MicroRNAs as biomarkers of diabetic retinopathy and disease progression
2019-07-18BridgetMartinezPhilipPeplow
Bridget Martinez , Philip V. Peplow
1 Department of Molecular & Cellular Biology, University of California, Merced, Merced, California, USA
2 Department of Medicine, St. Georges University School of Medicine, Grenada
3 Department of Physics and Engineering, Los Alamos National Laboratory, Los Alamos, New Mexico, USA
4 Department of Anatomy, University of Otago, Dunedin, New Zealand
Abstract Diabetes mellitus, together with its complications, has been increasing in prevalence worldwide. Its complications include cardiovascular disease (e.g., myocardial infarction, stroke), neuropathy, nephropathy, and eye complications (e.g., glaucoma, cataracts, retinopathy, and macular edema). In patients with either type 1 or type 2 diabetes mellitus, diabetic retinopathy is the leading cause of visual impairment or blindness. It is characterized by progressive changes in the retinal microvasculature. The progression from nonproliferative diabetic retinopathy to a more advanced stage of moderate to severe nonproliferative diabetic retinopathy and proliferative diabetic retinopathy occurs very quickly after diagnosis of mild nonproliferative diabetic retinopathy. The etiology of diabetic retinopathy is unclear, and present treatments have limited effectiveness. Currently diabetic retinopathy can only be diagnosed by a trained specialist, which reduces the population that can be examined. A screening biomarker of diabetic retinopathy with high sensitivity and specificity would aid considerably in identifying those individuals in need of clinical assessment and treatment. The majority of the studies reviewed identified specific microRNAs in blood serum/plasma able to distinguish diabetic patients with retinopathy from those without retinopathy and for the progresion of the disease from nonproliferative diabetic retinopathy to proliferative diabetic retinopathy. In addition,certain microRNAs in vitreous humor were dysregulated in proliferative diabetic retinopathy compared to controls. A very high percentage of patients with diabetic retinopathy develop Alzheimer's disease. Thus,identifying diabetic retinopathy by measurement of suitable biomarkers would also enable better screening and treatment of those individuals at risk of Alzheimer's disease.
Key Words: diabetes; retinopathy; diagnosis; disease progression; microRNAs; biomarkers; blood serum/plasma; vitreous humor; humans
Introduction
Diabetes mellitus (DM) is increasing in prevalence worldwide especially in developing countries (World Health Organization, 2018). The prevalence of DM in adults aged 20-79 years was estimated to be 8.8% in 2015 and forecast to increase to 10.4% in 2040 (Ogurtsova et al., 2017). While DM can be treated, and its consequences prevented or slowed with diet,physical activity, and medication, its complications have been increasing in prevalence. These complications include cardiovascular disease (e.g., myocardial infarction, stroke), neuropathy, nephropathy, and eye complications (e.g., glaucoma,cataracts, retinopathy, macular edema) (American Diabetes Association; NIH National Eye Institute, 2015; Solomon et al., 2017). The primary factor in the development of diabetic complications is usually considered to be lasting exposure to hyperglycemia (Aronson and Rayfield, 2002).
In patients with either type 1 DM (T1DM) or type 2 DM(T2DM), diabetic retinopathy (DR) is the leading cause of visual impairment or blindness. It is characterized by progressive changes in the retinal microvasculature (Cheung et al., 2010; Hammes et al., 2011; Yau et al., 2012). Currently DR affects approximately 150 million people worldwide and the number is likely to double by 2025 according to the World Health Organization (King et al., 1998; Gupta et al.,2013). As the first sign of diabetic complications, DR has been used as an indicator for the diagnosis of DM complications (Genuth et al. 2003). Research evidence indicates that nearly all patients with T1DM and 60% of patients with T2DM develop some degree of retinopathy within 20 years after diagnosis (Klein et al., 1989). Similarly, it was reported that DR affects up to 80% of all patients who have had DM for ≥ 20 years (Kempen et al., 2004). In a large prospective cohort study followed for over 10 years, 45% of patients with T2DM developed any type of DR (Yun et al., 2016). At the first diagnosis of nonproliferative DR (NPDR), the mean duration of diabetes was 14.8 years, but progression to a more advanced stage of DR (moderate to severe NPDR and proliferative DR (PDR)) was very fast, occurring within about 2 to 3 years after diagnosis of mild NPDR. These data highlight the need for early detection of DR to reduce the high morbidity of this disease. While major efforts have been made to elucidate the pathomechanism of DR, the exact causes remain largely unclear, and present treatments have limited effectiveness (Stitt et al., 2016). The prevalence of DR will continue to increase due to the increased lifespan and longer duration of diabetes in DM patients.
General risk factors for the occurrence and progression of DR are longer duration of diabetes, hyperglycemia, hypertension, poor glycemic control, and dyslipidemia. While these risk factors are helpful in stratifying a patient's risk for developing retinopathy, many patients without these traditional risk factors develop DR. Moreover, there are individuals with long diabetes duration who do not develop DR(Smio-Servat et al., 2016; Ting et al., 2016). In 2002, it was reported that 29% of diabetic patients develop DR, whereas 22% of individuals with a history of diabetes do not develop DR, regardless of glycemic exposure, indicating that genetic factors may be important in the development of DR (Cai and Boulton, 2002). Thus, identifying biomarkers to predict DR or to determine therapeutic response is important. An easily accessible, screening biomarker of DR with high sensitivity and specificity would aid considerably in detecting those individuals in need of clinical assessment and treatment (Pusparajah et al., 2016).
MicroRNAs are a major class of short (~22 nt) non-coding RNAs that function to block protein translation and/or degrade their messenger RNA targets. They bind to complementary sequences in the 3′-UTR (untranslated) region of the target messenger RNA (Krishnan and Damaraju, 2018).These small RNAs direct many important processes related to cellular growth, apoptosis, differentiation, metabolism and the immune response (Rodrigues et al., 2018). MicroRNAs (miRNAs) are involved in DR-related microvascularization, and miRNAs that exhibit increased or decreased expression during DR pathogenesis have been identified(Mastropasqua et al., 2014). Modulation of miRNA levels may be able to reverse dyslipidemia (Davalos and Fernandez-Hernando, 2013) and slow DR progression (Gong and Su, 2017), and could potentially be used in DR therapeutic strategies. However, few studies have investigated the progressive changes in miRNA levels in DR (Gong and Su,2017). In an article by Joglekar et al. (2016) the authors wrote “We hope that this important report of Zampetaki et al. is followed by clinical studies in which miRNAs are evaluated in larger study cohorts, across different ethnic groups, in different types of diabetes, and in a wide age range as well as different stages of DR. It would also be of interest to see small RNA-sequencing analyses from such trials and to follow up these or other identified miRNAs in longitudinal studies to determine their predictive value for DR progression and responses to treatments. As miRNAs themselves may be therapeutic targets or even therapeutic agents (as anti-miRNAs), further studies will help in identifying and assessing their therapeutic potential for the treatment of retinopathy in individuals with diabetes”. Additionally, a review by Ting et al. (2016)reported that the current studies on biomarkers are limited by the need for larger sample sizes, cross-validation in different populations and ethnic groups, and time-efficient and cost-effective analytical techniques.
We have performed a PubMed search for recently published studies on serum/plasma miRNAs in patients with early or late-stage DR to discover and validate miRNAs as possible diagnostic and prognostic biomarkers of DR. In particular, we have examined to what extent the limitations indicated by Joglekar et al. (2016) and Ting et al. (2016) have been addressed in the planning, implementation, and data analyses of these studies. In addition, we have searched for studies on miRNA expression in vitreous humor of eyes with PDR and compared the findings with those on miRNA expression in serum/plasma of patients with PDR.
Diabetic Retinopathy
PDR and diabetic macular edema (DME) are the two serious vision-threatening conditions in DR. DR progresses slowly before becoming symptomatic (Zou et al., 2017). The currently available treatments for DR are applicable only at advanced stages of the disease and are associated with significant adverse effects. The only therapeutic strategy in the early stages of DR is a tight control of the risk factors for DR. Therefore, new pharmacological treatments for the early stages of the disease are needed.
DR results from abnormal retinal blood vessels that are either nonproliferative or proliferative. The blood retinal barrier comprises the retinal vasculature and the retinal pigment epithelium. Endothelial cells are responsible for maintaining the blood retinal barrier, and their impairment causes increased vascular permeability (Ciulla et al., 2003).Exposure to high glucose and the resultant damage to the blood retinal barrier lead to the leakage of fluids and lipids into the retina and contribute to DR progression. DR eventually causes increasing hypoxia which stimulates abnormal neovascularization in the retina.
Various stages have been described in the progression of DR (Curtis et al., 2009; Cheung et al., 2010; NIH National Eye Institute, 2015; Solomon et al., 2017). Thus, stage 1 is mild NPDR, in which small areas of balloon-like swelling occur in the tiny blood vessels of the retina called microaneurysms, and which may leak fluid into the retina; stage 2 is moderate NPDR, as the disease progresses, blood vessels that nourish the retina may swell and distort, and may also lose their ability to transport blood. Both cause characteristic changes to the appearance of the retina and may contribute to DME; stage 3 is severe NPDR, many more blood vessels are blocked, depriving the blood supply to areas of the retina. These areas secrete growth factors that induce the retina to form new blood vessels; stage 4 is PDR, growth factors secreted by the retina stimulate the proliferation of new blood vessels which grow along the inside surface of the retina and into the vitreous humor, a gel-like fluid that fills the eye.These new blood vessels are fragile and more likely to leak and bleed. Accompanying scar tissue can contract and cause retinal detachment which can lead to permanent vision loss(NIH National Eye Institute, 2015). In an alternative classification of the stages, stage 1 is background DR (BDR) and comprises both mild and moderate NPDR, stage 2 is pre-PDR and corresponds to severe NPDR, while stage 3 is PDR(National Health Service UK, 2018). Vascular endothelial growth factor (VEGF) was shown to have a circumstantial role in the progression of DR (Boulton et al., 1998), but other growth factors such as insulin-like growth factor-1, angiopoetin-1 and -2, stromal-derived factor-1, fibroblast growth factor-2, and tumor necrosis factor may also be involved(Grant et al., 2004).
DME defines a swelling caused by the build-up of fluid(edema) in an area of the retina called the macula and is the most common cause of vision loss among individuals with DR, with ~50% of DR subjects developing DME. DME can form at any stage of the disease, although it is more likely as DR progresses (NIH National Eye Institute, 2015).
MicroRNA Expression in Diabetic Retinopathy
A total of 15 research articles were found in the PubMed search of blood microRNAs of which nine had measured miRNAs in serum, 4 had used plasma, 1 had used extracelular vesicles extracted from serum and plasma, and 1 had used early-outgrowth endothelial progenitor cells obtained from the in vitro culture of peripheral blood mononuclear cells (Table 1). Ten of these studies had used a validation method which was RT-qPCR, while in five studies no validation method was reported. While most studies had used quite large cohorts of DR and noDR patients (≥ 40/group),there were a few that had used much smaller cohorts (< 30/group). All of the studies except one had indicated exclusion criteria, and all except three had reported gender composition of the cohorts, which in general had similar numbers of male and female subjects. The mean duration of diabetes ranged from 2 to 28 years. Only a few studies reported on medications being used by the patients, which is a serious omission as the medications may constitute confounding factors. Only eight of the studies had used receiver operating characteristics (ROC) analysis with area under curve(AUC) values to establish which microRNAs are good or fair tests to distinguish DR from noDR. Many of the studies were performed with hospital patients in China, which is a country with a high prevalence of DR (Liu et al., 2017).
In addition, three research articles were found in a PubMed search on microRNAs in vitreous humor of patients with PDR (Table 2). Only one of these studies had used large cohorts of patients with PDR or macular hole (MH) (29 and 30, respectively). The other two studies were made with very small numbers of patients (3 or 4/group or 7 or 10/group).All of the studies had indicated exclusion criteria and, when reported, the gender composition of the PDR and MH groups was similar. None of the studies had carried out ROC analysis and measurement of AUC values.
Blood serum
A large-scale study by Liu et al. (2018) that included 40 T1D patients with DR and 40 T1DM patients without DR (NDR)showed that using RT-qPCR validation that miR-18b, miR-19b and miR-211 in DR were significantly upregulated compared to NDR. The AUC values with 95% CI calculated by ROC curves of miR-18b, miR-19b and miR-211 were 0.779,0.744 and 0.864, respectively, with significant diagnostic accuracy for DR, in which miR-211 is the most powerful.
A validation study by Liang et al. (2018) with 29 T2DM patients with DR and 50 T2DM patients with noDR revealed that let-7a-5p, miR-novel-chr5_15976, miR-28-3p were all significantly upregulated, while miR-151a-5p, and miR-148a-3p were all significantly downregulated between T2DM-DR and T2DM-noDR, and between early-stage T2DM-DR and T2DM-noDR. A combination of 3 miRNAs let-7a-5p, miR-28-3p, and miR-novel-chr5_15976 had an AUC value of 0.937 (sensitivity 0.923, specificity 0.950) for distinguishing T2DM-DR from T2DM-noDR, and AUC value of 0.901 (sensitivity 0.875, specificity 0.927) for distinguishing early-stage T2DM-DR from T2DM-noDR. Qin et al. (2017) examined the expression level of miR-126 in 42 DM patients with NPDR, 39 DM patients with PDR and 44 DM patients with no DR. The relative expression of miR-126 was not significantly different between NDR and NPDR groups; therefore, these two groups were combined. The level of expression of miR-126 in the PDR group was significantly lower than that in the combined NDR and NPDR group. By ROC analysis, miR-126 values differentiated PDR patients from healthy controls with an AUC of 0.976. The cut-off value was 5.02, which was associated with a sensitivity of 0.812 and a specificity of 0.903. Li et al. (2017) studied 255 patients with DR and 253 healthy controls and found by RT-qPCR that patients in DR group had significantly decreased miR-200b expression compared to healthy controls. Zampetaki et al. (2016) reported on a PREVENT-1 trial with 62 T1DM patients with DR and 64 T1DM patients without DR, and a PROTECT-1 trial with 93 T1DM patients with DR and 81 T1DM patients without DR. Penalized logistic regression analyses adjusted for age, gender, and diastolic blood pressure were performed to identify and evaluate the association of miRNAs with the incidence and progression of DR. The following miRNAs were identified in descending order of importance: miR-27b, miR-320a, miR-454, and miR-28-3p in PREVENT-1 and miR-320a, miR-122, miR-221, and miR-27b in PROTECT-1. MiR-27b and miR-320a were the two miRNAs most consistently associated with the incidence and progression of DR. Serum miR-27b was markedly lower in T1D-DR compared to T1D-noDR, while serum miR-320a was considerably higher in T1D-DR compared to T1D-no-DR. By ROC analysis, addition of miR-27b and miR-320a to a panel of variables associated with disease risk (i.e., age,sex, duration of diabetes, diastolic blood pressure, and level of HbA1c) improved the AUC by 0.087 for PREVENT-1 (P= 0.027) and by 0.034 for PROTECT-1 (P = 0.214). A study by Rezk et al. (2016) with 19 T2DM patients with DR and 14 T2DM patients without DR found by RT-PCR a significant decrease in miR-126 expression in T2DM-DR compared to T2DM-noDR. Qing et al. (2014) included 90 DM patients with NPDR and 90 patients with PDR, and found the expression of miR-21, miR-181c and miR-1179 was significantly higher in PDR than in NPDR. ROC analysis to assess the diagnostic sensitivity and specificity of the 3 miRNA signature for PDR gave AUC values of 0.83, 0.80, 0.87 and 0.89 in validation sets. A study by Rovira-Llopis et al. (2018) showed by RT-qPCR that levels of miR-31 in 13 T2DM patients with DR (2 with PDR) were similar to those of 18 T2DM patients without DR. Ma et al. (2017) found on RT-qPCR validation that levels of miR-3939 and miR-1910-3p were not statistically different between 45 T2DM patients with DR and 45 T2DM patients without DR.
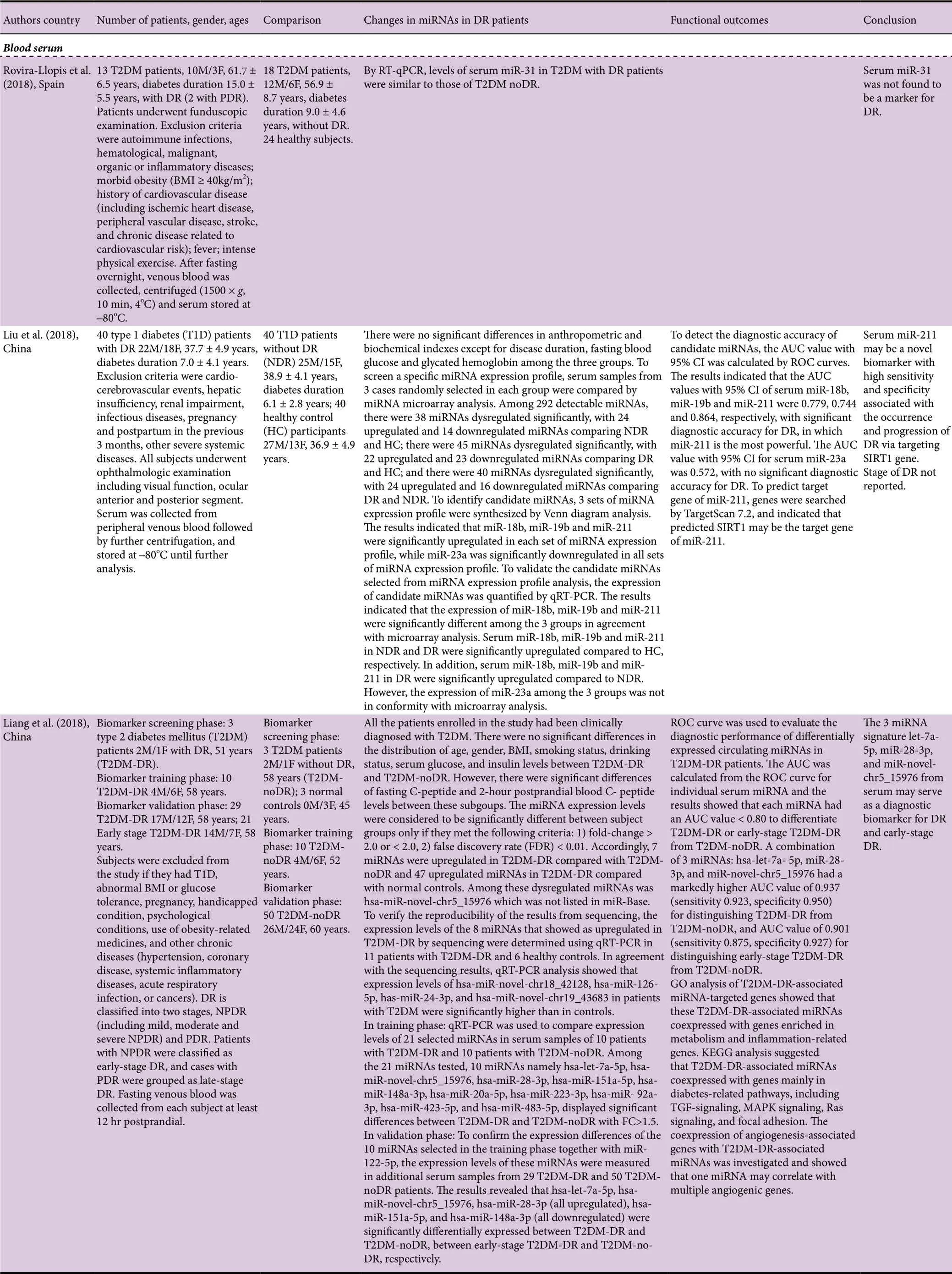
Table 1 MicroRNAs in blood serum, plasma, EVs and EPCs of human patients with DR
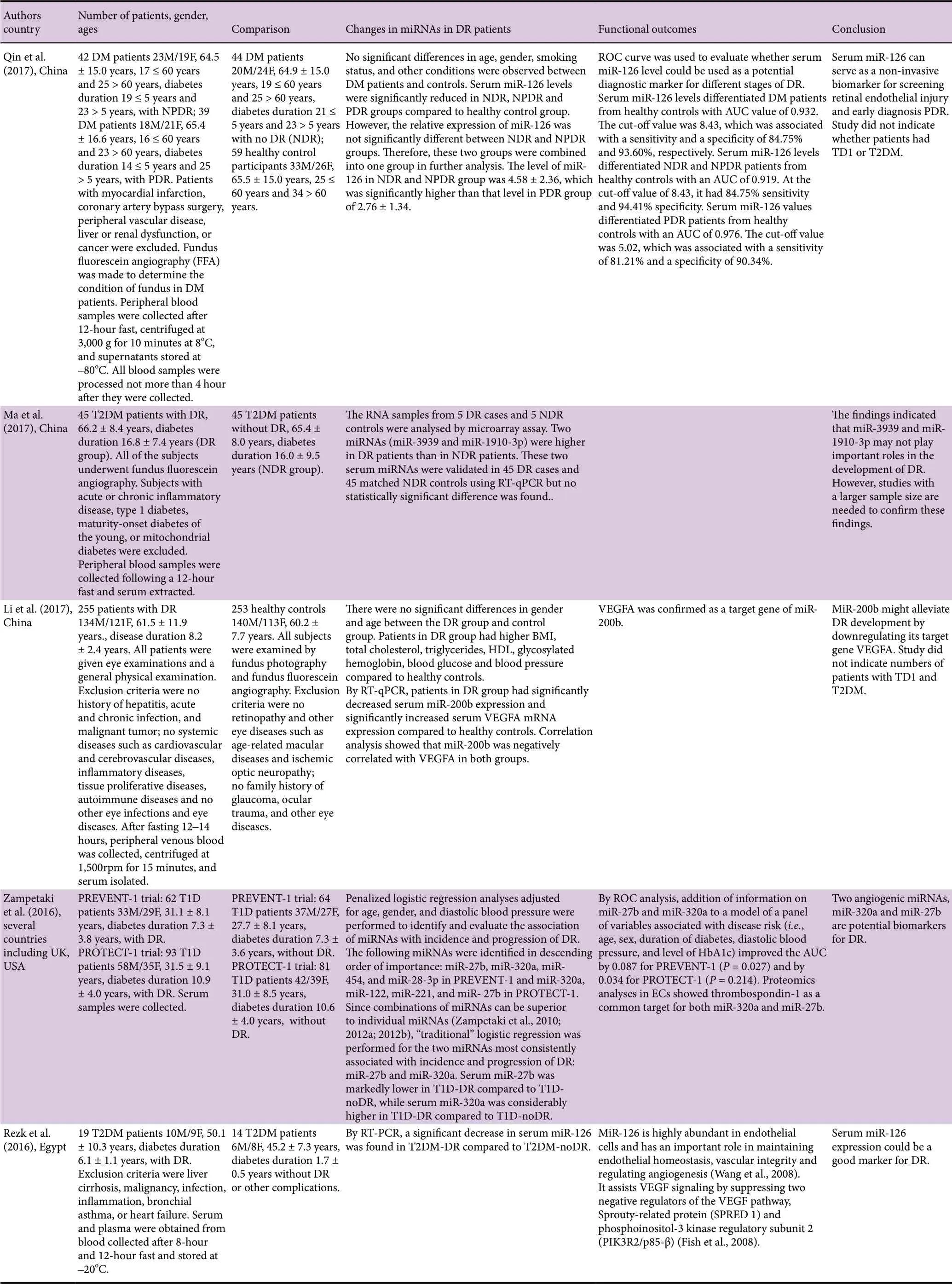
Table 1 Continued
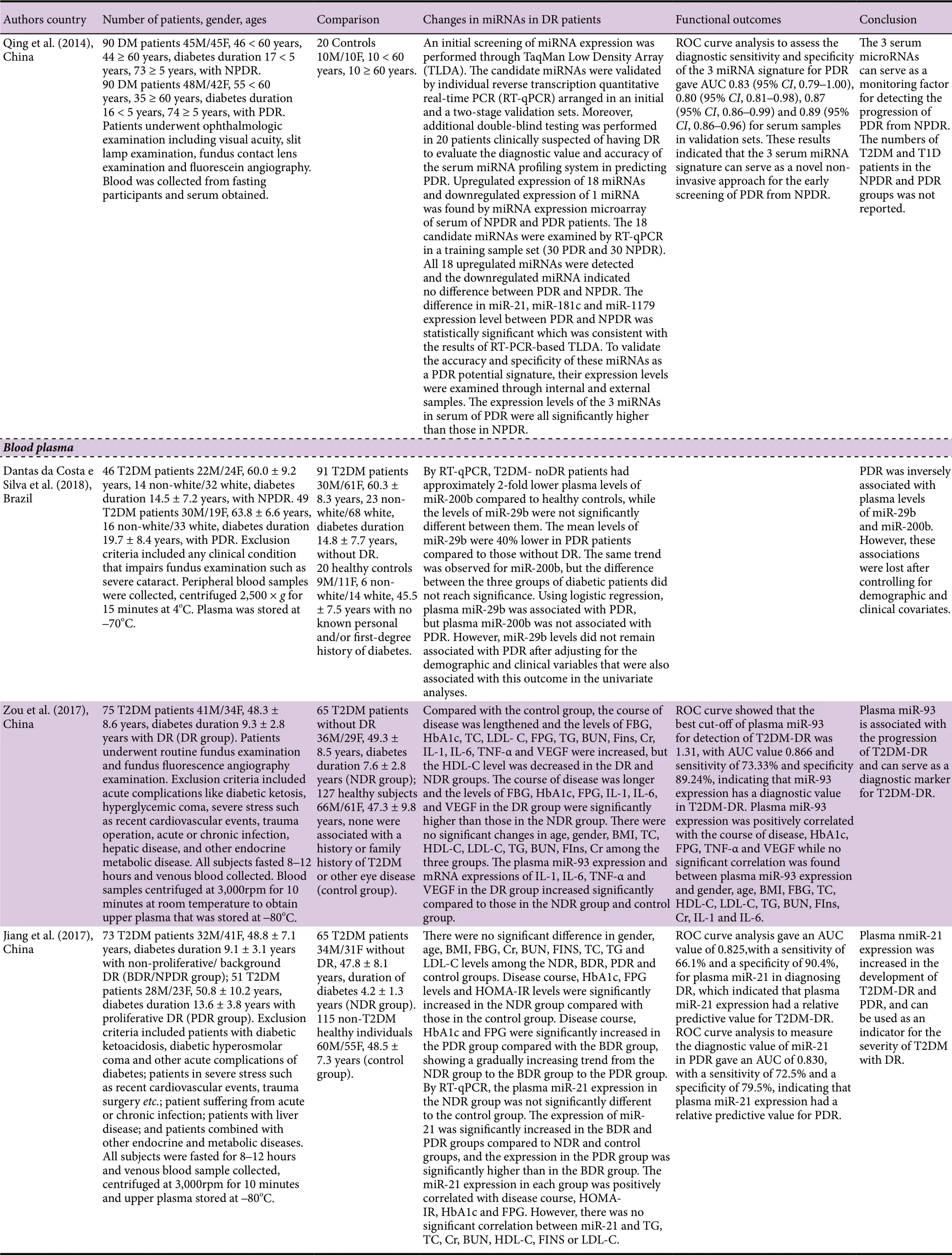
Table 1 Continued
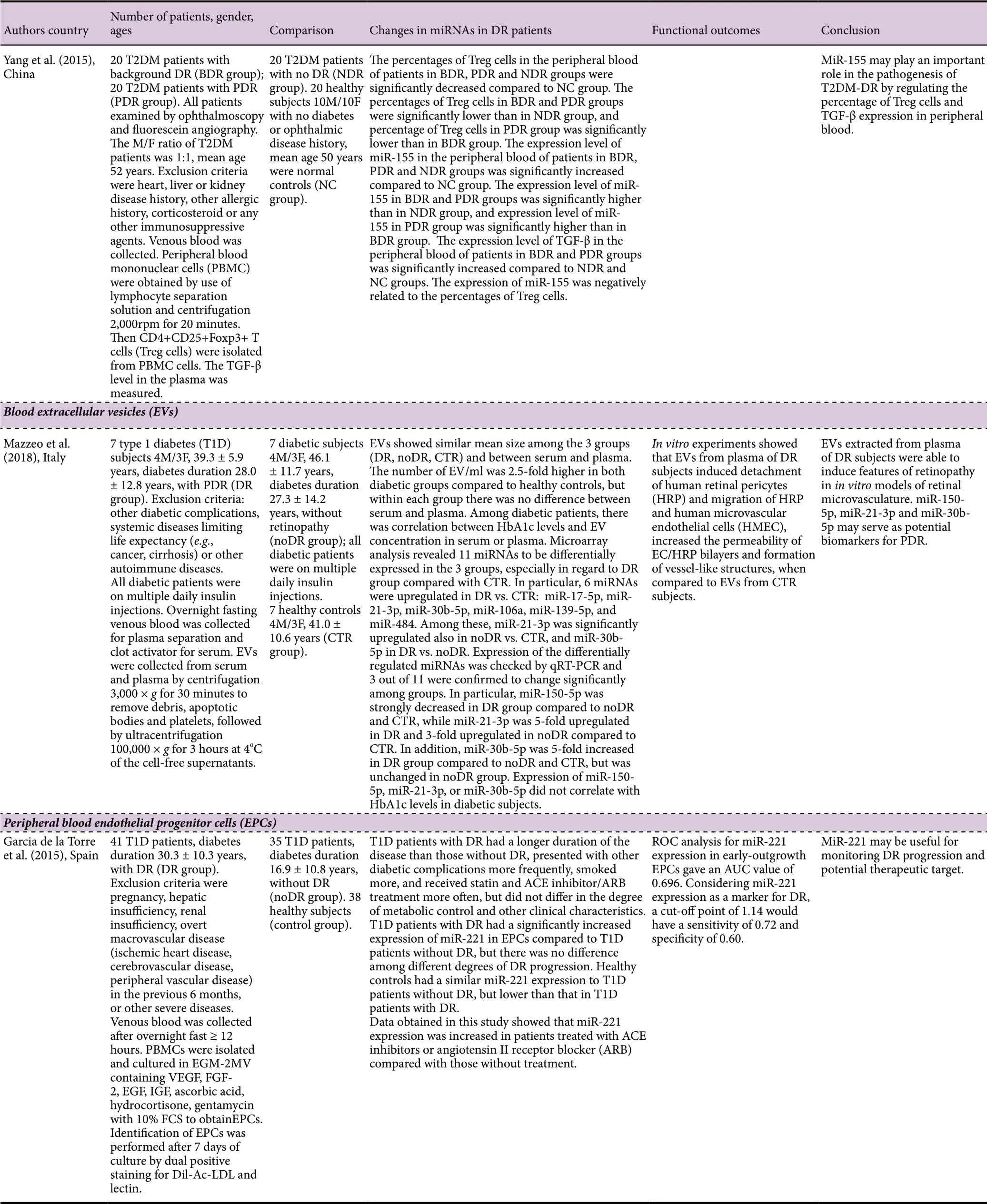
Table 1 Continued
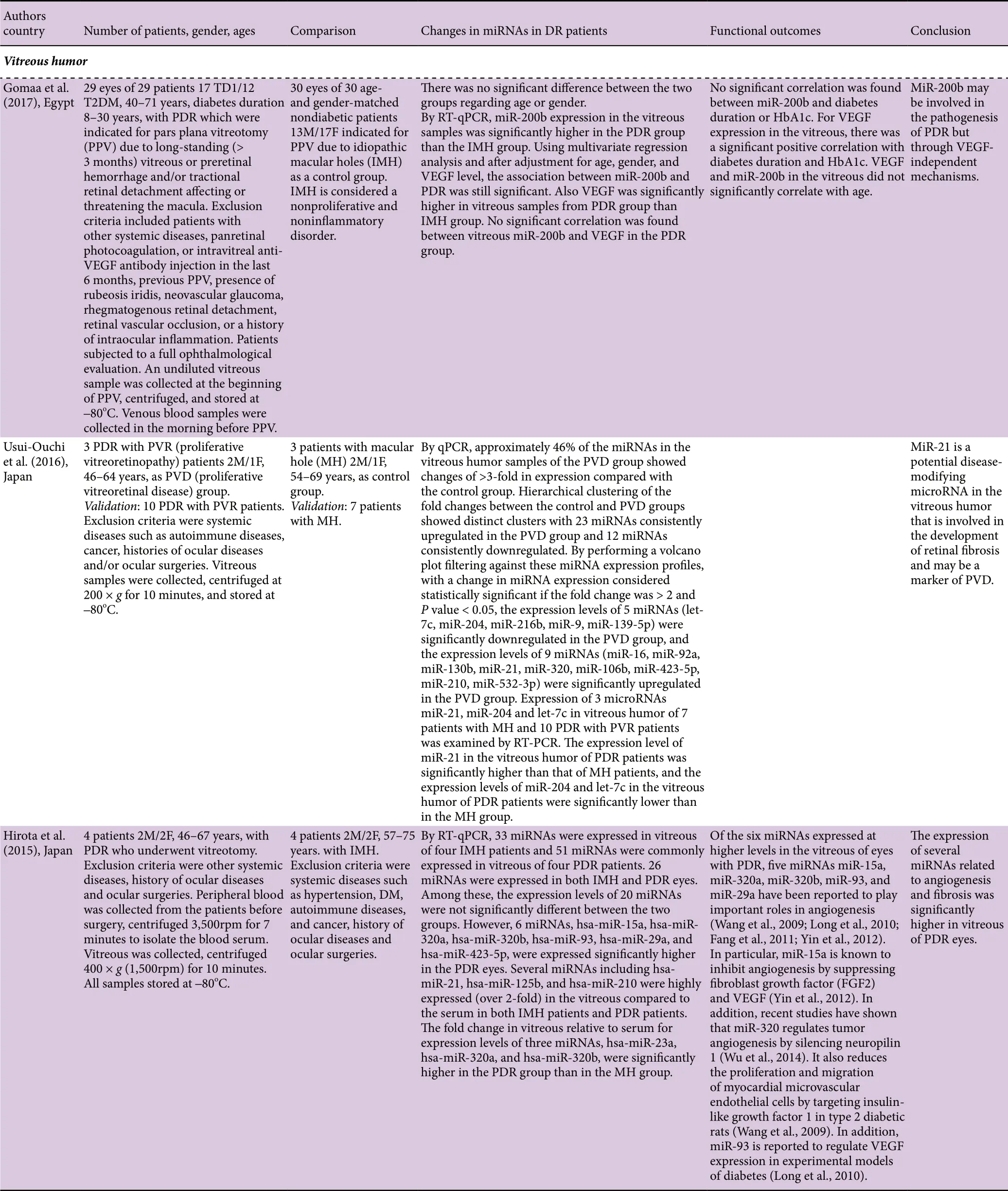
Table 2 MicroRNAs in vitreous humor of human patients with DR
Blood plasma
Dantas de Costa e Silva et al. (2019) performed a largescale study that included 46 T2DM patients with NPDR, 49 T2DM patients with PDR, and 91 T2DM patients without DR. This study was carried out in Brazil and most of the patients recruited were white. By RT-qPCR, the levels of miR-29b were significantly lower in PDR patients compared to those without DR. The same trend was observed for miR-200b, but the difference was not significant. Using logistic regression, miR-29b was associated with PDR, but miR-200b was not. However, miR-29b levels did not remain associated with PDR after adjusting for the demographic and clinical variables that were also associated with this outcome in the univariate analyses. A study by Zou et al. (2017) with 75 T2DM patients with DR and 65 T2DM patients without DR found using RT-qPCR that miR-93 was significantly increased in the DR group compare to the noDR group. ROC analysis showed that the best cut-off of miR-93 for detection of T2DM-DR was 1.31, with AUC value 0.866 (sensitivity 73.33%, specificity 89.24%, indicating that miR-93 expression has diagnostic potential for T2DM-DR. Another largescale study reported by Jiang et al. (2017) recruited 73 T2DM patients with BDR/NPDR, 51 T2DM patients with PDR, and 65 T2DM patients without DR. By RT-qPCR, the expression of miR-21 was significantly increased in the BDR and PDR groups compared to noDR, and the expression in the PDR group was significantly greater than in the BDR group. ROC analysis gave an AUC value of 0.825 (sensitivity 66.1%, specificity 90.4%) for miR-21 in diagnosing DR, which indicated that miR-21 expression had a relative predictive value for T2DM-DR. ROC curve analysis to measure the diagnostic value of miR-21 in PDR gave an AUC of 0.830 (sensitivity 72.5%, specificity 79.5%) indicating that miR-21 expression had a relative predictive value for PDR. MiR-21 can be used as an indicator for the severity of T2DM DR. Yang et al.(2015) examined miR-155 expression in 20 T2DM patients with BDR, 20 T2DM patients with PDR, and 20 T2DM patients without DR. By RT-PCR, the level of miR-155 in the BDR and PDR groups was significantly greater than in the noDR group, and the level of miR-155 in the PDR group was significantly greater than in the BDR group. Thus, miR-155 can serve to monitor the progression of DR.
Peripheral blood extracellular vesicles
Mazzeo et al. (2018) collected extracellular vesicles from serum and plasma of seven T1DM patients with PDR and seven diabetic patients without DR. By RT-qPCR validation,the expression of miR-150-5p was significantly lower in the DR group compared to noDR group, while the expression of miR-30b-5p was significantly greater in the DR group compared to noDR group.
Peripheral blood endothelial progenitor cells
Garcia de la Torre et al. (2015) examined miR-221 expression in early-outgrowth endothelial progenitor cells obtained from peripheral blood mononuclear cells of 41 T1DM patients with DR and 35 T1DM patients without DR. By RTPCR, the expression of miR-221 was significantly greater in the DR group compared to noDR group, but there was no difference among different degrees of DR progression. ROC analysis for miR-221 expression gave an AUC value of 0.696,and with a cut-off point of 1.14 had a sensitivity of 0.72 and specificity of 0.60 as a marker for DR.
Vitreous humor
Gomaa et al. (2017) performed a large-scale study with 29 eyes of 29 patients (17 T1DM, 12 T2DM) with PDR and 30 eyes of 30 nondiabetic patients with MH as a control group.By RT-qPCR, miR-200b expression was significantly greater in the PDR group than the MH group. Using multivariate regression analysis and after adjustment for age, gender, and VEGF level, the association between miR-200b and PDR was still significant. Usui-Ouchi et al. (2016) in a validation study with 10 PVD patients (PDR with PVR) and 7 patients with MH found using RT-PCR that the expression level of miR-21 in PDR patients was significantly greater than in MH patients, and the expression levels of miR-204 and let-7c in PDR patients were significantly lower than in MH patients.In addition, a small-scale study by Hirota et al. (2015) with four patients with PDR and four patients with MH observed by RT-qPCR that the expression levels of miR-15a, miR-320a, miR-320b, miR-93, miR-29a, and miR-423-5p, were significantly greater in the PDR eyes than in MH eyes. The fold changes in vitreous relative to serum for expression levels of three miRNAs, miR-23a, miR-320a, and miR-320b,were significantly greater in the PDR group than in the MH group.
Future Perspectives
DR is the leading cause of new cases of blindness and impaired vision in adults aged 20-65 years (Sokol-McKay). The basic pathological change of DR is retinal neovascularization and fibrosis hyperplasia. Recent studies have indicated that DR is an inflammatory disease, with oxidative stress, formation of advanced glycation end-products and increased expression of VEGF all contributing to the inflammatory response (Semeraro et al., 2015; Rubsam et al., 2018). The treatments for PDR are panretinal laser photocoagulation and vitrectomy in selected cases, and anti-VEGF injections have recently been used as another treatment option. However, serious systemic side-effects can develop and there may be reoccurrence of the neovascularization a few months after the anti-VEGF injection (Salam et al., 2011). There is an urgent need to develop new treatments for the management of PDR.
At present DR can only be diagnosed via formal examination of the eye by a trained specialist. Studies have shown that circulating miRNAs play a significant role in the development of diabetes and they may be a more sensitive way to predict development of the disease than the currently available tools (Jimenez-Lucena et al., 2018). To date, few studies have investigated the relationship between circulating miRNA levels and the development of retinopathy in diabetic patients, with most studies focussing on diabetic rat models or retinal endothelial cells cultured in high glucose conditions.Circulating miRNA levels can be used for the early prediction of DR with high sensitivity and specificity, and altered circulating miRNA levels may provide a novel minimally invasive biomarker for the early detection of DR (Ma et al.,2017). It has been shown that microRNAs have remarkable chemical and physical stability in various body fluids.
Most of the studies reviewed showed that circulating microRNAs could distinguish diabetic patients (T1DM and T2DM) with retinopathy from healthy controls and diabetic patients without retinopathy (T1DM noDR and T2DM noDR) (Table 1 and Figure 1). In addition, serum expression levels of miR-126, miR-21, miR-181c, miR-1179, and plasma expression levels of miR-21, miR-155, were dysregulated in PDR and therefore served as markers of disease and for monitoring disease progression. Interestingly, miR-21 expression was greater in vitreous humor of PDR patients than in MH patients (controls). Also, miR-320a and miR-320b had increased expression in vitreous humor in PDR than in MH, and Zampetaki et al. (2016) had identified a higher serum expression level of miR-320 in DR patients compared to patients without DR. However, several of the reviewed studies had small numbers of patients, especially those analyzing vitreous humor, and this is an important limitation of these studies. While vitreous humor cannot be collected from eyes of diabetic patients without DR due to ethical reasons,collection immediately postmortem from eyes of deceased diabetic patients with and without DR and from those of deceased healthy subjects could provide important information towards establishing biomarkers of DR, especially PDR, with high sensitivity and specificity.
The microRNA findings from screening/discovery studies need to be confirmed or discounted by validation studies with large group sizes. Very few of the validation studies reported relative fold change values for DR. Such quantitative information would emphasize miRNAs with the greatest evidence in support of their role as a biomarker of DR in humans. Currently qPCR is the favored method for determining miRNA expression due to its accuracy, simplicity, reproducibility and lower cost than other hybridization or sequencing-based systems (Git et al., 2010). Ma et al. (2017) found by microarray analysis that serum miR-3939 and miR-1910-3p were significantly greater in T2DM-DR patients than in T2DM-noDR patients, but on RT-qPCR validation no statistically significant difference was found for these two miRNAs. The choice of platform for the validation studies is also important. Farr et al. (2015) compared four different high-throughput platforms to validate a circulating miRNA signature for DR. Unprejudiced next generation smallRNA sequencing was carried out to identify a miRNA signature for DR, and the validation of the signature systematically assessed on clinical samples using each of four qPCR platforms which were a standard 96-well platform, a high-content microfluidics platform and two high content platforms. The ViA7 (96-well) platform is the“gold standard” among these qPCR platforms (Hardikar et al., 2014). Five plasma miRNAs (miR-376a-1, miR-132, miR-125b, miR-100, miR-221) were selected based on consistent differences between DR and noDR patients (age and gender matched) and the detection of such a signature was assessed using the four different platforms. The features of miRNA expression were retained across three qPCR platforms - ViA7,TaqMan Low Density Array and Open Array platforms -while the features appeared skewed for the Dynamic Array platform (Farr et al., 2015). Also those microRNAs found to be significantly dysregulated should be tested by ROC analysis and determination of AUC values to establish whether they are good candidates to identify DR and for monitoring disease progression. Further studies are warranted with large cohorts of NPDR and PDR patients and diabetic patients without DR, and of different ethnicities, to be performed in other countries as most of the studies reviewed had been carried out in China.

Figure 1 Altered expression of microRNAs in blood serum, blood plasma and vitreous humor of patients with DR.Blood serum and blood plasma expression levels for DR patients were compared to those of NDR patients, closely matched for age and gender. In the case of PDR, expression levels were compared to those of NDR, NPDR or BDR patients. Vitreous humor expression levels for PDR patients were compared to those of MH patients. Arrows pointing upwards or downwards indicate increased or decreased expression,respectively. miR-novel^ is miR-novelchr5_15976. DR: Diabetic retinopathy;NDR: no diabetic retinopathy; NPDR:nonproliferative diabetic retinopathy;BDR: background diabetic retinopathy;PDR: proliferative diabetic retinopathy;MH: macular hole.
The biological function of many of the dysregulated miRNAs in DR can be related to effects on retinal cells. The upregulation of miR-211 in DR, and shown in diabetic retinal tissue and hyperglycemic human umbilical vein endothelial cells, could suppress the expression of its downstream target gene SIRT1 via specifically binding to the target gene 3′-UTR,leading to retinal vascular disorder and endothelial apoptosis associated with DR (Liu et al., 2018). In addition, the upregulated expression of let-7a-5p in DR may be associated with increased proliferation of retinal microvascular endothelial cells (Liang et al., 2018). The downregulation of miR-126 in DR (Rezk et al., 2016) and PDR (Qin et al., 2017) may be related to endothelial damage as it has been reported that miR-126 provides protection for vascular endothelial cells(van Solingen et al., 2015; Christiakov et al., 2016). MiR-27b and miR-320a, the two miRNAs most consistently associated with the incidence and progression of DR (Zampetaki et al.,2016), have thrombosponin-1 as a target (Stenina-Adognravi,2013). Thrombosponin-1 inhibits endothelial cell proliferation, migration, and angiogenesis (Tolsma et al., 1997),with its antiangiogenic effect exerted via an interaction with VEGF and by inhibiting VEGF receptor-2 signaling (Kaur et al., 2010). The increased expression of miR-21 in DR and PDR (Qing et al., 2014; Jiang et al., 2017) may be associated with it inducing angiogenesis via targeting PTEN (phosphatase and tensin homologue), leading to activation of AKT and ERK1/2 signaling pathways, and thereby enhancing HIF-1α and VEGF expression (Liu et al., 2011). Moreover, miR-21 is overexpressed in response to high glucose and protects endothelial cells from apoptosis (Zeng et al., 2013). Also, the increased expression of miR-155 in PDR (Yang et al., 2015)may be related to miR-155 regulating the levels of Treg cells and transforming growth factor-β (Yang et al., 2015). The higher expression of miR-200b in PDR than in MH may be due to miR-200b overexpression downregulating the Oxr1(oxidation resistance 1) gene, which protects retinal cells against apoptosis and oxidative stress (Murray et al., 2013).
Of interest and clinically important, a very high percentage of patients with eye diseases such as glaucoma, age-related macular degeneration, and DR develop Alzheimer's disease(AD) (Lee et al., 2019). Patients with recent DR (diagnosed within 0-5 years) and established DR (> 5 years) were found to be at a higher risk of AD by 67% and 50% compared to those without DR (Lee et al., 2019). Identifying ophthalmic diseases by measurement of suitable biomarkers would enable better screening and treatment of those individuals at risk of AD (Romano et al., 2017; Lee et al., 2019).
Author contributions: Both authors contributed equally.
Conflicts of interest:There are no conflicts of interest.
Financial support:None.
Copyright license agreement: The Copyright License Agreement has been signed by both authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-Non-Commercial-ShareAlike 4.0 License, which allows others to remix, tweak,and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewer:Vance P. Lemmon, University of Miami Miller School of Medicine, USA.
杂志排行
中国神经再生研究(英文版)的其它文章
- Etomidate affects the anti-oxidant pathway to protect retinal ganglion cells after optic nerve transection
- Normal tension glaucoma: from the brain to the eye or the inverse?
- Mesenchymal stromal cell therapy for damaged retinal ganglion cells, is gold all that glitters?
- Diabetic neuropathy research: from mouse models to targets for treatment
- Potential therapeutic roles of retinoids for prevention of neuroinflammation and neurodegeneration in Alzheimer's disease
- Sigma-2 receptor as a potential therapeutic target for treating central nervous system disorders