Micromechanical adaptation as a treatment for spinal cord injury
2019-07-18Veronicaestrada,HanswernerMüller
Spinal cord injury:Thus far injury of the spinal cord is incurable and, in the majority of cases, a devastating and life-changing event. The worldwide incidence rate of spinal cord injury(SCI) ranges from 250,000 to 900,000 (www.who.int, 2013; Kumar et al., 2018) new cases per year. SCI outcome includes the damage of axons, demyelination of axons, loss of signal transduction, and consequential long-lasting motor and sensory deficits. Additionally, the non-use of muscles can lead to atrophy and joint contractures, thereby further reducing the possibility of recovery. Depending on the spinal level and the severity of the injury, the extent of the damage can vary and spontaneous recovery is possible to varying degrees. There is an enormous number and also a great variety of both experimental approaches (Estrada and Muller, 2014) and clinical trials (Badhiwala et al., 2018) for spinal cord trauma. Although the general pathomechanisms of primary and secondary traumatic injury events are known, the complicated multifactorial and multiphasic SCI outcomes have led to only little progress in the development of successful therapeutic treatments to achieve substantial degrees of axonal regeneration and consequently locomotor functional recovery - despite almost 40 years of extensive SCI research.
Current methods of bridging the injury site after spinal cord injury:The aim of bridging the injured area is generally based on the idea to provide a scaffold or matrix, which mimics the consistency of spinal cord tissue and at the same time provides growth support. While the list of therapeutic approaches keeps growing larger, one generally problematic issue remains: Many substrates, conduits, grafts etc. not only allow but even promote regenerative axon growth into the bridge. However, the dense scar, which is associated with many inhibitory molecules, leads to a general lack of axonal outgrowth from the bridge region into the adjacent undamaged host spinal tissue. The spinal lesion scar is composed of two major components: The fibrous scar (rich in extracellular matrix proteins) in the lesion core,and the surrounding glial scar formed of reactive astrocytes(Orr and Gensel, 2018). While the fibrous lesion scar is fully developed at 14 days after injury (Hackett and Lee, 2016), the glial scar is delayed and appears 1-2 months later (White et al.,2010). When making use of micromechanical tissue adaptation the time window of the full development of the SCI scar can be exploited to readapt tissue stumps not only after acute SCI but most likely also after scar tissue resection in chronic SCI. While the principle of bridging a tissue defect per se is not new and is an often used approach in the field of experimental SCI (Estrada et al., 2014), the idea of micromechanical tissue adaptation for SCI is still rather unique.
The mechanical microconnector system:The mechanical microconnector system (mMS, Figure 1A) is a tool, which has been developed specifically for the purpose of reconnecting severed spinal cord tissue stumps (Brazda et al., 2013, 2016). The device was designed, developed and fabricated by a consortium of neuroscientists, trauma clinicians and microtechnological engineers. It has two main features that can be utilized during and after its implantation (https://www.dr.hut-verlag.de/978-3-8439-0774-3.html):
1) Functional surface: The highly functional surface allows for a good tissue attachment to the mMS walls. The honeycomb-shaped duct holes are the most important feature. They provide maximum stability and at the same time the maximum free surface. The honeycombs' inner surface maximizes the forces, which hold the attached spinal cord tissue in place. At the same time it minimizes the area taken up by the device. The characteristic surface structure was achieved by an advanced silicon etching process for the side walls of the silicone mold ensuring optimal adherence of the tissue to the mMS walls after implantation and negative pressure application.
2) Internal supply system: The supply system is integrated into the honeycomb structure. It comprises the generation of negative pressure in the device chamber as well as the optional pharmaceutical treatment of the injury area after fixation of the spinal cord tissue in the honeycomb structures. The tissue is sucked into the mMS with gentle negative pressure (200-250 Pa) via an external vaccum pump. The negative pressure is adjustable in order to keep the stress exerted on the tissue as low as possible. A number of suction ducts ensure an even pressure distribution in the chamber and prevent blockage (caused by,e.g., premature forming of tissue bridges at the inlet). Attached to the duct for medicinal applications is a branched system/framework of very small channel structures. If intended, and depending on its respective mechanism of action, local drug treatment of the lesion core via this micro channel system can be provided either acutely (at the time of mMS implantation) or delayed. In either case, osmotic minipumps can also be attached to the mMS to allow medical manipulation of the lesion area over longer time.
Mechanical microconnector system implantation into small and large animal spinal cord lesions:Tissue adaptation with the mMS alone as a treatment for acute severe rodent SCI already achieves remarkable effects and can result in an improved locomotor functional outcome (Brazda et al., 2013; Estrada et al., 2018b).
After severe rat SCI, e.g., a complete transection of the thoracic spinal cord, the locomotor function of the hindlimbs is- if not lost completely - extremely impaired. When screening SCI literature it becomes obvious that maximum (Basso, Beattie,Breshnahan) BBB open field scores of rats with complete spinal cord transection generally range between BBB 0 (no observable hind limb movement) and BBB 3 (extensive movement of two joints of the hind limbs) for untreated control animals, and at maximum between BBB 6 (extensive movement of two joints and slight movement of the third) to BBB 8 (sweeping with no weight support/plantar placement of the paw with no weight support) for animals, which received some more or less effective treatment. The mMS implantation after complete spinal cord injury of the rat resulted in a thus far unmet locomotor functional recovery up to a BBB score of 10 (occasional weight supported plantar steps, no coordination) to 11 (frequent to consistent weight supported plantar steps and no forelimb-hindlimb coordination) (Estrada et al., 2018b). The range between BBB 8 to 11 is at present the “magical border/limit” for functional recovery after complete thoracic SCI in rat, which no existing SCI therapy can exceed. By using a modified version of the BBB open field test (the standard open field test for spinal cord injured rats)the frequency of certain gait parameters of the lower level of the BBB can be taken into account. Thereby a more precise distinguishment of hindlimb movements is possible reflecting low and intermediate BBB scores at a finer resolution. Both the animals'daily motivation and their muscle strength or the loss of either(due to muscle atrophy resulting from non-use of affected muscles) can, of course, hardly be influenced by the mMS nor any other local treatment/manipulation of the SCI lesion site. However, the mMS facilitates axonal regeneration across the injury area and additional rehabilitative treatment components such as, e.g., treadmill training and hind limb stretching can further increase the functional outcomes.
In comparison to rats, pigs need to support a higher bodyweight. They also naturally have the respective muscle mass for gait performance. Granted that the rate of muscle atrophy after SCI is comparable in both animal species, the task of achieving weight-supporting stepping even after a severe spinal cord transection is a challenging task, which will require combinatorial therapeutic treatment involving surgical, mechanical and rehabilitational measures. Depending on the susceptibility to a therapy, individual motivation and most likely other internal and external influences, there are good and poor performers in animal studies - not unlike it is the case also for human patients. This is also one of the reasons why SCI in particular will require personalized therapies in order to meet the need of every patient. The mMS is an ideal tool for personalized treatment because it can easily be adapted to any size and shape of defect and its internal microchannel system can be used for any kind of medication, which requires/involves the application of pharmaceutical drugs or cellular therapeutics into the lesion core(Estrada and Muller, 2016).
The stabilization of the injured spinal cord has an immensly positive effect on the gross anatomical SCI outcome in both rats and pigs. In the case of severe rat SCI both a flattening of the spinal cord tissue adjacent to the lesion center as well as the formation of large cysts in the lesion core are significantly reduced following acute mMS implantation. In the minipig transection SCI study (Estrada et al., 2018a) flattening of the spinal cord was not observed (Figure 1B), which might be explainable by the difference in diameter between these two animal species.The minipig spinal cord anatomy resembles human spinal cord more closely than rat spinal cord. The unavoidable maniluplation of the affected area is more likely during the microsurgical operation of the small rat spinal cord compared to “regular sized” surgical SCI intervention in pigs. Control minipigs that underwent only the complete thoracic spinal cord transection without subsequent mMS implantation revealed extremely large cysts in the lesion core, an outcome, which is similar to that after human SCI (Figure 1B). The mMS implanted minipigs,however, revealed significant degrees of tissue protection in the injury area because the device could generally prevent such strong cyst formation (Figure 1B). Thus, the importance of tissue preservation and stabilization by the mMS device could so far be demonstrated in both a rodent model and also in a preclinical large animal model of severe SCI.
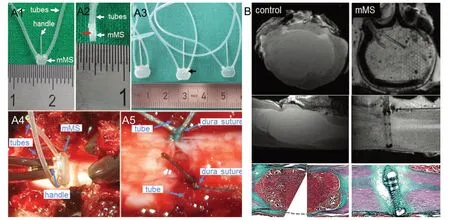
Figure 1 Mechanical microconnector system (mMS) and mMS implantation.(A) mMS for different animal species, and mMS implantation into the porcine spinal cord. (A1) Rat mMS with attached tubes and handle, front view; note the honeycomb structures of the mMS. (A2) rat mMS, side view, note the middle line (red arrow) where the two matching halfs of the mMS are attached. The thickness of both rat and minipig mMS devices is 1 mm. (A3) Minipig mMS, front view; arrow marks internal microchannel system. Minipigs displayed a larger variation in spinal cord diameters than the small rodents. Different mMS sizes were fabricated to allow high prescision fit. Note the size differences between rat and minipig mMS devices. (A4) mMS before SCI and implantation into the porcine spinal cord.(A5) Minipig lesion area after SCI and subsequent mMS implantation and suture of the dura. Tubes and handle are removed after mMS fixation,dura closure and vacuum application. Numbers on rulers in A1-A3 represent cm distances. (B) Minipig lesion area after complete thoracic spinal cord transection without (6 weeks post-operation, left side) and with (right side, 1 week post operation) subsequent mMS implantation. Black and white images: ex-vivo 7T MR images: Top row: coronal orientation, middle row: longitudinal orientation. The majority of the spinal cord tissue has been affected by the severe injury in the control animal, resulting in the formation of a large cavity in the lesion core. The mMS implantation prevents the large cyst formation in the spinal cord tissue. Note the good fit of the mMS in the spinal cord. The coronal MRI demonstrates that the mMS lumen is filled with spinal cord tissue. Moreover, both the internal microchannel system and the honeycomb structures in the mMS are clearly visible. Color Images (bottom row): Trichrome stain, due to methodological limitations the tissue of the lesion area of the control animal(left) was embedded and cut in two parts. The large cystic cavity is entirely filled with gelatin (from the embedding process), which is also stained strongly during the trichrome stain. Although a notable collagen (green) scar formation has occurred around the implant after mMS implantation,the spinal cord integrity is restored to a large extent. Modified from Estrada et al. (2018a).
On-going long-term minipig studies will need to show whether the mMS implantation can achieve a comparable functional outcome as it has been described for the rat model of complete transection SCI. First histological results from a short term (one to six weeks) minipig study are quite promising and confirm those of the previous rat studies: Regenerative axon growth of both descending motor and ascending sensory axons into and even beyond the mMS implant was promoted even as early as six weeks post implantation after acute complete thoracic spinal cord transection (Estrada et al., 2018a).
Currently, the mMS is implanted for the proper reconnection and readaptation of severed tissue stumps after SCI. However, in proof-of-principle studies the microchannel infusion system has so far also been successfully tested in the rat model by infusion of liquids (dyes and protein enzyme) into the lesion core (Brazda et al., 2013). To include the drug infusion feature into a medical intervention, it is important besides finding the ideal concentration and dosage, to work out optimal parameters regarding the timing of single or repetitive infusion treatments depending on the mechanism of action of the drug.
The mechanical microconnector system - a versatile tool for various treatment possibilities:Although mMS is initially developed specifically for the adaptation of severed spinal cord tissue stumps, its application is by no means limited to SCI alone. It is versatile and offers the possibility to adapt any kind of tissue after its implantation into an area of tissue defect. The mMS devices, which have been used so far, were made of the highly biocompatible material poly(methyl methacrylate). This material is already used in various clinical applications and has proven to be a generally safe material for medical applications.Poly(methyl methacrylate) is, however, a ridgid material, which is not or only minimally absorbed by the body. In order to avoid complications, which might arise from the rigid implant after longer times, on-going studies also include the use of bioresorbable mMS devices and three-dimensional printing approaches for their fabrication.
This work was supported by the DGUV (Deutsche Gesetzliche Unfallversicherung), BMBF (German Federal Ministry for Education and Research), DSQ (German Paraplegia Foundation),Manchot Foundation and Research Commission of the Medical Faculty of the Heinrich-Heine-University Düsseldorf.
Parts of the minipig experiments mentioned in this article were presented at the Society for Neuroscience meeting in San Diego in 2018 (Estrada et al., 2018a).
Veronica Estrada, Hans Werner Müller*Molecular Neurobiology Laboratory, Department of Neurology,Heinrich-Heine-University Medical Center Düsseldorf, Düsseldorf,Germany
*Correspondence to: Hans Werner Müller, PhD,hanswerner.mueller@uni-duesseldorf.de.
orcid: 0000-0001-6352-1418 (Hans Werner Müller)
Received: March 30, 2019
Accepted: May 17, 2019
doi: 10.4103/1673-5374.259605
Copyright license agreement:The Copyright License Agreement has been signed by both authors before publication.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewer:Eduardo Puelles, Universidad Miguel Hernandez, Spain.
杂志排行
中国神经再生研究(英文版)的其它文章
- Etomidate affects the anti-oxidant pathway to protect retinal ganglion cells after optic nerve transection
- Normal tension glaucoma: from the brain to the eye or the inverse?
- Mesenchymal stromal cell therapy for damaged retinal ganglion cells, is gold all that glitters?
- MicroRNAs as biomarkers of diabetic retinopathy and disease progression
- Diabetic neuropathy research: from mouse models to targets for treatment
- Potential therapeutic roles of retinoids for prevention of neuroinflammation and neurodegeneration in Alzheimer's disease