Normal tension glaucoma: from the brain to the eye or the inverse?
2019-07-18HuiJunZhangXueSongMiKwokFaiSo
Hui-Jun Zhang, Xue-Song Mi, , Kwok-Fai So
1 Department of Ophthalmology, the First Affiliated Hospital of Jinan University, Guangzhou, Guangdong Province, China
2 Guangdong-Hong Kong-Macau Institute of CNS Regeneration, Jinan University, Guangzhou, Guangdong Province, China
3 State Key Laboratory of Brain and Cognitive Sciences, Pokfulam, Hong Kong Special Administrative Region, China
4 Department of Ophthalmology, The University of Hong Kong, Pokfulam, Hong Kong Special Administrative Region, China
Abstract Glaucoma is a chronic, progressive optic neuropathy characterized by the loss of peripheral vision first and then central vision. Clinically, normal tension glaucoma is considered a special subtype of glaucoma, in which the patient's intraocular pressure is within the normal range, but the patient experiences typical glaucomatous changes. However, increasing evidence has challenged the traditional pathophysiological view of normal tension glaucoma, which is based only on intraocular pressure, and breakthroughs in central nervous system imaging may now greatly increase our knowledge about the mechanisms underlying normal tension glaucoma. In this article, we review the latest progress in understanding the pathogenesis of normal tension glaucoma and in developing imaging techniques to detect it, to strengthen the appreciation for the connection between normal tension glaucoma and the brain.
Key Words: nerve regeneration; normal tension glaucoma; open angle glaucoma; neurodegenerative diseases;visual field; cerebrospinal fluid pressure; imaging techniques; pathogenesis; magnetic resonance imaging;diffusion tensor imaging; metabolic changes; neural regeneration
Introduction
Glaucoma is a chronic, progressive optic neuropathy characterized by the loss of peripheral vision first and then central vision. Glaucoma is second only to cataracts among causes of visual impairment worldwide and is the leading cause of irreversible blindness worldwide (Kingman, 2004;Liu and Lee, 2016). It is predicted that the number of patients with glaucoma will reach approximately 80 million by 2020, of whom more than half will have open angle glaucoma (OAG) (Quigley and Broman, 2006). Normal tension glaucoma (NTG), as a special subtype of glaucoma, occurs in the absence of elevated intraocular pressure (IOP) and,in fact, challenges the traditional pathophysiological viewpoints of glaucoma that are based only on IOP. According to the Collaborative Normal-Tension Glaucoma Study Group,although lowering IOP in NTG was beneficial, the disease still progressed in some patients (No authors listed, 1998).In fact, many recent studies have revealed that IOP-independent risk factors, including vascular factors, trans-laminar pressure difference (TLPD), immune-related disorders,gene problems, and myopia-related biome-chanical factors,etc., play important roles in the development of NTG (Kim and Park, 2016). Lately, researchers have focused on the relationship between glaucoma and cognitive impairment,especially that due to Alzheimer's disease (AD) and Parkinson's disease (PD) (Lin et al., 2014; Eraslan et al., 2015;Maurano et al., 2018). In the absence of obvious visual deterioration and clinical signs in the early stage, NTG is likened to a ‘silent thief' of vision because the vision of NTG patients erodes without symptoms. Therefore, it is important to intervene as early as possible in the disease course,before significant, irreversible damage occurs. Accordingly,more effective structural and functional measurements for the early diagnosis of NTG are needed. We believe the new investigative techniques, such as functional magnetic resonance imaging (MRI), diffusion tensor imaging (DTI)and optical coherence tomography (OCT) angiography, will lead to improvements in early detection of NTG and better follow-up of patients after diagnosis (Brown et al., 2016;Kim and Park, 2016). The relevant questions are where the thief comes from, how it works and what we can do to recognize it. In order to answer those questions, we review here the latest advances in understanding NTG pathogenesis and in the development of investigative technologies for NTG (Figure 1).
Literature Search
We performed an electronic search of the PubMed database for articles published from 1998 to 2019, using the following search terms: “normal tension glaucoma”, “normal pressure glaucoma” and “open angle glaucoma”. The results were further screened by title and abstract to isolate papers using the words: “brain”, “magnetic resonance imaging”, “cerebrospinal fluid pressure”, “vascular factor”, “immune”, “neurodegenerative diseases”, “central nervous system” and “mechanisms”. In total, 52 related articles are cited in this article.
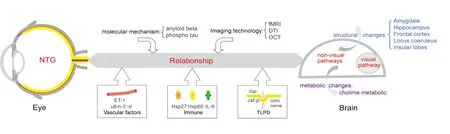
Figure 1 The potential relationship between the eye and brain in the development of NTG.The intraocular-pressure-independent mechanisms in NTG include vascular factors, immune and cerebrospinal fluid factors, etc. It is now known that the NTG-related damage is not limited to the eye, but extends to the entire visual pathway, as well as to some nonvisual pathways in the brain.In addition, NTG and central neurodegenerative diseases share similar structural and metabolic changes in the brain, including similar molecular mechanisms. Those pathophysiological changes in both the eye and brain can be detected with new imaging technologies. NTG: Normal tension glaucoma; ET-1: Endothelin-1; Hsp27: heat shock protein 27; Hsp60: heat shock protein 60; IL-6: interleukin-6; csf-p: cerebrospinal fluid pressure;TLPD: trans-laminar pressure difference; fMRI: functional magnetic resonance imaging; DTI: diffusion tensor imaging; OCT: optical coherence tomography.
Normal Tension Glaucoma and Neurodegenerative Diseases: Where Does theThief Come From?
Recently, many studies have reported on the relationship between primary OAG (POAG) and neurodegenerative dis-eases, inspiring the question of whether glaucoma should be considered a brain disease. According to the Hodapp/Bascom Palmer classification, POAG patients had early, moderate, and severe stages. A multimodal MRI study in patients with all three stages of POAG showed disrupted anatomical connectivity, compared with that in normal controls, along the visual pathway and in nonvisual white matter tracts of the central nervous system. In ad-dition, patients with severe POAG showed brain atrophy in gray matter regions (the hippocampus and frontal cortex) (Frezzotti et al., 2016). Further, in both POAG patients and animal models, the locus coeruleus and other brain regions (i.e., the parahippocampal gyri and frontal and insular lobes) seem to act as a network in POAG pathogenesis including IOP regulation and behavioral changes (Yuan et al., 2018). These findings suggest that the POAG-related damage might not be limited to the retinal ganglion cells (RGCs) or to the eye, but might extend to the entire visual pathway and some nonvisual brain regions. There are many similarities in mechanisms between glaucoma and neurodegenerative diseases, such as the loss of RGCs and the deposition of abnormal proteins in specific anatomical areas (e.g., the hippocampus). Findings in a study of cognitive function showed that patients with glaucoma had a reduction in cognition that was similar to the cognitive impairment in subjects with AD, regardless of whether the glaucoma was mild or advanced (Maurano et al., 2018).A recent pathology study provided further evidence that chronic high IOP impairs learning and memory in rats by increasing amyloid beta and phospho-tau expression in the hippocampus; similar issues are thought to contribute to cognitive and behavioral impairments in AD (Yuan et al.,2017). Thus, more and more scholars support that POAG should be considered a neurodegenerative disease of the retina and the brain.
As a subtype of POAG, NTG shares similar clinical manifestations and disease progression with POAG, therefore, the pathogenesis of NTG is likely similar to that of the neurodegenerative diseases. Evidence has, in fact, shown that there is a close pathogenetic link between neurodegenerative disorders, POAG and NTG (Bulut et al., 2016), as we describe below.
Epidemiological research demonstrated that there is a high percentage of NTG cases in the Japanese glaucoma population (Iwase et al., 2004). Recently, findings in a brain conformational study showed that there was a very high incidence of structural changes in the white matter of Japanese NTG patients (Boucard et al., 2016), which may indi-cate that NTG can be included under the broad grouping of neurodegenerative disorders. Furthermore, similar retinal changes, such as reduction of the retinal nerve fiber layer(NFL) and ganglion-cell-complex thicknesses, were found in both NTG and AD patients (Eraslan et al., 2015), suggesting that the pathogenesis of NTG may be similar to that in the development of neurodegenerative diseases. Thus, we can hypothesize that NTG and AD may originate from sim-ilar pathophysiological mechanisms, but in different regions of the central nervous system and showing different clinical manifestations.
Similar to AD, PD is a progressive neurodegenerative disorder with selective loss of dopaminergic neurons in the nigrostriatal pathway. Previous studies showed that PD patients with peripapillary retinal nerve fiber thinning are more likely to develop glaucomatous-like visual field loss than controls (Garcia-Martin et al., 2012). However, no causal link between POAG and PD was demonstrated in a large population-based study (Lin et al., 2014). More and more research suggests that NTG could be classified as a disease of the brain, as well as the eye. Unfortunately, until now there has been controversy regarding whether changes in the brain occur before, simultaneously with, or after the development of glaucoma (Prins et al., 2016). Future studies with large subject populations are warranted to identify the common pathological mechanism that contributes to the neurodegeneration in NTG and other disorders.
IOP-Independent Mechanisms in NTG: How Does the Thief Work?
Although IOP plays an important role in the pathogenesis of glaucoma, including NTG, increasing evidences reveals that IOP-independent mechanisms, such as vascular factors,TLPD and immune-related disorders, may be particularly important in the development of NTG.
The role of vascular factors in NTG
Among the various IOP-independent factors, vascular factors have been suggested as central to the pathogenesis of glaucomatous optic neuropathy in NTG, as many studies have found vascular structural changes or dysregulation in NTG patients. For example, endothelin-1, a potential vessel constrictor, was first found to be involved in NTG(Grieshaber et al., 2007). Our previous studies found that transgenic mice with overexpression of endothelin-1 in blood vessel endothelial cells can progressively lose RGCs,which is consistent with the development of NTG (Mi et al.,2012, 2014). Vascular dysregulation, which has been identified as a causal factor underlying NTG, has re-cently been named “Flammer syndrome” (a phenotype characterized by primary vascular dysregulation, together with a set of symptoms and signs, including cold hands and/or feet, low blood pressure, prolonged sleep-onset time, increased blood flow resistance in retro-ocular vessels and so on (Konieczka et al., 2014)). Optic disc hemorrhage, which occurs with a high incidence in NTG patients, is frequently associated with the“nonphysiologic” nocturnal blood pressure dips known as overdips (Kwon et al., 2017). Vascular-related mechanisms may be persistent risk factors in the development of NTG.In a previous experiment, NTG patients were classified into distinct subgroups: low-teen IOP (IOP ≤ 15 mmHg) and high-teen IOP (15 mmHg < IOP ≤ 21 mmHg). In that study,a higher prevalence of the Raynaud phenomenon was observed in the low-teen IOP group, which led the authors to speculate that the progression of NTG in low-IOP patients may be more affected by peripheral vasospasm, such as the Raynaud phenomenon than in high-teen IOP patients (Kim et al., 2014). Furthermore, the prevalence of other vascular comorbidities (like hypertension, hyperlipidemia, ischemic heart disease, stroke and metabolic syndrome) was significantly higher among participants with low-teen NTG than among normal participants. This supports the hypothesis that vascular factors may contribute more to the pathogenesis of NTG, especially in low-teen NTG than high-teen NTG subjects (Lee et al., 2017). Thus, the pathophysiology of NTG in patients with low-teen and high-teen baseline IOP may be dif-ferent. In addition, Himori et al. (2016) evaluated the association between ocular blood flow and biomarkers of sys-temic oxidative stress in patients with NTG, finding that increase in urinary 8-hydroxy-2′-deoxyguanosine and skin autofluorescence were associated with decreasing ocular blood flow, which may be contribute to NTG. However,an-other recent study showed the opposite result, with no statistically significant differences in ocular perfusion pressure and ophthalmic artery flow between the NTG patients and non-glaucoma controls, suggesting that vascular insufficiency or dysregulation may not account for the pathogenesis of NTG (Samsudin et al., 2016). Although samples were small, it warns us that the definite vascular mechanism is still not clear.
The role of cerebrospinal fluid factors in NTG
Two opposite pressures act on the lamina cribrosa of the optic nerve: the anterior IOP and the posterior cerebrospinal fluid (CSF) pressure. The TLPD is defined as the difference between the IOP and the optic nerve CSF pressure (Liu et al., 2018). Recently, attention has been paid to the role of the CSF, and circulatory dysfunction of the CSF has been suggested as contributing to the pathogenesis of NTG. Based on the associations between CSF and eye anatomy, an elevated TLPD could be due to an imbalance between CSF pressure and IOP, and CSF pressure should not be over-looked as a factor in the pathogenesis of glaucomatous optic neuropathy in NTG (Jonas et al., 2015). In clinical practice, the first case of obvious worsening of glaucoma after ventriculoperitoneal shunt placement, to decrease CSF pressure, was reported in a 93-year-old Caucasian woman, who was previously diagnosed with stable NTG. That case suggests that low CSF pressure may increase TLPD of the optic nerve, thereby contributing to glaucomatous progres-sion in NTG (Chen et al., 2016). A retrospective cohort study examined the TLPD in Caucasian patients with NTG, to document the possible relationship between TLPD and progressive visual field loss,but false-negative results were obtained. There was no significant correlation between TLPD and the mean defects (MD)of fields. Hence, the esti-mation of TLPD is best performed as close as possible to the optic nerve (Pircher et al., 2017a).A further study by the same group measured optic canal cross-sectional areas with computed tomographic images in 56 Caucasian NTG patients and the same number of control subjects without known optic nerve diseases. In the patients,the optic canal cross-sectional area was significantly smaller than that in the controls. A smaller optic canal area might act as a bottleneck between the intracranial space and the orbital subarachnoid space and, therefore, contribute to a discontinuity of CSF flow, which might impact NTG (Pircher et al., 2017b). Recently, there was a case report of a 27-yearold male who had an early onset of NTG, combined with intracranial hypotension and recurrent left optic disc hemorrhages. Since the normalization of his TLPD, by the dual approach of decreasing IOP, with 0.005% Latanoprost, and increasing CSF pressure, with implantation of a programmable gravity-assisted valve, his optic disc hemorrhages have not recurred (Yusuf et al., 2017). Increasing evidence shows a relationship between low CSF pressure and NTG, driving speculation that an abnormal decrease in CSF pressure leads to changes in the TLPD, resulting in optic nerve injury in NTG (Jonas et al., 2015; Chen et al., 2016; Pircher et al.,2017a, b; Yusuf et al., 2017). Lately, the combined effects of IOP and intracranial pressures (ICP) on the microstructure of the lamina cribrosa (locus coeruleus) were reported by Wang et al. (2017a), based on a monkey model that accounted for the potential effect of IOP-ICP interactions. However,the role of CSF in NTG is still unclear and more experimental models need to be established for further research.
The role of immune factors in NTG
Recent studies have demonstrated that POAG is associated with changes in the immune system (Bell et al., 2013), so abnormalities in the immune system may play an important role in NTG. There have been reports of alterations in serum antibody profiles against many optic nerve and retinal proteins, such as increased expression of heat shock protein 27,heat shock protein 60 and tumor necrosis factor-α (Tezel and Wax, 2004). However, research has not yet clarified whether the autoantibodies seen in glaucoma are an epiphenomenon or are causative (Rizzo et al., 2017). In an experimental autoimmune glaucoma model, animals were immunized with ocular antigens which led to a loss of RGCs and to optic nerve damage. Shortly after that, early remodeling of extracellular matrix proteins, including tenascin-C and phosphacan, was observed; that occurred before the loss of RGCs (Reinehr et al., 2016). Interleukin-6, one type of chemokine of innate immunity which participate in the body's immune response,is a potent initiator of the extrinsic apoptosis of neurons.Serum levels of interleukin-6 were borderline higher in NTG patients than in con-trols, which suggests that serum interleukin-6 levels might be associated with the severity of NTG(Wang et al., 2017b). These findings regarding natural autoimmunity offer new insight into the pathogenesis of NTG.
In addition to the findings above, other IOP-independent mechanisms, such as gene-mutation-related mechanisms and several risk factors (such as age, sex and race) have also been shown to be important in the development of NTG (Mi et al., 2014). Up through the present day, the complicated etiologies of NTG are still not completely understood.
Detections of Structural, Functional, and Metabolic Changes: What Should We Do to Find the Thief?
Since patients have no obvious symptoms at the early stage of NTG, determining optimal tools, including structural and functional assessments, is essential for early diagnosing of NTG and monitoring of disease progression. As de-scribed above, studies have shown that changes in the structure and function in glaucoma go beyond the eye and the visual pathway, suggesting that glaucoma can result in widespread modifications of the brain (Mastropasqua et al., 2015). Specifically, MRI studies have confirmed that there are brain structural alterations, including damage to the hippocampus,amygdala and locus coeruleus, where the structural abnormalities reflected the degree of glaucoma-tous damage in the eye (Wang et al., 2016; Yuan et al., 2018). In an MRI study,patients with NTG showed abnormali-ties in the anterior visual pathway involving the optic nerve diameter, height of the optic chiasm and lateral geniculate nucleus volume(Zhang et al., 2012). Based on extensive improvements in neuroimaging technological, DTI has been developed as a noninvasive method to sensitively quantify axonal and myelin injury in glaucoma using diffusivity parameters that cannot be examined with conventional MRI (Zhang et al.,2015). Recent research found that there were microstructural alterations along the visual pathway in glaucoma patients,suggesting that widespread changes in DTI parameters (e.g.,fractional anisotropy and mean diffusivity) could serve as quantifiable indicators of glaucoma severity (Jiang et al.,2017). In a cross-sectional study, when the cut-off value of DTI-derived fractional anisotropy was set at 0.34, mild glaucoma could be screened out, suggesting that changes in fractional anisotropy in the optical pathway have high sensitivity and specificity for distinguishing mild glaucoma from severe cases (Sidek et al., 2014). In particular, by comparing the occurrence of visual cortex injury, as measured by functional MRI, and clinical visual field loss, Murphy et al. (2016)demonstrated that structural and functional glaucomatous changes occur earlier in the brain than in the eyes. More importantly, DTI-derived indices not only indicated the degree of whole-brain white matter damage in NTG patients, but also showed a positive correlation with retinal NFL thickness(Sidek et al., 2014; Wang et al., 2018).
Advanced statistical modeling can also further the analysis of the interactions between eye and brain in patients with varying degrees of visual impairment (Kasi et al., 2019).For example, Murphy et al. (2016) demonstrated that the higher-order visual brain areas showed less severe functional damage than did the primary visual cortex in glaucoma,indicating that glaucoma-driven degeneration in the retina might be more closely associated with changes in the primary visual cortex than in the higher-order visual areas. These in vivo imaging studies are expected to disrupt the traditional perception of early glaucoma mechanisms and to address the question of whether the accompanying brain changes are causes or consequences.
Recently, Nasaruddin et al. (2017) calculated the correlation between visual field changes and brain activation in visual cortex (Brodmann areas 17, 18 and 19) in glaucoma suspects and suggested that functional MRI is suitable for detecting early glaucoma in glaucoma suspects. Of particular note, by comparing POAG with NTG, Giorgio et al.(2018) demonstrated that independent and widespread brain pathogenic processes seem to occur not only in POAG, but also in NTG, which could explain the MRI findings of widespread brain structural damage and functional changes in NTG. These results make multimodal MRI techniques an appropriate method for the study of mechanisms in NTG,to document alterations in the visual pathways in glaucoma and to gain new insights into the pathophysiology of glaucoma (Brown et al., 2016). Recent advances in ocular imaging have also enriched our understanding of vascular-related mechanisms in NTG. For example, Igarashi et al. (2017)found that there is no significant difference between the diagnostic power of isolated-check visual evoked potentials and that of RGC-inner plexiform layer analysis using optical coherence tomography. OCT angiography has been praised for allowing thorough observation of the radial peripapillary capillaries. The disappearance angle of the radial peripapillary capillaries is a novel morphological indicator of visual defects due to NTG that might predict the progression of glaucoma (Igarashi et al., 2017).
Apart from structural and functional brain changes, metabolic changes in the brain may also be involved in trans-neuronal degeneration in glaucoma, and such changes can been detected non-invasively. It has been shown that choline metabolism in the visual cortex is disturbed with increased retinal structural damage and reduced visual field (Murphy et al., 2016). Magnetic resonance spectroscopy is a novel non-invasive imaging technique that identifies metabolic changes throughout the visual pathway, by identifying and quantifying changes in metabolites in brain tissue (Aksoy et al., 2018). Finally, in the early stage of OAG, proton magnetic resonance spectroscopy can demonstrate changes in the primary visual cortex (Guo et al., 2018). However, the practical application of these techniques needs further study, with the early detection of NTG still an unresolved problem. A combination of structural, functional and metabolic assessments might enhance the detection of early glaucoma (Chen and Zhao, 2017), and only then can we implement early preventative measures.
Conclusions
In summary, to our current knowledge, NTG is still a multifactorial and complicated disease that requires large-scale,prospective research to investigate the relationship of the eye to the brain and even to systemic diseases. In the future, using multimodal MRI examinations, we need not only focus on the visual pathway, but also pay more attention to non-visual pathways and look for biological markers in NTG. It would be of great interest to discover more in-depth relationships between NTG and central neurodegenerative diseases, which helps to better understand the pathogenesis of NTG. In addition, we believe that advanced exploration of the mechanisms of NTG would promote the progress of novel techniques to recognize subtle changes in the early stage of NTG.
Author contributions: Manuscript writing: HJZ; manuscript reviewing:XSM, KFS. All authors approved the final manuscript.
Conflicts of interest: There are no conflicts of interest.
Financial support:This study was supported in part by the National Basic Research Program of China, No. 81300766 (to XSM); the Cultivation and Innovation Fund from the First Affiliated Hospital of Jinan University, China, No. 802168 (to XSM); the fund of Leading Talents of Guangdong Province, China, No. 87014002 (to KFS); the fund of Ningxia Key Research and Development Program (Yinchuan, Ningxia Hui Autonomous Region, China), and Programme of Introducing Talents of Discipline to Universities, China, No. B14036 (to KFS).
Copyright license agreement:The Copyright License Agreement has been signed by both authors before publication.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-Non-Commercial-ShareAlike 4.0 License, which allows others to remix, tweak,and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
杂志排行
中国神经再生研究(英文版)的其它文章
- Etomidate affects the anti-oxidant pathway to protect retinal ganglion cells after optic nerve transection
- Mesenchymal stromal cell therapy for damaged retinal ganglion cells, is gold all that glitters?
- MicroRNAs as biomarkers of diabetic retinopathy and disease progression
- Diabetic neuropathy research: from mouse models to targets for treatment
- Potential therapeutic roles of retinoids for prevention of neuroinflammation and neurodegeneration in Alzheimer's disease
- Sigma-2 receptor as a potential therapeutic target for treating central nervous system disorders