Antibody-based immunotherapies for Parkinsonian syndromes
2019-07-18LarsTnges,MariaAngelaSamisZella
What is the rationale for immunotherapies in Parkinsonian syndromes (PS)? PS are neurodegenerative diseases which are clinically characterized by a hypokinetic phenotype in combination with additional motor and non-motor symptoms. One major pathological hallmark of all PS consists of a non-physiological detrimental accumulation of protein aggregates which appear intracellularly in neurons and glial cells but also in the extracellular space (Wong and Krainc, 2017). Depending on the pathogenic protein, PS can be divided into synucleinopathies, characterized by aggregation of the protein alpha-Synuclein (aSyn), and tauopathies, characterized by aggregation of the protein Tau (Levin et al., 2016; Poewe et al., 2017).Clinical syndromes of synucleinopathies include Parkinson's disease(PD), multiple system atrophy (MSA) and dementia with Lewy bodies, and tauopathies include progressive supranuclear palsy (PSP)and corticobasal degeneration.
At the neuropathological level, the progression of the disease is associated with a spread of the pathogenic agents aSyn or Tau, which disseminate in different patterns in the central nervous system (Levin et al., 2016). Importantly, not only neuronal cell populations but also glial cells are affected and exhibit specific cellular responses to aggregated proteins that can be subsumed as neuroinflammatory reactions(Zella et al., 2019).
The identification of the disease-causing agents and their pathophysiologic importance have led to the development of immunotherapeutic approaches that target the proteins and try to reduce or even halt the spread of disease. It has recently been shown that immunotherapies effectively reduce the burden of disease and lead to improved motor function in animal models of synucleinopathies and tauopathies. In initial human trials, the drugs showed good safety and tolerability and are considered to be promising disease modifiers for PS (Zella et al., 2019).
The immunotherapies for neurodegenerative PS a) represent a novel curative therapeutic approach to the disease, which has long been awaited, b) target central nervous system neuroinflammation,c) can be monitored for their effectiveness in reducing the activity of glial cell populations and d) reduce the load of pathogenic protein aggregates.
What types of immunotherapy are currently being studied and what are safety issues?The two principal types of anti-Parkinsonian immunotherapy are active immunization or vaccination, which recruits the immune system to generate itself antibodies against a protein, and passive immunization, which consists of the administration of antibodies directed against different domains of the particular protein (Zella et al., 2019).
Possible side effects of both active and passive immunization are local or systemic inflammatory reactions as well as allergenic reactions. In earlier trials for other neurological diseases like Alzheimer's disease active immunization with pre-aggregated amyloid-beta(1-42), the adjuvant QS21 and the emulsifier polysorbate 80 had provoked severe adverse reactions such as aseptic meningoencephalitis. It was supposedly caused by a strong shift from a Th2 humoral response to a proinflammatory Th1 response. Next-generation amyloid-beta vaccination trials were designed to target more specifically pathological conformations and used other adjuvants - so far without a severe side reaction as found in the first trials. Peer-reviewed study data from phase I trials in PS with active immunization against aSyn or Tau have not been published so far.
Results of two phase I trials with a passive immunization against aSyn with the antibody PRX002 in healthy persons and PD patients have recently been presented. The primary outcome measures safety and tolerability were successfully met with different dosages of the anti-aSyn antibody and up to three intravenous infusions. Treatment was generally safe and well tolerated; no serious or severe treatment-related adverse events were reported. In several cases, constipation, infusion-related reactions, diarrhea, headache and upper respiratory tract infections occurred (Jankovic et al., 2018). There are also passive immunization trials ongoing against the protein Tau.According to phase I study data of the anti-Tau antibody ABBV-8E12 in 30 patients with PSP, it showed an acceptable safety profile with no clinically concerning trends in the number or severity of adverse events between the placebo and dosed groups. One patient encountered transient headache and one patient had an episode of agitation.Pharmacokinetic modelling showed that the antibody has a plasma half-life and cerebrospinal fluid/plasma ratio that was consistent with other humanized antibodies, and there were no signs of immunogenicity (West et al., 2017; Boxer et al., 2018).
What is the evidence from preclinical and clinical studies on passive immunization therapies?Several monoclonal antibodies targeting different epitopes of aSyn have been investigated in preclinical models of PD. The principal targets for anti-aSyn antibodies are the aSyn C-terminal region and the aSyn N-terminal region. Three C-terminally directed antibodies were administered to transgenic PD mouse models in separate studies. In all three of these, aSyn pathology was alleviated, dopaminergic cell loss and neuroinflammatory responses were reduced, and tests for motor performance were improved. N-terminally directed antibodies were studied in viral vector-based models or in mice that had been intrastriatally injected with aggregates of aSyn. The outcome was similarly positive with reduced neuronal loss and improved motor function. A third approach consists in the application of antibodies that preferentially target pathologic aggregation forms of aSyn such as oligomers, protofibrils or fibrils. Their application to transgenic mouse models has also been successful with reduction of neuronal cell loss, partial block of aSyn spreading and diminished microgliosis. These studies have all been summarized in a recent review (Zella et al., 2019).
Based on the promising results from the PD animal models, several phase I/II human trials are currently planned or are already ongoing that primarily test the tolerability and safety of these antibodies: the C-terminal and aggregation-directed humanized IgG1 monoclonal antibody PRX002 (NCT03100149), the N-terminallyand aggregation-directed fully human IgG1 monoclonal antibody BIIB054 (NCT03318523) and the oligomer/protofibril-directed monoclonal antibody ABBV-0805 (Zella et al., 2019) (Figure 1A and B). Study results have been published for earlier PRX002 studies(NCT02095171, NCT02157714) with good performance in safety and tolerability measures in a multiple ascending-dose trial in patients with early stages of idiopathic PD. Here, serum PRX002 levels increased in an approximately dose-proportional manner and could significantly reduce serum levels of free-to-total aSyn. In the cerebrospinal fluid, the PRX002 concentration increased with the PRX002 dose but reached only 0.3% relative to serum levels. Remarkably, no change in the level of cerebrospinal fluid aSyn was detected, which was attributed to the relatively low antibody affinity for monomeric,as opposed to aggregated forms of aSyn (Jankovic et al., 2018).
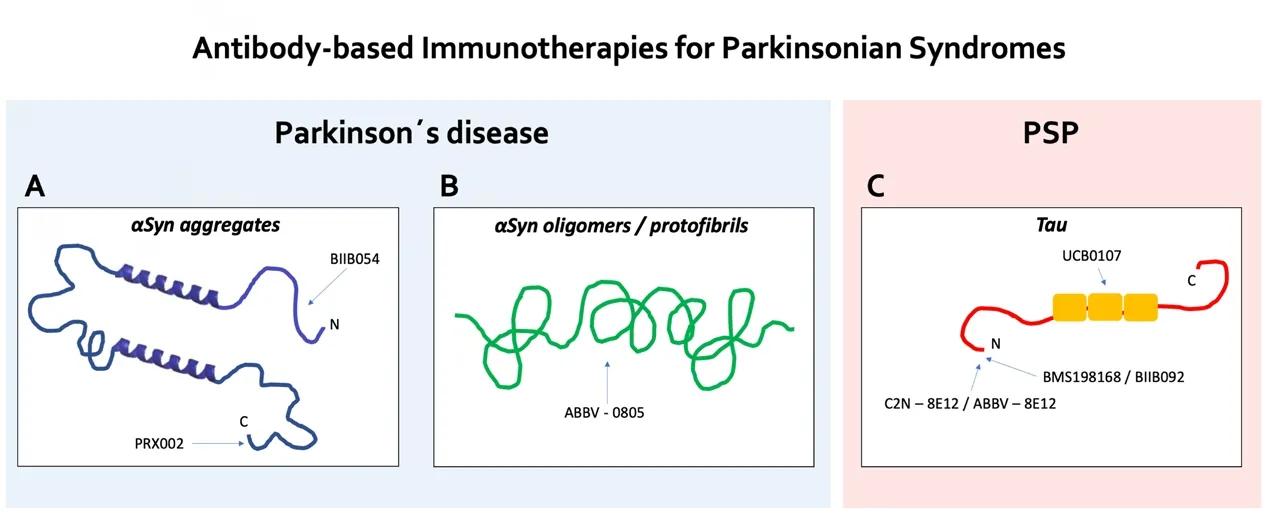
Figure 1 Schematic representation of the antibody targets for aSyn and Tau immunotherapies.(A) The humanized IgG1 monoclonal anti-aSyn antibody PRX002 is C-terminal- and aggregation-directed,the fully human IgG1 monoclonal anti-aSyn antibody BIIB054 is N-terminal- and aggregation-directed.(B) The monoclonal anti-aSyn antibody ABBV-0805 is oligomer/protofibril-directed. (C) The humanized IgG4 monoclonal anti-Tau antibodies BMS-198168/BIIB092 and C2N-8E12/ABBV-8E12 are N-terminal-directed, the monoclonal anti-Tau antibody UCB0107 targets the mid-region of the protein. aSyn:Alpha-synuclein; PSP: progressive supranuclear palsy.
There exist also preclinical models for MSA, another synucleinopathy which primarily affects oligodendrocyte cells but later also spreads to other cell types in the brain. In one study in a transgenic mouse model for MSA, a single-chain antibody against aSyn was administered via a lentiviral vector construct in combination with an anti-inflammatory agent. Positive effects on neuroinflammation and aSyn accumulation were observed and were strongest if the antibody and anti-inflammatory were combined (Valera et al., 2017). So far,no passive immunization studies in humans are underway. Only one active immunization trial was completed (NCT02270489) for which results are now expected.
PSP is a tauopathy with a 4-repeat Tau neuropathology that is clinically characterized by a variety of motor and behavioral syndromes(Boxer et al., 2018). In passive immunization studies in transgenic mouse tauopathy models, there was reduced Tau pathology and improved motor function when the antibody was given prior to the onset of pathology. In another approach, anti-Tau monoclonal antibodies were intraventricularly infused after disease onset and could markedly reduce hyperphosphorylated, aggregated, and insoluble Tau and blocked the Tau seeding activity in brain lysates (Yanamandra et al., 2013). At present, several human anti-Tau passive immunotherapy clinical trials are being conducted in PSP-RS patients with the goal to target extracellular Tau specimen and prevent transneuronal spreading. The N-terminally directed humanized IgG4 monoclonal antibodies BMS-198168/BIIB092 and C2N-8E12/ABBV-8E12 both have shown good safety and tolerability in phase I studies (West et al., 2017; Boxer et al., 2018). BMS-198168/BIIB092 was able to dose-dependently reduce free Tau in cerebrospinal fluid(NCT02460094). Both antibodies are now evaluated in phase II clinical trials (NCT03068468, NCT02985879). UCB0107 is another anti-Tau directed monoclonal antibody which targets the mid-region of the protein and is currently studied in a phase I trial (NCT03464227),a phase II study is planned (Figure 1C).
Essential requirements and unmet needs for an optimized study performance:An essential requirement for future studies with passive immunization in PS is to recruit patients who have a very precisely defined clinical syndrome that fits into our current definition of disease. It is very good that the clinical diagnostic criteria for PD and PSP have been updated recently and are under continuous validation. So far, the immunization studies have been designed to include patients in early stages of disease, which could to some extent make diagnostic certainty more difficult because symptoms may be very subtle and not specific. Therefore, the development of diagnostic but also of progression biomarkers is essential. In particular, MRI technology has made some progress lately, but biomarkers for fluids or tissues still need to be significantly improved. In the near future,nuclear medicine imaging techniques such as PET or single-photon emission computed tomography, which are currently being developed at high speed for PS, will certainly play an important role. The molecular target engagement of anti-aSyn or -Tau antibodies must be accurately demonstrated both quantitatively and qualitatively.However, if the almost ubiquitous proteins aSyn or Tau are adversely manipulated, it could also be a significant risk for severe side effects(Espay et al., 2019). It will definitively be worthwhile to closely monitor immunization techniques in other neurodegenerative diseases such as Alzheimer's disease, as Tau pathology is also crucial there. Finally, the value of preclinical therapeutic data remains to be considered with caution, since the neurodegenerative PS of humans differ in many ways from animals or disease models, and immunological mechanisms in particular differ significantly between species (Diederich et al., 2019).
Lars Tönges*, Maria Angela Samis Zella
Department of Neurology, St. Josef-Hospital, Ruhr-University Bochum, Bochum, Germany (Tönges L, Zella MAS)Neurodegeneration Research, Protein Research Unit Ruhr (PURE),Ruhr University Bochum, Bochum, Germany (Tönges L)Department of Neurology, St. Josef-Hospital, Katholische Kliniken Ruhrhalbinsel, Contilia Gruppe, Essen, Germany (Zella MAS)
*Correspondence to: Lars Tönges, MD, lars.toenges@rub.de.
orcid: 0000-0002-0815-9820 (Maria Angela Samis Zella)0000-0001-6621-144X (Lars Tönges)
Received: February 21, 2019
Accepted: April 12, 2019
doi: 10.4103/1673-5374.259613
Copyright license agreement:The Copyright License Agreement has been signed by both authors before publication.
Plagiarism check:Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewer:Aysegul Yildiz-Unal, Muğla Sıtkı Koçman University,Turkey.
Additional file:Open peer review report 1.
杂志排行
中国神经再生研究(英文版)的其它文章
- Etomidate affects the anti-oxidant pathway to protect retinal ganglion cells after optic nerve transection
- Normal tension glaucoma: from the brain to the eye or the inverse?
- Mesenchymal stromal cell therapy for damaged retinal ganglion cells, is gold all that glitters?
- MicroRNAs as biomarkers of diabetic retinopathy and disease progression
- Diabetic neuropathy research: from mouse models to targets for treatment
- Potential therapeutic roles of retinoids for prevention of neuroinflammation and neurodegeneration in Alzheimer's disease