G protein-coupled receptor 37(GPR37) emerges as an important modulator of adenosinergic transmission in the striatum
2019-07-18XavierMorató,RodrigoA.Cunha,FranciscoCiruela
G protein-coupled receptor 37 (GPR37), also known as parkin associated endothelin-like (Pael) receptor, is an orphan G protein-coupled receptor, which suffers a defective parking ubiquitination in autosomal recessive Parkinson's disease promoting its endoplasmic reticulum aggregation and stress, neurotoxicity and neuronal death (Takahashi and Imai, 2003). Interestingly,we have demonstrated previously that GPR37 heteromerizes with adenosine A2Areceptor (A2AR) in the striatum (Morató et al., 2017; Sokolina et al., 2017). In addition, we also reported some functional consequences of this direct interaction,whereby GPR37 deletion enhanced striatal A2AR cell surface expression with a concomitant increase in A2AR agonist-mediated cAMP accumulation (Morató et al., 2017); accordingly,an enhancement of A2AR agonist-induced catalepsy and antagonist-induced locomotor activity was observed upon GPR37 deletion (Morató et al., 2017). Overall, it has been hypothesized that GPR37 might hold a chaperone-like activity controlling A2AR cell surface targeting and function. However, the precise physiological function of GPR37 still is unidentified. The current findings now provide additional evidence for the role of GPR37 as a repressor of A2AR function. Thus, while chronic A2AR antagonist treatment (i.e., SCH58261, 1 mg/kg per day,intraperitoneal administration, 10 days) of mice lacking GPR37 did not affect the striatum-dependent cued learning, it enhanced locomotor sensitization (Morató et al., 2019). Moreover,chronic A2AR blockade boosted striatal long-term depression(LTD) in corticostriatal synapses of GPR37-/-but not of wild type mice; this observation correlated well with the adenosinergic neurochemical modifications present in GPR37-/-mice,namely an increased density of A2AR and decreased levels of adenosine (Morató et al., 2019). Overall, GPR37 emerged as key contestant controlling A2AR function particularly upon chronic A2AR blockade, thus delineating A2AR-dependent long-term plastic changes in corticostriatal synapses.
Adenosine is an key neuromodulator in the central nervous system regulating two important brain processes, namely neurotransmission and neural metabolism. Indeed, adenosine modulates the basal levels and release of many neurotransmitters including glutamate, γ-aminobutyric acid (GABA), dopamine and serotonin, thus fine-tuning synaptic transmission.Derived from the breakdown of intra- and extracellular adenosine triphosphate, adenosine is released upon some physiological and pathological stimuli, thus exerting its effects through four G protein-coupled adenosine receptors. In the brain, the density of A2AR is far greater in regions heavily innervated by dopaminergic terminals, namely in the striatum, nucleus accumbens and olfactory tubercle. Interestingly, the striatum is a large forebrain region involved in action initiation, motor skill learning and development and cued action-selection memory. Moreover, this structure could be involved both in reward-association memory and visual recognition memory. In Parkinson's disease, a progressive degeneration of midbrain dopamine neurons is associated with an aberrant striatal activity producing marked motor impairments, including bradykinesia,tremor, and rigidity. Indeed, dopamine replacement therapy with levodopa is the “gold standard” Parkinson's disease treatment. While initially effective in treating motor deficits, most Parkinson's disease patients develop drug-induced involuntary movements (i.e., levodopa-induced dyskinesia). This clinical problem triggers the urgent need of identifying the circuit dysfunction resulting from dopamine loss and consequent levodopa replacement. Mostly, the information flows into the striatum through glutamatergic cortical terminals innervating medium spiny neurons. At the same time, corticostriatal synapses are modulated by the nigrostriatal dopaminergic system implicated in action control and learning (Figure 1). Conversely, there are two main GABAergic output circuits controlling locomotion:the direct and indirect pathways. Interestingly, although both pathways are not anatomically separated, they express a different set of GPCRs (Figure 2). Thus, the indirect-pathway neurons selectively express dopamine D2receptor (D2R) and A2AR,and the neurons from the direct pathway contain the dopamine D1receptor and muscarinic acetylcholine M4receptor. Importantly, intrinsic cholinergic interneurons, which represent only 1% of total striatal neurons, play an important role in modulating GABAergic neuron excitability by tuning their response to glutamatergic inputs through postsynaptic muscarinic acetylcholine M1receptor/muscarinic acetylcholine M4receptor. In addition, at the presynaptic level cholinergic interneurons exert a tonic inhibitory effect over incoming information through muscarinic acetylcholine M2receptor/muscarinic acetylcholine M4receptor activation located on cortical and thalamic glutamatergic terminals.
Interestingly, many different A2AR containing heteromers has been described within the brain, thus establishing molecular and functional receptor-receptor interactions allowing neurotransmission harmonization (Fuxe et al., 2014) (Figure 2).Precisely, in the dorsolateral striatum, the dopaminergic and adenosinergic transmission is tuned by D2R/A2AR heteromers.Indeed, the control of motor function by adenosine is based on the ability of A2AR to tightly control D2R function, both at the level of intracellular signaling as well as by an heteromer-dependent A2AR-D2R negative allosteric modulation. Importantly,GPR37, through its chaperone-like activity, controls A2AR cell surface targeting in vivo, thus limiting the plasma membrane density and function of A2AR (Morató et al., 2017). Therefore,its heteromerization with GPR37 may sculpt the ability of A2AR to modulate D2R and therefore dopaminergic transmission.Indeed, GPR37 deletion prompts an enhanced A2AR membrane trafficking and response to adenosinergic drugs (Morató et al.,2017, 2019). Thus, chronic blockade of A2AR induced a sensitization of locomotor activity, which is associated with elevated dopamine concentrations and synaptic changes in the striatum.These striatal plastic changes powerfully regulate basal ganglia circuitry by setting the gain on cortical and thalamic inputs,thus formatting motor function. Indeed, long-lasting alterations in striatal plasticity have been proposed to play a key role in movement disorders such as Parkinson's disease, Huntington's disease and dystonia.
Interestingly, while long-term potentiation in the hippocampus and LTD in the cerebellum are well established models of synaptic plasticity, the molecular mechanisms underlying striatal plasticity remain elusive. Eventually, the study of synaptic plasticity in the striatum has been limited by the difficulty in triggering reliable, long-lasting long-term potentiation in striatal brain slices under physiological conditions. Certainly,the mechanisms governing LTD induction and expression vary across brain regions. Thus, in medium spiny neurons, low-frequency stimulation of corticostriatal afferent fibers leads to a different form of LTD (Ronesi and Lovinger, 2005). This form of striatal long-term synaptic plasticity involves glutamate and dopamine. Indeed, the best-studied form of striatal plasticity is the homosynaptic endocannabinoids (EC)-dependent LTD (eCBLTD) at glutamatergic synapses (Figure 2). Typically evoked by persistent low frequency stimulation, eCB-LTD relies in the activation of postsynaptic receptors couped to protein Gq/11(i.e.,metabotropic glutamate type 5 receptors) triggering phospholipase C (PLC) activation and synthesis of ECs (2-arachidonoyl glycerol and anandamide) which diffuses presynaptically to activate CB1 cannabinoid receptors and suppress synaptic transmission (i.e., lower glutamate release probability) (Figure 2) (Gerdeman et al., 2002). Interestingly, eCB-LTD is observed mainly at glutamatergic synapses onto indirect-pathway medium spiny neurons (Kreitzer and Malenka, 2008), precisely where D2R/A2AR heteromers are expressed and interact with metabotropic glutamate type 5 receptors (Figure 2) (Cabello et al., 2009). Furthermore, presynaptic A2AR are powerful regulator of CB1 receptor-mediated synaptic depression in corticostriatal synapses of the dorsal striatum and they also control metabotropic glutamate type 5 receptor function at these same synapses (Cunha, 2016). Likewise, post-synaptic A2AR also control these same receptor systems at postsynaptic compartments,as well as D2R. This prompts several still unanswsered questions on the fine-tuning of LTD in the indirect-pathway by adenosine and dopamine, such as: i) how does A2AR blockade enhances LTD? ii) why does eCB-LTD requires D2R activation? Indeed,dopamine depletion, D2R antagonists and D2R deletion prevent striatal LTD induction.
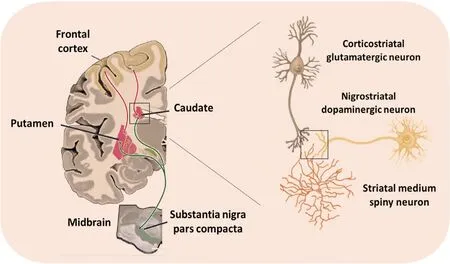
Figure 1 Striatal medium spiny neurons are modulated by corticostriatal and nigrostriatal neurons.Collateral axons from glutamatergic cortical neurons (pink traces) establish synapses with the head of dendritic spines of medium spiny neurons, the most common neuron in the striatum. Simultaneously, dopaminergic afferents from the substantia nigra pars compacta (green traces) make synaptic contact with the neck of the medium spiny neuron dendritic spine.
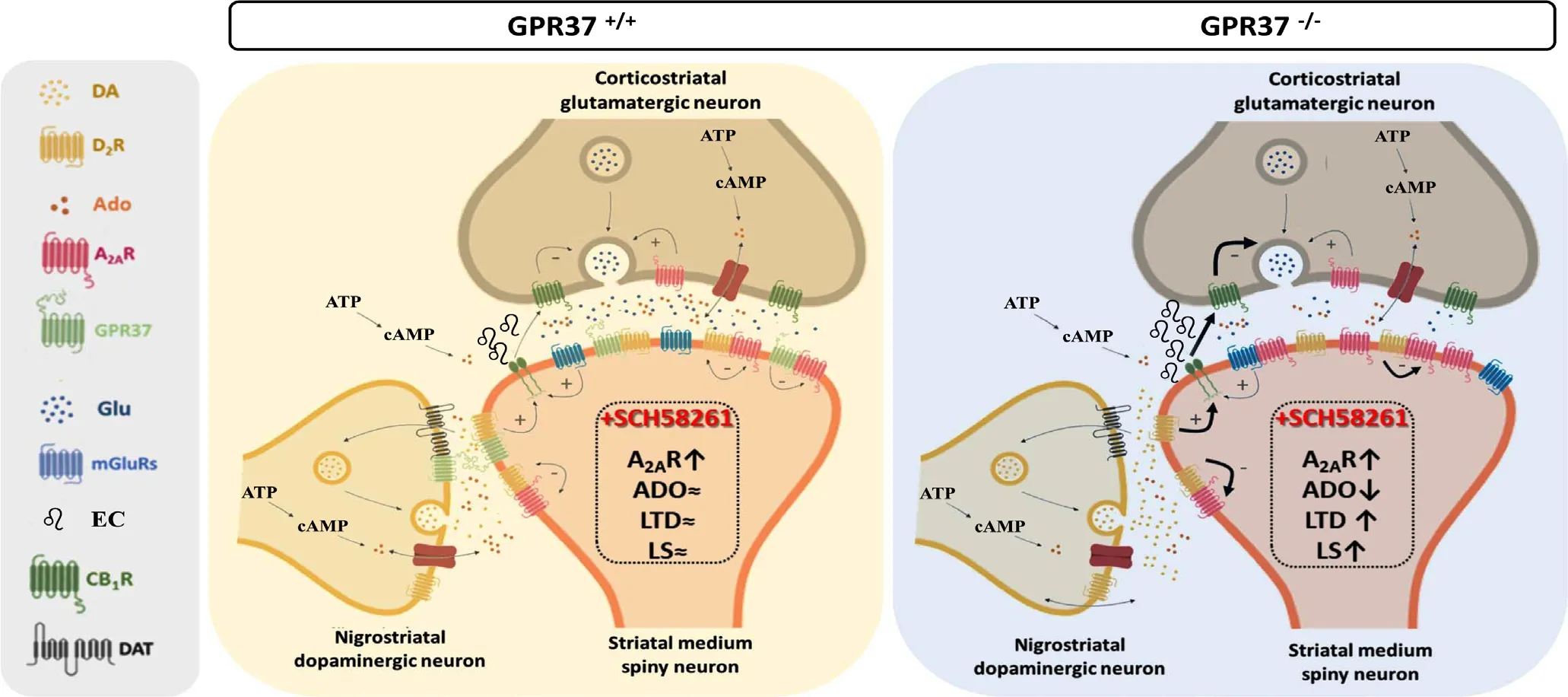
Figure 2 Indirect pathway striatal spiny module.The medium spiny neuron dendritic spine, the glutamatergic terminal and the dopaminergic terminal constitute the so-called striatal spiny module (SSM). The segregation of DA and adenosine receptors within the two subtypes of medium spiny neurons suggests the existence of at least two subtypes of SSMs, which could be termed as enkephalin-SSM (i.e., indirect pathway) and dynorphin-SSM (i.e., direct pathway). Thus, while dynorphin-SSM predominantly express DA D1 receptor (D1R), enkephalin-SSM express DA D2 receptors (D2Rs) and A2ARs. The endocannabinoid(EC)-dependent long-term depression (LTD) model at enkephalin-SSM requieres activation of postsynaptic Gq/11-protein coupled receptors(i.e., metabotropic glutamate type 5 receptors (mGlu5Rs)) propmting ECs synthesis and release. Co-activation of postsynaptic D2R enhances ECs production. ECs activate presynaptic cannabinoid 1 receptor (CB1R) which induce LTD. GPR37, by modulating A2AR and D2R, play a key role balancing ECs production and thus LTD. Chronic A2AR antagonist treatment (i.e., SCH58261, 1 mg/kg per day, intraperitoneal administration, 10 days) induced an increase of A2AR density in the striatum of wild-type and GPR37-/- mice. Interestingly, under the same experimental conditions a reduction in striatal adenosine (Ado) levels and a potentiation of striatal LTD and locomotor sensitization was observed only in GPR37-/- mice (right panel). ATP: Adenosine triphosphate; cAMP: cyclic adenosine monophosphate; DA: dopamine; DAT: DA transporter; Glu: glutamate; LS: locomotor sensitization.
Although prosaposin has been postulated as ligand, GPR37 still is an orphan GPCR (i.e., not yet been linked to endogenous ligands). In addition, there is a lack of highly selective GPCR37 ligands allowing ex vivo and in vivo functional studies, thus currently research efforts are concentrated in GPR37-based drug discovery. Hence, experiments in mice lacking GPR37 are unique in providing some hints about its function. Also, the assessment of the GPR37 interactome revealing GPCR partners(i.e., A2AR and D2R) aid to understand the biological relevance of this orphan receptor particularly in the central nervous system (Sokolina et al., 2017). Indeed, since GPR37 deletion does not affect striatal LTD and hippocampal LTD (Lopes et al.,2015; Morató et al., 2019), we assume that GPR37 function per se do not play a role in synaptic plasticity. However, the proteomic studies revealing the ability of GPR37 to heteromerize with A2AR suggested that this orphan receptor may function in a ligand-independent manner (i.e., chaperone-like) modulating A2AR function (i.e., repressor) in different brain regions (i.e.,striatum and hippocampus). It is important to highlight that GPR37-dependent physiological effects (i.e., increased striatal LTD and locomotor sensitization) were observed only upon chronic A2AR blockade, thus reinforcing the role of GPR37 in long-term neuroadaptations mediated by the adenosinergic system. Also, these results consolidate the idea that A2AR-mediated control of motor sensitization is a consequence of the ability of A2AR to control corticostriatal plasticity. This selective impact of GPR37 ablation on long-term adaptive changes of A2AR function may be of particular relevance for striatal-related neurodegenerative diseases. In fact, there is an abnormal over function of A2AR coupled with perturbed corticostriatal plasticity at the onset of striatal-related neurodegenerative diseases, such as Parkinson's or Huntington's diseases (Cunha, 2016); this prompts considering a prominent role for GPR37 at the onset of these disorders, through its ability to control A2AR trafficking to the plasma membrane.
This work was supported by Fondo Europeo de Desarrollo Regional (FEDER)/Ministerio de Ciencia, Innovación y Universidades-Agencia Estatal de Investigación (SAF2017-87349-R) and Instituto de Salud Carlos III (ISCIII) (PIE14/00034), the Catalan government (2017 SGR 1604), Fundació la Marató de TV3 (Grant 20152031), Fonds Wetenschappelijk Onderzoek (FWO) (SBO-140028) to FC and Centro 2020 (projects CENTRO-01-0145-FEDER-000008:BrainHealth 2020 and CENTRO-01-0246-FEDER-000010), and Fundação para a Ciência e a Tecnologia (FCT)(projects PTDC/NEU-NMC/4154/2014 and POCI-01-0145-FEDER-031274) to RAC.
Xavier Morató, Rodrigo A. Cunha, Francisco Ciruela*
Unitat de Farmacologia, Departament Patologia i Terapèutica
杂志排行
中国神经再生研究(英文版)的其它文章
- Etomidate affects the anti-oxidant pathway to protect retinal ganglion cells after optic nerve transection
- Normal tension glaucoma: from the brain to the eye or the inverse?
- Mesenchymal stromal cell therapy for damaged retinal ganglion cells, is gold all that glitters?
- MicroRNAs as biomarkers of diabetic retinopathy and disease progression
- Diabetic neuropathy research: from mouse models to targets for treatment
- Potential therapeutic roles of retinoids for prevention of neuroinflammation and neurodegeneration in Alzheimer's disease