New perspectives in iron chelation therapy for the treatment of Parkinson's disease
2019-07-18MarcoT.Nuez,PedroChana-Cuevas
Neurodegenerative diseases with an iron accumulation component:A wide variety of neurological diseases are characterized by the accumulation of iron in different areas of the central nervous system, include Parkinson's disease, Huntington disease, Alzheimer's disease, Friedreich's ataxia, amyotrophic lateral sclerosis,pantothenate kinase-associated neurodegeneration and other neuropathologies associated with brain iron accumulation (Hayflick et al., 2018).
In this perspective, we focus on Parkinson's disease, a neurodegenerative disorder characterized by the death of dopaminergic neurons of the sustantia nigra pars compacta, which results in the decreased release of the neurotransmitter dopamine at the centers of movement control in the striatum. These neurons are particularly vulnerable to degeneration because of their extensive branching and the large amounts of energy required to keep ionic gradients along this network: a prototype sustantia nigra dopaminergic neuron is estimated to have an average total neuritic tree length of about 4.5 meters and to give rise to 1-2.4 million synapses (Mamelak, 2018).
Iron accumulation and iron-induced damage in idiopathic Parkinson's disease:Idiopatic Parkinson's disease is the best characterized form of this disease in which a link between iron accumulation and its associated pathology has been established (Ward et al., 2014). In dopaminergic neurons, iron acts as a double-edged sword. These neurons require an ample supplement of iron for energy production in the mitochondrion and for dopamine synthesis.Notwithstanding, iron in its redox-active form reacts with hydrogen peroxide produced by dopamine catabolism and mitochondria as a byproduct of the electron transport chain, generating through the Fenton reaction the noxious hydroxyl radical. The Fenton reaction is a fast, thermodynamically favorable nonenzymatic reaction that obeys mass action law. These characteristics of the reaction result in a direct relationship between the concentration of redox-active iron and the production of hydroxyl radical (Uranga and Salvador, 2018).
Idiopathic Parkinson's disease treatment:The introduction of levodopa, the natural precursor of dopamine, was the most important milestone in the current therapy paradigm of idiopathic Parkinson's disease. In the central nervous system levopoda generates dopamine through the enzyme 3,4-dihydroxyphenyl-l-alanine(DOPA) decarboxylase. The association of levodopa with a peripheral DOPA decarboxylase inhibitor (carbidopa or benserazide),increases the bioavailability of cerebral dopamine especially at the striatal level, and substantially improves the tolerance of patients to levodopa treatment. The combination of levodopa/carbidopa or benserazide is currently the most potent therapy for treating idiopathic Parkinson's disease patients, whom when receiving this treatment experience an improvement in motor symptoms that remains stable throughout the day and persists during the first months or years of levodopa treatment (Chana, 2009). Unfortunately, this initial success decays after a few years of treatment due to the development of motor and mental complications, which do not respond to levodopa therapy. Among the alternative therapies available, good control of motor symptoms is achieved using dopamine agonists, MAOB and COMT inhibitors (https://www.mayoclinic.org/diseases-conditions/parkinsons-disease/diagnosis-treatment/drc-20376062). Alternative treatments include deep brain stimulation and spinal cord stimulation.
Considering the neurodegenerative nature of Parkinson's disease,and the purely symptomatic treatment of current therapies, the first question we must ask ourselves is how to advance from symptomatic to neuroprotective treatments.
The current standard for the validation of a neuroprotective effect is a delayed-start design, in which the Unified Parkinson's Disease Rating Scale score is used as the dependent variable (Figure 1A). A neuroprotective effect is evidenced if at month 9 of treatment the curve presents differences between the early onset (9-month treatment) and the delayed onset (6-month treatment) groups. If these curves come together the treatment effect is just symptomatic. In all groups there may be an initial small improvement due to a placebo effect.
To date, no neuroprotective treatment with demonstrable efficacy has been demonstrated, although its search is a very active focus of current research (Kulisevsky et al., 2018). Because of the heterogeneous and multifactorial nature of the disease, probably there is no single treatment that will stop the neurodegenerative process. Nevertheless, the earlier we can intervene the progress of the disease,ideally in pre-clinical stages, the greater the probability of success.A recent analysis suggests that all attempts to develop an effective therapy have failed because the molecular determinants of the disease will manifest differentially in each individual patient. The authors propose the use of drug cocktails to attack different molecular targets, and the selection of sub-populations of patients enriched in the intended drug target (Lang and Espay, 2018).
Clinical trials using iron chelation:Positive experiences validate the use of iron chelation therapy for the treatment of systemic diseases such as thalassemia major, sickle cell disease and cardiomyopathy associated with hereditary hemochromatosis. Recently, iron chelation has been introduced as a new therapeutic concept for the treatment of neurodegenerative diseases that have a component of iron accumulation (Nunez and Chana-Cuevas, 2018).
A current search in (https://clinicaltrials.gov.) yielded 16 ongoing or finished trials of iron chelation for the treatment of neurodegenerative diseases: 5 trials for Parkinson's disease, 3 for Friedreich's ataxia, 3 for amyotrophic lateral sclerosis, 1 for mild Alzheimer's disease, 2 for pantothenate kinase-associated neurodegeneration and 2 for neurodegeneration with brain iron accumulation. These numbers disclose the present times relevance of the use of iron chelation therapy for the treatment of neurodegenerative diseases that have an iron accumulation component.
Until now, the results on the use of chelators for the treatment of neurodegenerative diseases are largely inconclusive. Clinical trials using the iron chelator deferiprone for the treatment of Parkinson's disease almost unanimously draw the same conclusion: results in slowing disease progression are sufficiently significant to prompt larger studies that might provide clinical benefits to Parkinson's disease patients (Devos et al., 2014). In this respect, a probe of concept that iron chelation therapy is here to stay is the FAIR PARK II trial,now in the recruiting phase (www.fairpark2.eu). The FAIR PARK II is a large (338 patients) phase 2 European clinical trial aimed at evaluating whether deferiprone can slow Parkinson's disease progress in patients (Moreau et al., 2018).
Putative neuroregenerative properties of iron chelators:Progressive neuronal death in Parkinson's disease occurs by a process of dying-back, characterized by progressive neurite loss and axon shortening; the death of the neuronal body is the last step in this process. Accordingly, at the onset of motor symptoms, the loss of dopaminergic fiber density in the dorsal putamen was found to be considerably larger compared to the loss of neuron bodies in the sustantia nigra (Grosch et al., 2016). If cell body death is delayed compared to axonal degeneration, alive but dysfunctional neurons could be rescued by neuritogenic stimuli.
Only a few studies have reported the putative capacity of iron chelators to generate neurite growth. In recent years, the group of Moussa Youdim at Technion - Israel Institute of Technology,has developed a family of iron chelating agents, represented by M30. M30 stabilizes hypoxia-inducible factor-1α, by inactivating the prolyl hydrolase that initiates its degradation, which activity depends on oxygen and Fe. In cell systems, the stabilization of hypoxia-inducible factor-1α by M30 induces the expression of the neurotrophic factors glial cell line-derived neurotrophic factor and brain-derived neurotrophic factor. M30 treatment also induces survival signaling factors such as protein kinase C, extracellular signal-regulated kinase 1/2, Akt and GSK-3β. Therefore, by chelating Fe, M30 has a large neuroprotective effect through stabilization of hypoxia-inducible factor-1α.
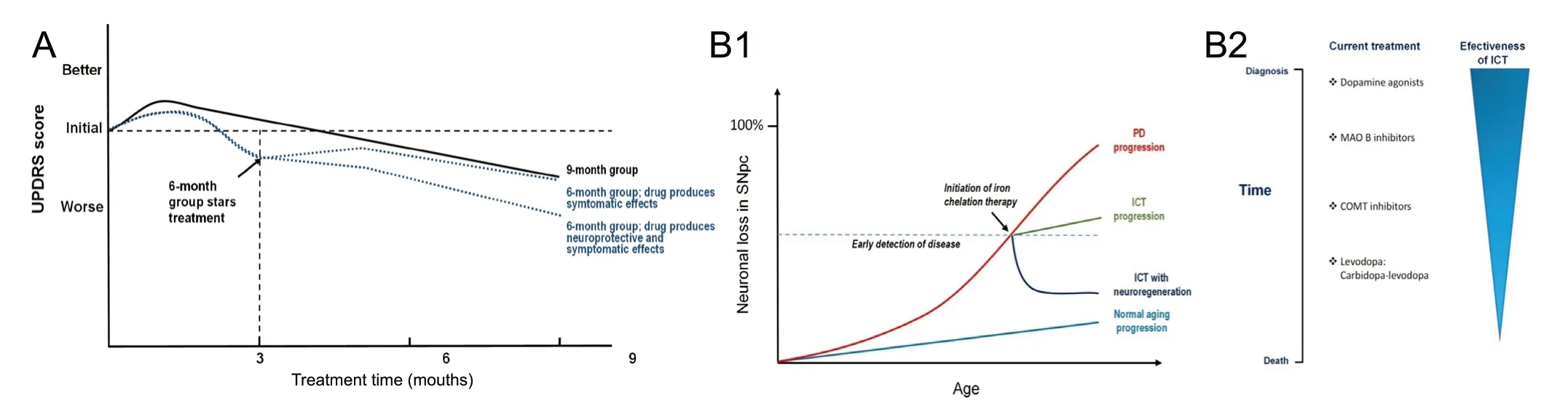
Figure 1 Delayed-start design for new drug validation for the treatment of idiopathic Parkinson's disease (PD) and putative relevance of iron chelation therapy (ICT) as a function of disease progress.(A) Schematic representation of delayed-start design trials in idiopathic PD. A delayed-start design trial is usually applied to untreated patients in the symptomatic stages of the disease. Patients are randomized into two groups, one in which the treatment is maintained for 9 months (T1) and the other in which the treatment is delayed for 3 months and then is maintained for the next 6 months (T2). During the first 3 months the T2 group is treated with placebo. Figure modified from (Clarke, 2008). (B1) Hypothetical time progression of neuronal loss in sustantia nigra without and with iron chelation therapy. Light blue line: normal neuronal loss due to aging; red line: neuronal loss in PD; green line: rate of neuronal loss if iron chelation therapy decreases PD neuronal loss rate; dark blue line: rate of neuronal loss if iron chelation therapy is neuritogenic. Bulk graph parameters based on (Lang, 2007). (B2) Since the neuroregenerative effect of iron chelation is effective on alive neurons, the relevance of ICT decreases with disease progression. Thus, treatment upon early symptoms detection is advisable.
If the capacity of iron chelators to produce neuritogenesis holds in in vivo treatments, the expected effect of iron chelation therapy will be a slow-down of the neurodegeneration process (Figure 1B1). Moreover, if connections between damaged neurons in the substantia nigra and movement control centers in the striatum are reestablished, then a reverse of the neurodegenerative process is to be expected. Considering the process of dying-back, the earlier the iron chelation therapy starts the better will be the neuroregenerative outcome (Figure 1B2).
Perspective: Iron chelation therapy offers the chance of providing a critical change in the paradigm for the treatment of Parkinson's disease, from a symptomatic treatment to another that slows or stops the disease progression. Nevertheless, because of the multifactorial nature of the disease, a “single target drug”, as an iron chelator,may not be sufficient to induce complete neuroprotection. In this context, clinical evaluation of multifunctional iron chelators that in animal models slow or deter the neurodegenerative process is needed (Nunez and Chana-Cuevas, 2018). Also needed is an early diagnosis of the disease, since the neuroprotective effects of these compounds will be higher if treatment starts when neuronal death is lower.
This work was supported by FONDEF 17I10095 grant from Comisión Nacional de Investigación Científica y Tecnológica (CONICYT, to MTN).
Marco T. Nuñez*, Pedro Chana-Cuevas Faculty of Sciences, Universidad de Chile, Santiago, Chile(Nuñez MT)Movement Disorders Center, Universidad de Santiago de Chile,Santiago, Chile (Chana-Cuevas P)
*Correspondence to: Marco T. Nuñez, PhD, mnunez@uchile.cl.
orcid: 0000-0002-1967-8570 (Marco T. Nuñez)
Received: March 30, 2019
Accepted: April 15, 2019
doi: 10.4103/1673-5374.259614
Copyright license agreement:The Copyright License Agreement has been signed by both authors before publication.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewers:Mónica Sanchez, Universidad Nacional de Córdoba,Argentina; Basant K. Puri, Imperial College London, London, UK.
杂志排行
中国神经再生研究(英文版)的其它文章
- Etomidate affects the anti-oxidant pathway to protect retinal ganglion cells after optic nerve transection
- Normal tension glaucoma: from the brain to the eye or the inverse?
- Mesenchymal stromal cell therapy for damaged retinal ganglion cells, is gold all that glitters?
- MicroRNAs as biomarkers of diabetic retinopathy and disease progression
- Diabetic neuropathy research: from mouse models to targets for treatment
- Potential therapeutic roles of retinoids for prevention of neuroinflammation and neurodegeneration in Alzheimer's disease