Mesenchymal stromal cell therapy for damaged retinal ganglion cells, is gold all that glitters?
2019-07-18FernandoLucasRuizCaridadGalindoRomeroDavidGarcBernalMarNorteMuozKristyRodrguezRamrezManuelSalinasNavarroJoseMillRiveroManuelVidalSanzMartaAgudoBarriuso
Fernando Lucas-Ruiz , Caridad Galindo-Romero , David García-Bernal , María Norte-Muñoz Kristy T. Rodríguez-Ramírez Manuel Salinas-Navarro Jose E. Millán-Rivero , Manuel Vidal-Sanz Marta Agudo-Barriuso
1 Grupo de Oftalmología Experimental, Instituto Murciano de Investigación Biosanitaria Virgen de la Arrixaca (IMIB-Arrixaca), Murcia, Spain
2 Department of Talmología, Universidad de Murcia, Murcia, Spain
3 Unidad de Terapia Celular y Trasplante Hematopoyético, Instituto Murciano de Investigación Biosanitaria Virgen de la Arrixaca (IMIB-Arrixaca),Murcia, Spain
4 Department of Medicina Interna, Universidad de Murcia, Murcia, Spain
Abstract Mesenchymal stromal cells are an excellent source of stem cells because they are isolated from adult tissues or perinatal derivatives, avoiding the ethical concerns that encumber embryonic stem cells. In preclinical models, it has been shown that mesenchymal stromal cells have neuroprotective and immunomodulatory properties, both of which are ideal for central nervous system treatment and repair. Here we will review the current literature on mesenchymal stromal cells, focusing on bone marrow mesenchymal stromal cells, adipose-derived mesenchymal stromal cells and mesenchymal stromal cells from the umbilical cord stroma, i.e.,Wharton's jelly mesenchymal stromal cells. Finally, we will discuss the use of these cells to alleviate retinal ganglion cell degeneration following axonal trauma.
Key Words: stem cells; adipose stem cells; umbilical cord; bone marrow; Wharton's jelly; optic nerve axotomy;neuroprotection
Introduction
Mammalian central nervous system (CNS) neurons are not replaced upon death. Furthermore, adult CNS neurons have a limited spontaneous axonal regenerative capacity which together with the complexity of CNS circuits, make CNS lesions very difficult to repair. Thus, neurodegenerative diseases and insults that cause neuronal death or axonal interruption (axotomy, i.e., nerve injury) lead to an irreparable loss of function.
To date, no therapies are available to either stop or, at the very least to delay, neuronal degeneration nor to boost axonal regeneration. In search of neuroprotective therapies,stem cell therapy may be an ideal tool for CNS repair.
Cell therapy has two different goals: i) cell replacement,when the transplanted cells not only survive and integrate into the host tissue but also differentiate and acquire the functional properties of the lost cells, and ii) cell protection,which is the ability of the transplanted cells to ameliorate the degeneration of the host tissue.
Stem cells self-renew and differentiate into different cell types. Embryonic/induced pluripotent stem cells (ESCs) are pluripotent, and thus they are able to differentiate into cells of the three germinal layers. Progenitor cells derived from adult tissues are multipotent, and so they have the potential to differentiate into specialized cell types present in a specific tissue or organ. Adult stem cells maintain adult tissue by homeostatic cell replacement (Wagers and Weissman, 2004).They are also the reserve for repairing adult tissues by homing to damaged areas.
Mesenchymal stromal cells (MSCs) derive from the mesoderm, and were first identified in the bone marrow (BMMSCs) (Friedenstein et al., 1974). Later, MSCs have been found in many other adult organs such as the brain, intestine, dental tissue, heart and adipose tissue (Ad-MSCs), as well as in perinatal derivatives (PnD) such as the umbilical cord (UC) and the amniotic membrane (da Silva et al., 2006;Lim, 2017; Argentati et al., 2018). MSCs are able to differentiate to adipocytic, chondrocytic, osteocytic and myogenic lineages (Pittenger et al., 1999; Zuk et al., 2001). Moreover,they can be driven into non-mesodermic fates and differentiate into different cell-types such as neurons (Woodbury et al., 2000; Lin et al., 2003; Salehi et al., 2016; Venkatesh and Sen, 2017; Alizadeh et al., 2019).
MSCs are considered the ideal source for numerous regenerative therapeutical approaches. While ESCs have pluripotency and a high capacity of replication, their high division rate may form tumors (Brederlau et al., 2006; reviewed in Caplan and Dennis, 2006; Carson et al., 2006). In addition,they are immunogenic and may cause severe immune rejection. Furthermore, there are not reports on ESC secretion of protective trophic factors as there are for MSCs (reviewed in Fu et al., 2017). Finally and most importantly, ESCs are encumbered with numerous ethical and regulatory problems while MSCs are free from ethical concerns, they are accessible with minimal patient discomfort or pain, and can be culture-expanded in vitro. Lastly, because MSCs do not express human leukocyte antigen-class II molecules, their immunogenicity is low which, together with their demonstrated immunomodulatory properties, allow their autologous or allogeneic transplantation (reviewed in De Miguel et al., 2012).
Here we aim to review the latest developments on Ad-MSCs, BM-MSCs, and Wharton's jelly-MSCs (WJ-MSCs)therapy for retinal ganglion cell (RGC) degeneration induced by optic nerve damage. We have performed a PubMed literature search of articles on mesenchymal stem cells or mesenchymal stromal cells AND retina or central nervous system. Within this search, we centered on Ad-MSCs, BM-MSCs, and WJ-MSCs, and optic nerve trauma.As shall be discussed, MSCs from different sources elicit different outcomes.
Which Phenotypic Marker(s) Define Mesenchymal Stromal Cells?
There are not specific markers for MSCs, thus the International Society for Cellular Therapy (Horwitz et al., 2005; Dominici et al., 2006) set the minimal criteria to define them:i) Plastic adherence, ii) expression of the surface markers CD105, CD90 and CD73, low expression of human leukocyte antigen-class I and negative expression of hematopoietic cell markers such as CD45, CD34, CD14 or CD11b, and human leukocyte antigen-class II and co-stimulatory molecules and, iii) capacity to differentiate into mesodermal lineages under appropriate in vitro culture conditions.
Mesenchymal Stromal Cell Characteristics for Clinical Application
Gimble proposed in 2003 the following criteria for stem cells to be considered for clinical application: i) must be found in abundant quantities, ii) must be harvested by a minimally invasive and painful procedure, iii) must be able to differentiate along multiple cell lineages in a regulated and reproducible way, iv) must be safely and effectively transplanted to autologous or allogeneic hosts, and v) it must be possible to manufacture them in accordance with current Good Manufacturing Practice guidelines (Gimble, 2003).
Mesenchymal Stromal Cells from the Bone Marrow, Adipose Tissue, and Umbilical Cord
BM-MSCs were the first to be isolated and described (Friedenstein et al., 1974). However, they might not be the best choice for therapy: i) They are isolated from bone marrow aspirate, which is an invasive procedure that is painful for the patient and is accompanied by a risk of infection. ii) The titers of BM-MSCs in the bone marrow are quite low and decrease with the age of the donor from a 1:10,000 ratio in newborns, to 1:2,000,000 in 80-year-old donors (Caplan,2007). iii) Their use is sometimes hampered because of the risk of viral infection, and finally. iv) BM-MSCs display chromosomal abnormalities during their ex vivo expansion(Miura et al., 2006; Redaelli et al., 2012; Borgonovo et al.,2015; Stultz et al., 2016).
These drawbacks led to the search of other sources for MSCs, and so, MSCs have been found in many tissues such as skeletal muscle, peripheral blood, skin, adipose tissue, and PnD (Musina et al., 2005).
Ad-MSCs are isolated mainly from the subcutaneous deposits of white adipose tissue (Zuk et al., 2001). Adipose depots are abundant, replenishable and accessible by liposuction aspiration, a minimally invasive procedure with low risk, low morbidity or patient discomfort and cheaper than bone marrow aspiration. Furthermore, although lipoaspirates are considered medical waste, they are an excellent and abundant source for autologous Ad-MSCs obtainment. In addition, the yield of MSCs from the adipose tissue is 500-fold higher than from the bone marrow (Fraser et al., 2006),although this yield may vary depending on the donor age and location of adipose tissue harvesting. For instance, while the greatest numbers are collected from the arms compared to the abdomen, those isolated from the abdominal region are more resistant to apoptosis (Kolaparthy et al., 2015).Finally, Ad-MSCs seem to have more potent immunomodulatory properties than BM-MSCs (Garcia-Olmo et al., 2005;DelaRosa et al., 2009; Melief et al., 2013).
PnD such as UCs, possess significant advantages over MSCs obtained from adult donors: i) They are an inexhaustible source of stem cells. ii) They are non-invasively harvested after birth, with no harm to the baby or the mother. iii)UCs can be collected from underrepresented ethical groups.iv) UC-MSCs show an improved proliferative capacity, life span and differentiation potential compared to BM-MSCs(Baksh et al., 2007) without signs of senescence over serial passages (Weiss et al., 2006). v) MSC titers from the UC are higher than those from bone marrow aspirates (Karahuseyinoglu et al., 2007). vi) UC-MSCs have been demonstrated to be more immunoprivileged than BM-MSCs, reducing the probability to be rejected by the recipient after their allogeneic or xenogeneic transplantation. For instance, although the expression of immune-stimulatory ligands in MSCs from the UC stroma (WJ-MSCs) is very similar to that of BM-MSCs, human leukocyte antigen-class II expression is induced substantially in BM-MSCs in a pro-inflammatory milieu but not in WJ-MSCs (Deuse et al., 2011). Also, it has been reported that after in vitro exposure with pro-inflammatory cytokines WJ-MSCs release higher amounts of immunomodulatory mediators than BM-MSCs (Deuse et al.,2011).
Finally, although it has been demonstrated that MSCs isolated from the whole UC or specific sections of the UC have similar biological characteristics including CD marker expression, multilineage differentiation capacity, immunomodulatory properties, and hematopoiesis supporting profile (Zhou et al., 2013; Bharti et al., 2018), WJ-MSCs could be considered the best MSC source from the UC.
Subramanian et al. (2015) compared the histology, fresh and cultured cell numbers, morphology, proliferation, viability, stemness characteristics and differentiation potential of MSCs isolated from the amnion, subamnion, perivascular,and WJ of several UC. They concluded that MSCs from the WJ offer the best clinical utility as they have less non-stem cell contaminants, can be generated in large numbers with minimal culture avoiding changes in phenotype and karyotype, their derivation is quick and easy to standardize, they are rich in stemness characteristics and have a high differentiation potential.
Mesenchymal Stromal Cell Mechanisms of Neuroprotection
Even though MSCs can differentiate into neurons (Woodbury et al., 2000; Lin et al., 2003; Salehi et al., 2016; Venkatesh and Sen, 2017; Alizadeh et al., 2019), rather than using them for cell replacement in the CNS these cells are being actually investigated for their neuroprotective and immunomodulatory properties. The neuroprotective potential of MSCs lays in part on their capacity to secret trophic factors (reviewed in Caplan and Dennis, 2006; Caplan, 2007). The secretome of MSCs changes depending on their developmental pathways.Indeed, MSCs secrete various bioactive molecules that reflect their functional status and their local microenvironment (Lin et al., 2011; Zhang et al., 2017; Millan-Rivero et al., 2018).In addition, the neuroprotection mediated by MSCs may be due to their immunomodulatory and anti-inflammatory properties (Chaudhary et al., 2018). Neuroinflammation is crucial in several neurodegenerative diseases, and it is caused by over-activated microglial cells. In the CNS, MSC immunomodulation drives microglial cells to secrete neuroprotective substances, changing from M1 (deleterious) to M2(beneficial) phenotype, and producing anti-inflammatory instead of pro-inflammatory mediators (reviewed in Laroni et al., 2015).
Retinal Ganglion Cell Degeneration by Axonal Damage
The retina is a layered structure of the CNS where neurons and glia are beautifully organized. RGCs are located in the innermost layer of the retina, and they are the only retinal neurons that send their axons outside the retina to the brain through the optic nerve to convey the luminous information gathered by photoreceptors.
RGC degeneration occurs in advanced stages of photoreceptor degeneration or after ischemic insults (Vidal-Sanz et al., 2000; Garcia-Ayuso et al., 2018) but, because of their long projecting axons, RGCs are especially sensitive to optic nerve insults. The optic nerve can be injured by trauma, ischemia or degenerative diseases such as optic neuritis, glaucoma or Leber's neuropathy. Independently of the etiology of the damage, optic nerve injuries cause specifically the retrograde death of RGCs (Nadal-Nicolas et al., 2015). As abovementioned, central axons in mammals are unable to regenerate back to their targets and neuronal replacement in the CNS is very limited and restricted to specific areas (Inta et al., 2015).Consequently, to date, it is not possible to restore the system and thus blindness ensues.
In an effort to understand the underlying molecular and anatomical triggers by which optic nerve injuries cause RGC death, and to assess the goodness of different neuroprotective strategies, several preclinical models have been developed. In our group, we have analyzed in depth the course of RGC death caused by axotomy (optic nerve crush (ONC) or transection), and raised intraocular tension in rats and mice.Ocular hypertension mimics the main risk factor of glaucoma but is not as reproducible as axotomy. Furthermore,axotomy is cleaner than ocular hypertension because it only causes axonal damage, while ocular hypertension causes an ischemic insult as well, thus complicating drawing conclusions (reviewed in Vidal-Sanz et al., 2017, and references therein).
Using automated routines to quantify the whole population of RGCs, we have reported that RGC loss after optic nerve crush occurs in two lineal phases. The first one is very quick (9 or 14 days, in mice or rats, respectively) and drastic, disappearing 85% of the RGCs. Within these 9-14 days,RGC loss is first significant at 3 or 5 days, and 50% of RGCs have died at day 5 or 7 (in mice or rats). The second phase is much slower and lasts at least 6 months, by then only 1-4% of RGCs are still alive (Nadal-Nicolas et al., 2015; Sanchez-Migallon et al., 2018).
Thus, optic nerve crush is a particularly suitable model because is highly reproducible and fairly quick for proof of concept experiments, and so many groups have used it to assess the neuroprotective potential on RGCs of drugs, trophic factors or cell transplantation, as we will discuss below.
Retinal Ganglion Cell Neuroprotection by Mesenchymal Stromal Cells
Most neuroprotective strategies for axotomized RGCs are based on intravitreal delivery of neurotrophic factors, or anti-apoptotic drugs (Sanchez-Migallon et al., 2016). To date, brain-derived neurotrophic factor (BDNF) is the best neuroprotectant for RGCs, but even with prolonged delivery (Di Polo et al., 1998) RGC rescue lasts not longer than 14-21 days, and this occurs because RGCs down-regulate Trk receptors when a high amount of BDNF is administered at once (Sommerfeld et al., 2000). Thus, some groups have engineered MSCs to express a given neuroprotective trophic factor to get a steady and prolonged delivery (Levkovitch-Verbin et al., 2010; Harper et al., 2011). We will not discuss here RGC therapy using genetically modified MSCs because their use in the clinic is hindered by safety concerns.
Bone marrow mesenchymal stromal cells
In organotypic rat retinal explants, an in vitro model of optic nerve axotomy, human BM-MSCs increase RGC survival by 2-3-fold at day 3 in vitro compared to untreated explants(Mead et al., 2014). In a following up paper, the same authors showed in vivo and in vitro, that this effect is mediated by mi-RNAs delivered by human BM-MSCs exosomes (Mead and Tomarev, 2017).
Adipose tissue mesenchymal stromal cells
Mead et al. (2014) observed that human Ad-MSC are able to promote both, RGC survival and neurite outgrowth in organotypic rat retinal explants. While the titers of BDNF and vascular endothelial growth factor secreted by these cells are higher than those secreted by human BM-MSCs, the RGC elicited neuroprotection is not as marked as that achieved by human BM-MSCs (Zhou et al., 2013; Mead et al., 2014). Regarding in vivo studies using these cells, we did not find any published report.
In our lab, we have tested the possible toxicity of an intravitreal syngeneic transplant of Ad-MSCs as well as its putative neuroprotective potential after optic nerve crush in rats and mice (Figure 1). It is important to highlight here that safety assays are hardly reported. Toxicity and safety assays are essential to exclude neuronal death or tumor formation by the transplant. These assays should be done in vivo in healthy hosts before translating a given cell therapy into the clinic.
In mice, we isolated Ad-MSCs-green fluorescent protein(GFP) from the abdominal fat of C57/BL/6 Tg(CAG-EGFP)adult animals and transplanted them into the vitreous chamber of C57/BL/6 wild type mice. We followed the transplant in vivo using optical coherence tomography, and as shown in Figure 1A Ad-MSCs-GFP are observed in the vitreous cavity up to 21 days after the transplant. Ex vivo, Ad-MSCs-GFP are found on the retinal surface forming an epi-retinal membrane (Figure 1B). Regarding their toxicity (Figure 1C) 21 days after intravitreal administration of 10,000 Ad-MSCs-GFP into intact retinas the total number of Brn3a+R GCs does not differ from control values. In addition, we did not observe tumor formation. When we looked at RGC survival at 5, 9 or 21 days after ONC, we found no differences between Ad-MSCs-GFP and vehicle-treated retinas (Figure 1C, left graph), neither with a dose of 10,000 cells nor doubling the dose.
In rats, 20,000 Ad-MSCs are not toxic for RGCs at least up to 1 month after the transplant (Figure 1C, right graph).This dose was chosen to be able to compare these results with those recently published using human WJ-MSCs (hWJMSCs) (Millan-Rivero et al., 2018). In the current experiment, albino Sprague Dawley rats received an intravitreal injection of Ad-MSCs extracted from the abdominal fat of animals of the same strain. Using the same cell dose, we also assessed RGC neuroprotection. After ONC, we observed a significant increase of RGC survival in the transplanted retinas (142% of vehicle), but this protection is transient and at 14 days the population of surviving RGCs is the same in Ad-MSC and vehicle-treated retinas (Figure 1C, right graph).
These data indicate that at least at these doses and in a syngeneic scenario, Ad-MSCs are neither toxic for RGCs nor they form tumors, but on the other hand they are not very efficient to neuroprotect RGCs in rats and show no neuroprotective effects in mice.
Human Wharton's jelly mesenchymal stromal cells
The neuroprotective potential of hWJ-MSC has been studied in retinal degenerations (Leow et al., 2015), ocular hypertension (Ji et al., 2018), and we have recently reported their effect after optic nerve crush (Millan-Rivero et al., 2018). In our work, we xenotransplanted hWJ-MSCs into the vitreous cavity of albino rats. Using in vitro mixed human lymphocyte cultures, we observed that hWJ-MSCs were able to suppress T-cell proliferation, and that this effect was mediated, at least in part, by production of prostaglandin E2, tumor growth factor beta and indoleamine 2,3-dioxygenase. In vivo, we investigated hWJ-MSCs safety, neuroprotective potential, and secretion of trophic factors and immunomodulatory mediators. Before performing the cell transplants, we assessed the viability of isolated hWJ-MSCs in two vehicles, and we observed that they were viable for a longer time in Dulbecco's modified Eagle's medium (> 180 minutes) than in phosphate buffer saline (< 150 minutes). Next, we determined that different doses of these cells were not toxic for RGCs up to 7 days post-transplant.
Regarding hWJ-MSC neuroprotective potential, we assayed hWJ-MSCs from three different UCs because, due to the high genetic variability among human donors, each cord could have different properties.
Our results show that hWJ-MSC transplant increases RGC survival 1.8- and 3-fold compared to vehicle retinas at 7 and 14 days post-ONC, respectively. However, at 30 days there was no difference between treated and control groups.
When it comes time in my life to explain the reality of Santa Claus to my children, I pray to the spirit of Christmas that I will be as eloquent12 and loving as my dad was the day I learned that the spirit of Santa Claus doesn’t wear a red suit. And I hope they will be as receptive as I was that day. I trust them totally and I think they will.
Despite the transient neuroprotection, which is the common denominator of all therapies assayed in this model of neuronal damage, hWJ-MSC transplant produces the higher rescue reported to date in in vivo models of RGC axonal damage treated with MSCs, either derived from the bone marrow (160% higher than no treatment at 14 days after optic nerve crush (Zaverucha-do-Valle et al., 2011), or the whole UC (22% after ocular hypertension) (Ji et al., 2018).Interestingly, hWJ-MSCs from the three UC elicited the same response, which is a very promising result for translational medicine.
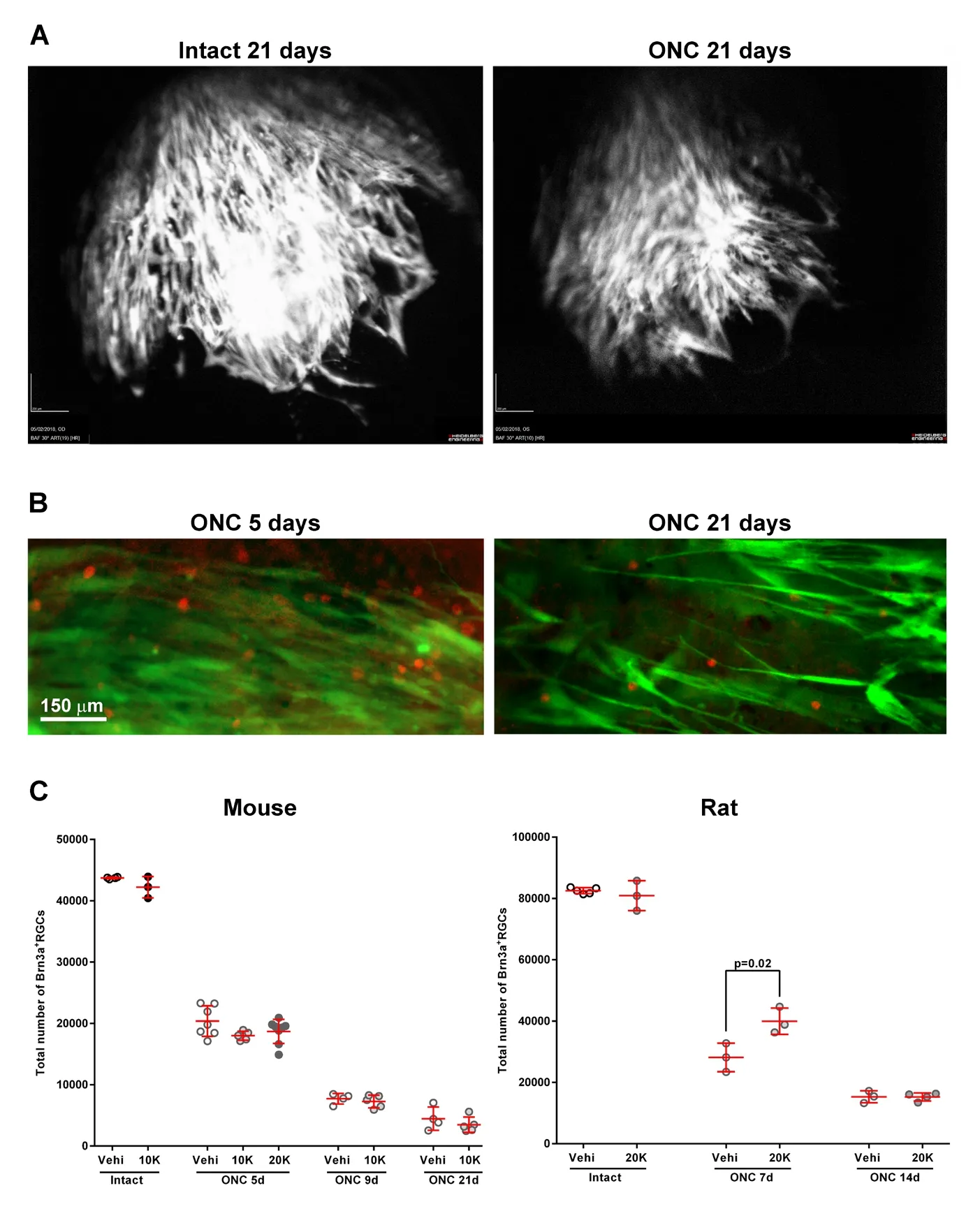
Figure 1 Syngeneic intravitreal transplant of adipose-derived mesenchymal stromal cells in mice and rats: toxicity and effect on retinal ganglion cell survival after optic nerve axotomy.(A) In vivo optical coherence tomography images showing the grafted Ad-MSCs-GFP in the vitreal cavity 21 days after intravitreal injection into intact (left) or ONC-injured (right) mice. (B) Ex vivo visualization of Ad-MSCs-GFP cells (green)and Brn3a+RGCs (red) 5 or 21 days after intravitreal administration in mice. Ad-MSCs-GFP do not integrate into the retina, rather they form a layer covering it. (C) Scatter graphs showing the total number of Brn3a+ RGCs in mice (left)and rats (right) in intact or ONC-injured retinas treated with vehicle (vehi) or Ad-MSCs. Each dot represents a retina, and the red lines are the mean ± SD. In intact retinas, an intravitreal transplant of 10,000 (mice) or 20,000 (rats) Ad-MSCs does not cause RGC cell death compared to vehicle. These retinas were analyzed 21 (mice)or 30 (rats) days after the transplant. In mice,Ad-MSC administration does not improve RGC survival after ONC at any time point or dose. In rats, Ad-MSC treatment increases significantly the number of surviving RGCs at 7 days post-lesion (T-test, vehicle vs. Ad-MSC), but at 14 days the neuroprotection has ceased. The total number of Brn3a+RGCs was automatically quantified as reported (Galindo-Romero et al., 2011; Nadal-Nicolas et al., 2015; Sanchez-Migallon et al.,2016). Ad-MSCs: Adipose tissue mesenchymal stromal cells; GFP: green fluorescent protein;ONC: optic nerve crush; RGCs: retinal ganglion cells; d: days post-lesion.
Anatomically, once transplanted hWJ-MSCs gather first close to the retinal arteries, spreading latter across the retina,where they remain at least up to 30 days. We also observed that few hWJ-MSCs integrated into the ganglion cell layer.
Next, we measured the levels of several human proteins in retinal extracts and observed that in the injured transplanted retinas there was an increase of BDNF, vascular endothelial growth factor, nerve growth factor, ciliary neuroptrophic factor, tumor growth factor beta and prostaglandin E2. We suggested that RGC neuroprotection by hWJ-MSCs is at least mediated by their capability of secreting these neurotrophic factors, all of which are known neuroprotectants.
Interestingly, these levels were higher in the injured transplanted retinas compared to intact transplanted ones, denoting that the environment modulates hWJ-MSCs secretome.
Finally, we saw that this xenotransplant produced a massive migration of ionized calcium binding adapter molecule 1 postive cells (macrophages) from the choroid into the retina, which caused retinal folding. Ionized calcium binding adapter molecule 1 postive cells were found close to the hWJ-MSCs, indicating that the transplanted cells triggered a response from the innate immune system, both local (microglial cells) and systemic, (macrophages). Tissue rejection is not surprising for two reasons, firstly, because MSCs express a variety of macrophage attracting-chemokines (Chen et al., 2008) and secondly, because although the retina is fairly immunoprivileged and hWJ-MSCs are immunomodulatory, we are transplanting human cells into a rat environment.
Conclusions
MSCs are, to date, the best candidates for clinical translation of cell therapy strategies. Those MSCs isolated from adult human donors can be cryopreserved for future autologous transplants. In addition, adult MSCs as well as MSCs from PnD, can be cryopreserved, banked, and used for allotransplants.
Currently, six clinical trials using MSC therapy for the retina are registered at https://clinicaltrials.gov/. All of them are based on BM-MSCs, either autologous transplant of the cells or their exosomes to treat age-related macular degeneration, glaucoma, or retinitis pigmentosa. These trials are still ongoing, and we shall wait until their results are published to know whether the patients improve.
Finally, more research is needed to fully translate cell therapy into the clinic: from cell isolation, banking and cryopreservation protocols, to safety studies and establishment of the best cell type for each disease, the best administration route and the most effective posology.
Acknowledgments:This article contributes to the COST Action CA17116 “International Network for Translating Research on Perinatal Derivatives into Therapeutic Approaches (SPRINT)”, supported by COST(European Cooperation in Science and Technology).
Author contributions:Conceptualization: MAB, MVS; methodology:FLR, CGR, DGB, MNM, KTRR, MSN, JEMR; data analysis and representation: FLR, CGR, MNM, KTRR; original draft: MAB; writing review& editing: MVS, DGB, MAB; supervision: DGB, MAB; funding acquisition: MVS, MAB.
Conflicts of interest:All authors disclose any conflicts of interest.
Financial support:This work was supported by the Spanish Ministry of Economy and Competitiveness, Instituto de Salud Carlos III (ISCIII),Fondo Europeo de Desarrollo Regional “Una Manera de Hacer Europa”(SAF2015-67643-P to MVS and PI16/00031 to MAB) and Fundación Séneca, Agencia de Ciencia y Tecnología Región de Murcia (19881/GERM/15 to MVS).
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-Non-Commercial-ShareAlike 4.0 License, which allows others to remix, tweak,and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewer:Evgeniya Pushchina, Zhyrmunskii Institute of Marine Biology, Far East Division, Russian Academy of Sciences, Russian Federation.
杂志排行
中国神经再生研究(英文版)的其它文章
- Etomidate affects the anti-oxidant pathway to protect retinal ganglion cells after optic nerve transection
- Normal tension glaucoma: from the brain to the eye or the inverse?
- MicroRNAs as biomarkers of diabetic retinopathy and disease progression
- Diabetic neuropathy research: from mouse models to targets for treatment
- Potential therapeutic roles of retinoids for prevention of neuroinflammation and neurodegeneration in Alzheimer's disease
- Sigma-2 receptor as a potential therapeutic target for treating central nervous system disorders