lines were significantly reduced by RA treatment. Also, RA was found to sensitize the 5-Fu treated tumoral cell lines as well as facilitate apoptosis of 5-Fu-resistant cell line. Further Western blot results revealed that Wee1 expression level was dampened by RA (13 μg/mL)treatment and that the combinational effects of RA (13 μg/mL) and 5-Fu (35 μM) were associated with a group of cell cycle- and apoptosis-related factors. The results collectively suggest that the anti-bile duct cancer effects of RA may involve a series of regulatory mechanisms on the cell proliferation and apoptosis.
Wee1, a member of the family of protein kinases, is related to the regulation of G2checkpoint in response to DNA damage[26,27]. Increasing evidence in recent years demonstrates that the overexpression of Wee1 kinase is associated with various malignancies, such as breast cancer[28], hepatocellular carcinoma[15], and malignant melanoma[16]. In this study, RA treatment was found to be related to decreased Wee1 protein level in RBE cell line, which indicates that the effects of RA potentially relies on the Wee1-dependent mechanism. Downregulation of Wee1 has been reported to contribute to the apoptosis of ovarian tumor[29]and neuroblastoma cells[30]. In particular, the checkpoint in the cell cycle is an important self-check mechanism to terminate the cell cycle process in response to DNA damage[27]. Despite DNA damage,the down-regulated Wee1 expression in tumor cells plays essential roles in maintaining cell proliferation via halting cell cycle arrest, thereby facilitating apoptosis and mitotic arrest during mitosis[27,31]. Collectively, RA-induced decreased cell viability and impaired cell functions in our study may result from the activation of Wee1 signaling that triggers apoptosis of cholangiocarcinoma cells.
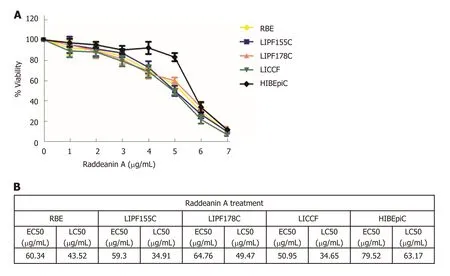
Figure 1 Effects of raddeanin A on cholangiocarcinoma cell lines (n = 3 independent experiments). A: Increasing concentrations of raddeanin A (RA) were administered to cholangiocarcinoma cell lines for 24 h before being analyzed by the ATPlite assay. The percentage cell viability normalized to control is shown and data are expressed as the mean ± standard deviation; B: The half-maximal effective concentration and the half-maximal lethal concentration values for cholangiocarcinoma cell lines treated with RA. RA: Raddeanin A; EC50: Half-maximal effective concentration; LC50: Half-maximal lethal concentration.
5-Fu is a worldwide used chemotherapeutic treatment for many tumors, such as ovarian, breast, pancreatic, and colorectal cancers[32]. It has been well-documented that 5-Fu generates DNA damage through breaking DNA double-strand structure[33]and exerts anticancer effects via suppression of thymidylate synthase[34]. However,increasing reports revealed that the drug resistance has been becoming a significant limitation to the clinical application of 5-Fu in cancer treatment[34], which was also observed in cholangiocarcinoma[35,36]. In this study, the results suggest that RA sensitized cholangiocarcinoma cell lines and also enhanced the anti-cancer effects of 5-Fu. Furthermore, the combinational effects of RA and 5-Fu were found to be associated with alterations of a group of the cell cycle and apoptosis protein expression, such as decreased expression of Cox-2, Bcl-2, and Wee1 and increased levels of Bax, cyclin D1, and cyclin E.
As an important inducible enzyme, Cox-2 has been demonstrated to be involved in angiogenesis and tumorigenesis[37]. Cox-2 expression can be activated by various factors, such as mitogens, oncogenes, as well as carcinogens[38]. Selective Cox-2 inhibitors, for example, rofecoxib and celecoxib, are capable of suppressing established tumor growth and preventing tumorigenesis[37,39]. The down-regulated expression of Cox-2 induced by the combinational treatment in this study suggests that cox-2 may play key roles in the inhibition of cholangiocarcinoma cell growth.
Mitochondrial apoptosis pathway is an important programmed cell death pathway[40]and involves a great number of morphological changes and pathological alterations, including cancers[41,42]. Activation of the mitochondrial apoptotic pathway causes alterations of mitochondrial membrane properties through the Bcl-2 family,including translocation of Bax and suppression of Bcl-2. Thus, Bax/bcl-2 ratio is widely used as a predictive marker for evaluation of cancer therapy[43,44]. In this study,RA treatment in cholangiocarcinoma cell lines is associated with increased Bax/Bcl-2 ratio, namely, increased Bax and decreased bcl-2 level, which is consistent with the findings from previous studies[4,45]. The results indicate that the mitochondrial apoptosis pathway may be involved in the anti-tumor effects of RA in bile duct cancer.
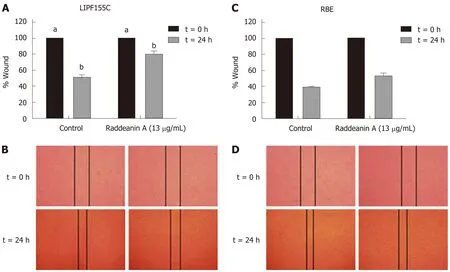
Figure 2 Wound healing assay of RBE and LlPF155C cell lines treated with raddeanin A (13 µg/mL) (n = 3 independent experiments). A and C: The percentage of wound width normalized to the controls is shown and data are expressed as the mean ± standard deviation. Unique letters shared by the bars suggest significant differences between groups and P < 0.05 was regarded to be statistically significant; B and D: Representative histograms for the percentage of wound width.
In addition to apoptosis, the cell cycle was also found to be related to the effects of RA on the cholangiocarcinoma cell lines in this study. It is well-studied that cell cycle progression is associated with sophisticated molecular and cellular cascades[46]and participates in the development of various types of cancer[27]. During these processes,the cyclin family and their catalytic subunits cyclin-dependent kinases form a series of molecular complexes in every phase of the cell cycle to regulate this complicated cell cycle progression[27]. The cyclin D-Cdk4/6-cyclin E-Cdk2 signaling pathway plays an essential role in G1-S transition and the response to DNA damage[47,48]. Some evidence reveals that increased cyclins E and D1 protein levels are highly related to the initiation of apoptosis and sensitization to radiation in tumor cells[49,50], suggesting that increased cyclin E and D1 may act as proapoptotic factors in cholangiocarcinoma cells simultaneously treated with RA and 5-Fu.
In conclusion, our results suggest that RA treatment causes increased apoptosis and impaired cell functions in cholangiocarcinoma cell lines via a Wee1-dependent mechanism and that RA is an enhancer of 5-Fu in bile duct cancer through activating multiple cell cycle and apoptosis-related factors, such as Cox-2, Bax, Bcl-2, and cyclins E/D1. These findings together indicate that RA is a potential novel therapeutic treatment for bile duct cancer.
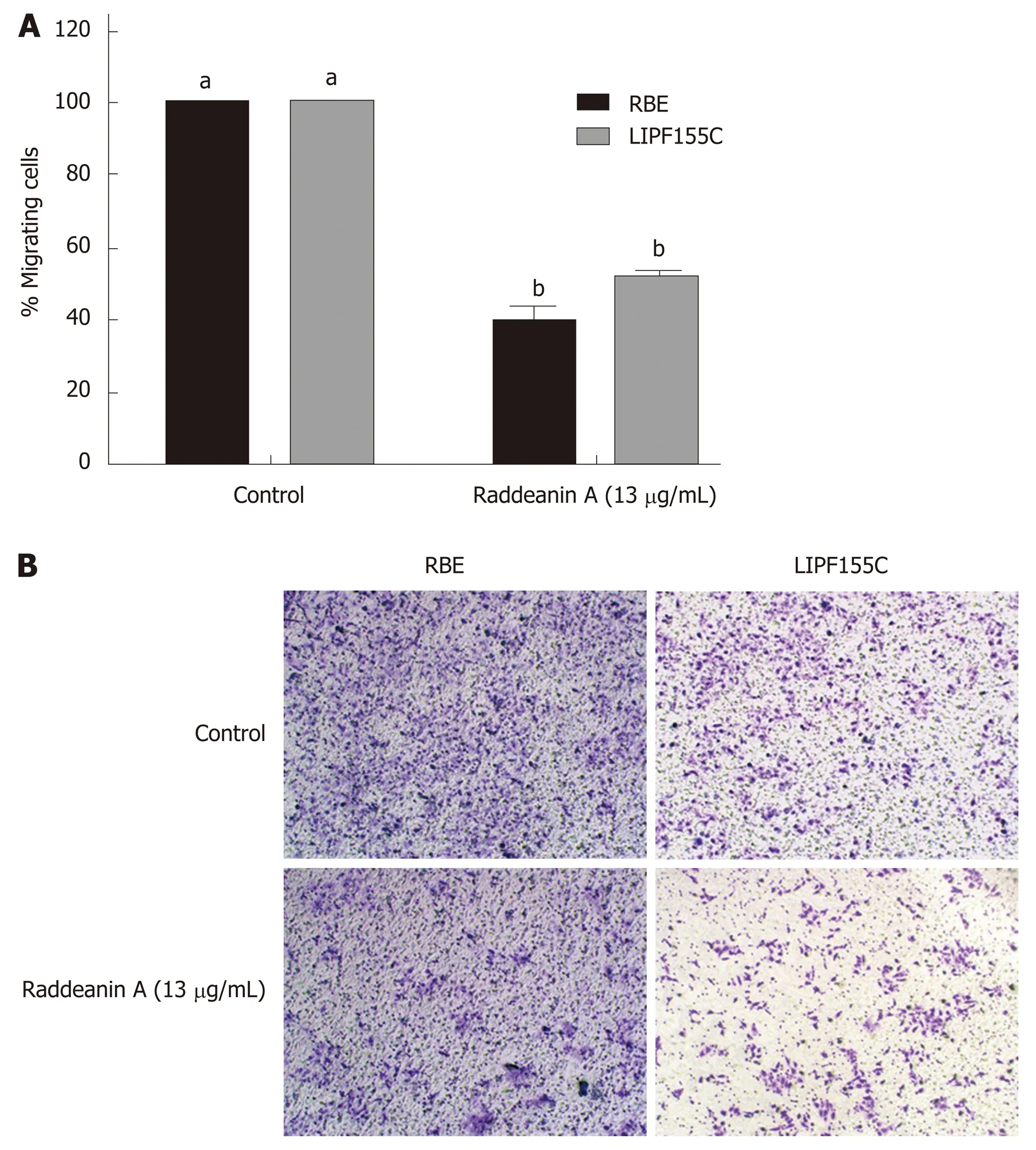
Figure 3 Transwell migration and clonogenic assays of RBE and LlPF155C cell lines treated with raddeanin (13 µg/mL) (n = 3 independent experiments). A:The percentage of migration cells normalized to the controls are shown and data are expressed as the mean ± standard deviation. Unique letters shared by the bars suggest significant differences between groups and P < 0.05 was regarded to be statistically significant; B: Representative histograms for cell colony formation.
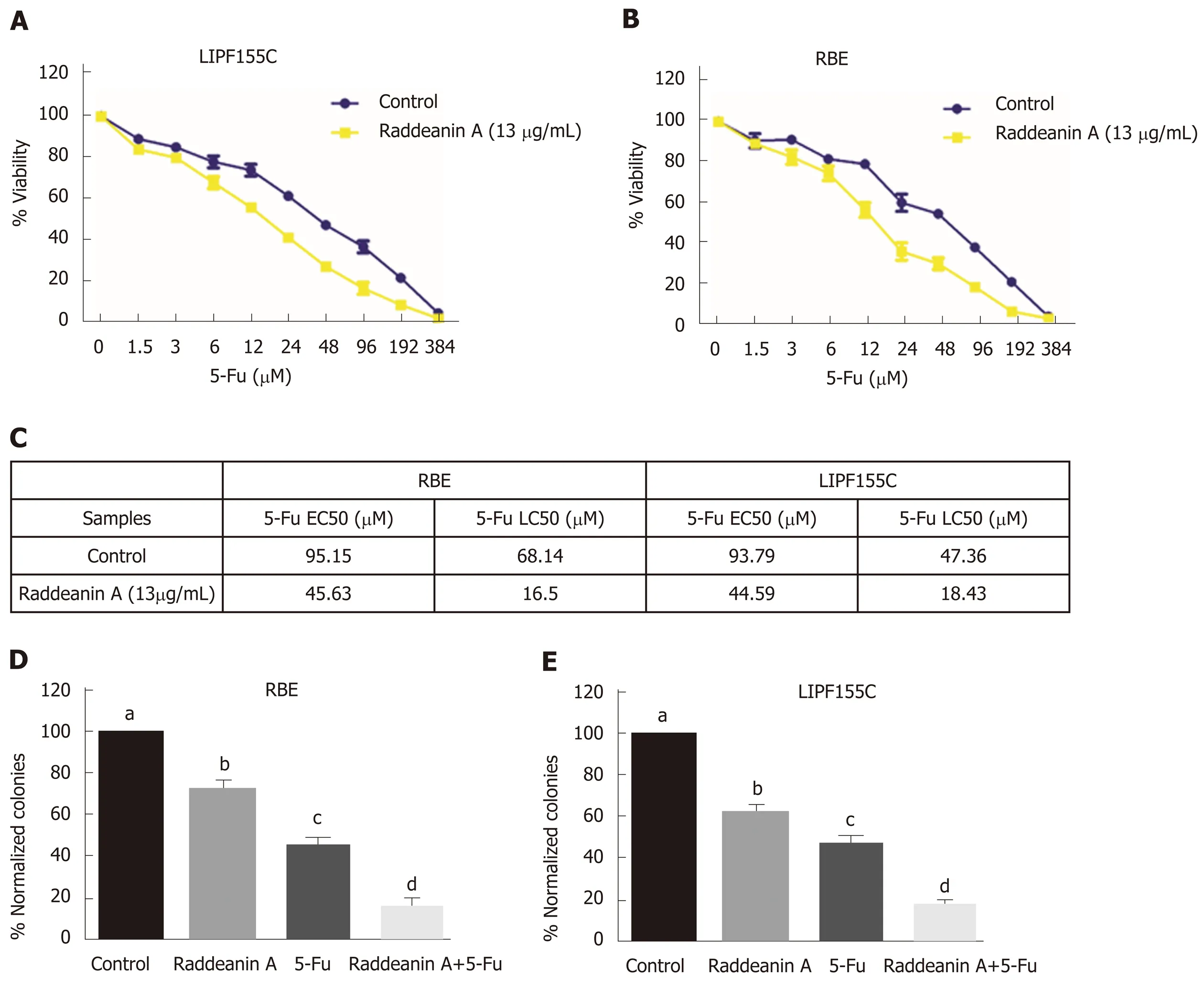
Figure 4 Effects of raddeanin A on 5-fluorouracil effectiveness in RBE and LlPF155C cell lines. A and B: RBE and LIPF155C cell lines treated with either control or raddeanin A (13 μg/mL) in combination with increasing doses of 5-Fu; C: The half-maximal effective concentration (EC50) and half-maximal lethal concentration values for RBE and LIPF155C cell lines; D and E: The cell colony formation ability in RBE and LIPF155C cell lines. Unique letters shared by the bars suggest significant differences between groups and P < 0.05 was regarded to be statistically significant. 5-Fu: 5-fluorouracil.
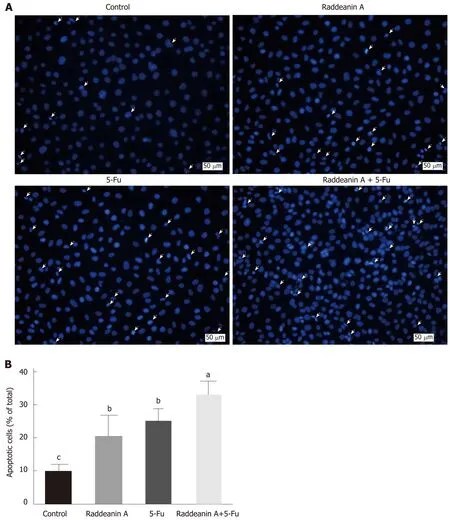
Figure 5 Effects of raddeanin A on 5-fluorouracil cell line RBE/5-Fu. A: Representative graphs for apoptotic RBE/5-Fu cells treated with 13 μg/mL raddeanin A; B:The percentage of apoptotic cells is shown and data are expressed as the mean ± standard deviation. Unique letters shared by the bars suggest significant differences between groups and P < 0.05 was regarded to be statistically significant. 5-Fu: 5-fluorouracil.
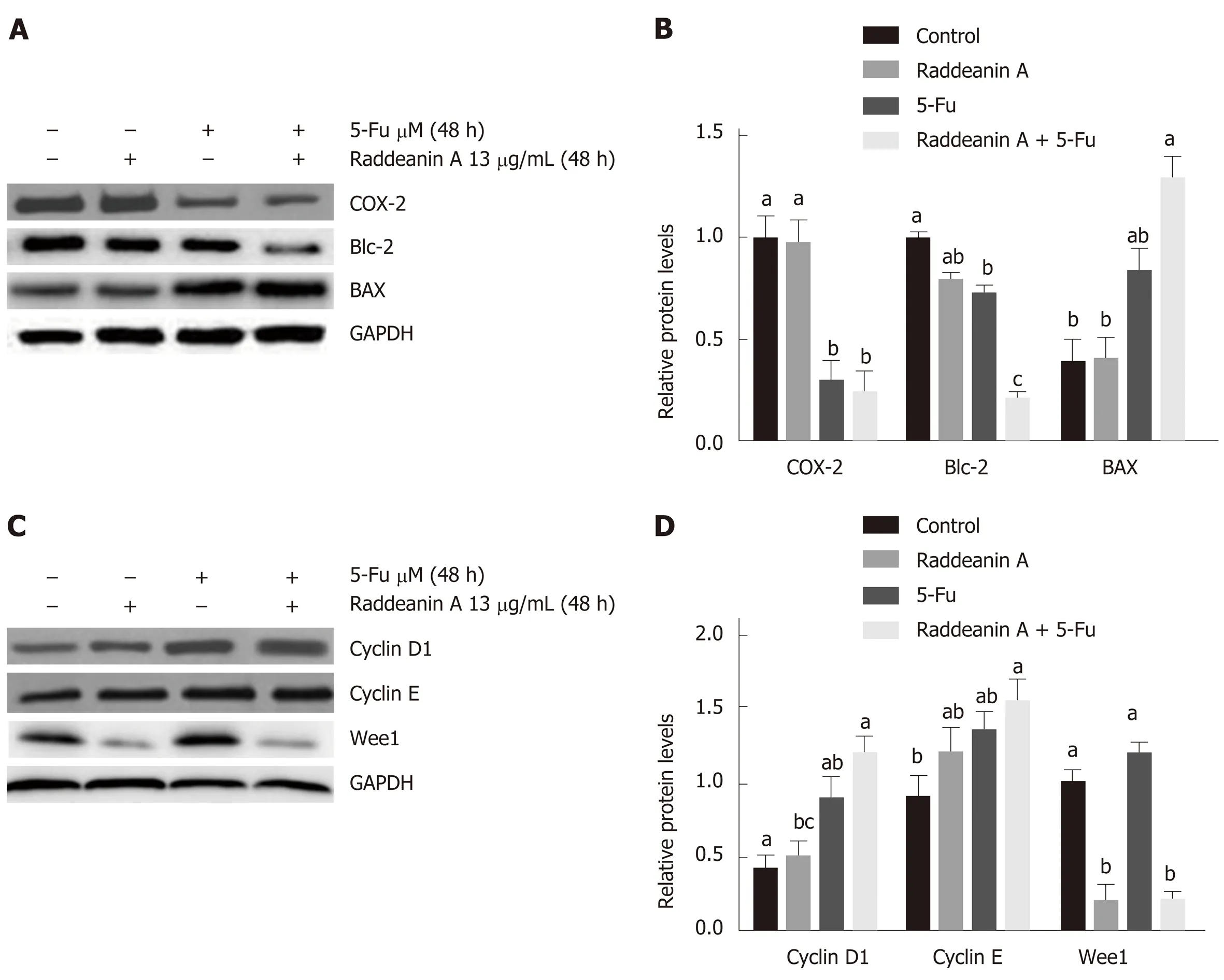
Figure 6 Effects of raddeanin A on cell cycle- and apoptosis-related protein expression in RBE cell line treated with 13 μg/mL raddeanin A for 48 h. A and B: Apoptosis-related protein expression detected by Western blot; C and D: Cell cycle-related protein expression detected by Western blot. Data are expressed as the mean ± standard deviation. Unique letters shared by the bars suggest significant differences between groups and P < 0.05 was regarded to be statistically significant.RA: Raddeanin A; 5-Fu: 5-fluorouracil; COX-2: Cyclooxygenase-2.
ARTICLE HIGHLIGHTS
Research background
Bile duct cancer is characterized by fast metastasis and invasion and has been thought of as aggressive cancer due to the lack of effective diagnosis at an early stage. The 5-year survival rate of bile duct cancer has not substantially improved in clinical practice. In addition, it is reported that 5-fluorouracil (5-Fu) has been widely applied in treatments for various cancers, achieving a great therapeutic effect. Furthermore, raddeanin A (RA) plays essential proapoptotic roles in various types of tumor cells.
Research motivation
Exploring and developing effective diagnostic ways and therapies for bile duct cancer is greatly urgent for both basic science and clinical management.
Research objectives
The objective of this study was to determine the effects of RA on bile duct cancer cells and the underlying mechanisms.
Research methods
In this study, RA at different concentrations was administered to four cholangiocarcinoma cell lines (RBE, LIPF155C, LIPF178C, and LICCF). Cell viability, Wound-healing migration,Transwell invasion, and Hoechst staining assays were performed to evaluate cell activities.Western blot analysis was performed to the apoptosis-related pathway.
Research results
RA inhibited cell viability in a dose-dependent pattern in the four cell lines. In RBE and LIPF155C cell lines, the migration and colony formation abilities were impaired by RA. Also, RA sensitized cell lines to 5-Fu treatment, promoting the effects of 5-Fu in cholangiocarcinoma cell lines. The role of RA was associated with reduced Wee1 expression. Furthermore, the combinational effect of RA and 5-Fu led to the inhibition of cyclooxygenase-2, B cell lymphoma 2, and Wee1 as well as the elevation of Bax, cyclin D1, and cyclin E.
Research conclusions
RA administration could effectively regulate apoptosis and cell functions in cholangiocarcinoma cell lines in a Wee1-dependent pattern and promote the effect of 5-Fu in bile duct cancer.Collectively, RA is a promising treatment for bile duct cancer.
Research perspectives
This study was aimed to provide clues to the application of RA for the therapy of bile duct cancer.
杂志排行
World Journal of Gastroenterology的其它文章
- Diuretic window hypothesis in cirrhosis: Changing the point of view
- Fluoroquinolones for the treatment of latent Mycobacterium tuberculosis infection in liver transplantation
- Reactivation of hepatitis B virus infection in patients with hemolymphoproliferative diseases, and its prevention
- Current status of endoscopic retrograde cholangiopancreatography in patients with surgically altered anatomy
- Choledochal cysts: Similarities and differences between Asian and Western countries
- Gastro-duodenal disease in Africa: Literature review and clinical data from Accra, Ghana
