Inducing α-synuclein compaction: a new strategy for inhibiting α-synuclein aggregation?
2019-07-18FranciscaPinheiro,Salvadorventura
Proteins might misfold during translation and folding or even once they are in their native states, due to stochastic fluctuations, destabilizing mutations or cellular stress. Aberrant protein species are usually detected and either refolded or cleared by the protein quality control machinery(Ciechanover and Kwon, 2015). When misfolded protein conformers cannot be degraded, they tend to self-assemble to form aggregates, a characteristic of many neurodegenerative diseases.
Aggregated α-synuclein (α-syn) is the main component of Lewy bodies and Lewy neurites, proteinaceous inclusions that constitute the major histopathological hallmarks in Parkinson's disease, the second most common neurodegenerative disorder after Alzheimer's disease (Spillatini et al., 1997). α-syn is a small protein, highly abundant in the presynaptic terminal of dopaminergic neurons, with a high conformational plasticity. The precise role of α-syn remains unknown, but several functions have been ascribed to this polypeptide, including the regulation of the presynaptic vesicular pool and the protection of nerve terminals against injury. In terms of structure, it is largely believed that monomeric α-syn behaves as an intrinsically disordered protein under physiological conditions in the neuronal cytosol. This unordered conformation shifts to an amphipathic helical structure upon binding to membranes (Uversky and Eliezer, 2009). Moreover, it has been reported that α-syn can occur also as an alpha-helical folded tetramer under native conditions (Bartels et al., 2011), but the prevalence of this assembly is still under debate.Although the precise cause of Parkinson's disease onset remains to be deciphered, α-syn is seen as one of the most compelling targets for therapeutic intervention. Indeed, interfering with the conversion of the above described functional α-syn conformations into misfolded ones able to self-assemble and form toxic amyloid aggregates within dopaminergic neurons appears as one of the most promising strategies to fight this devastating disease.
α-syn primary structure consists of the N-terminal domain, a central region known as the non-amyloid β component (NAC), and the C-terminal domain (Uversky and Eliezer, 2009). The N-terminal domain(residues 1-60) contains an imperfect conserved repeat KTKGEV and it acts as the membrane anchor region of α-syn (Fusco et al., 2014).The NAC domain (residues 61-95) is highly hydrophobic and prone to aggregation, being responsible for the ability of α-syn to undergo a conformational change from random coil to β-sheet structure. Additionally,it is thought that this domain regulates the affinity of α-syn for cellular membranes (Fusco et al., 2014). Finally, the C-terminal domain (residues 96-140) is rich in acidic and disorder-promoting residues, counteracting the tendency to aggregate of the NAC domain.
In vitro, the kinetics of α-syn amyloid fibril formation is best described by a nucleation/polymerization reaction that renders the typical sigmoidal curve, in which an initial lag phase is followed by an exponential phase, ending on a plateau. The formation of the nuclei is the rate limiting step of the reaction and accounts for the lag phase. Nuclei are the smallest stable aggregates for which growth by monomer incorporation becomes faster than monomer dissociation. Monomer association to the fibril ends occurs during the elongation phase, until the depletion of monomers leads to a decline in the rate of polymerization and, ultimately, to the plateau phase (Uversky and Eliezer, 2009). Much effort has been devoted to understanding the structural properties of the final amyloid assemblies, especially in the last years, with the emergence of high resolution cryo-electron microscopy. We begin to have atomic information on the molecular determinants of fibrils structure and stability. However, we know little on the conformational features of the ensemble of on-pathway and off-pathway oligomeric and proto-fibrillar assemblies that populate the early stages of fibrillogenesis. The identification of these species is extremely important as it may help in the early diagnosis of the disease. Additionally, accumulating evidence suggests that the oligomers, rather than the mature fibrils, may be the neurotoxic species (Winner et al., 2011).
The lack of a well-defined native structure in monomeric α-syn and the lack of high-resolution structures of α-syn oligomers poses an obstacle for the use of rational drug design to target the early events of α-syn aggregation. Recently, a high-resolution three-dimensional structure of a fibril formed by full-length human α-syn was solved by solid-state nuclear magnetic resonance, revealing that the NAC domain constitutes the core of the fibril and arranges into a Greek-key β-sheet motif (Tuttle et al., 2016). This motif was also observed in the structure of two α-syn fibrils determined by cryo-electron microscopy. To investigate if the Greek-key β-sheet motif already exists at the early stages of aggregation Carija et al. (2019) conducted a study in which they engineered an artificial disulfide bound between two residues within the NAC domain, which will thermodynamically favor the formation of the Greek-key β-sheet in the mutant protein (α-synCC), if this motif is populated in the initial assemblies (Figure 1). Importantly, they confirmed that the double cysteine mutation did not alter the predicted intrinsic aggregation propensity, hydrophobicity and disorder properties of the sequence, when compared with that of the wild type protein (wt α-syn). On the other hand, the authors verified that, as intended, the two proteins were conformationally distinct, with α-synCC adopting a more compact conformation. This conformational difference impacted dramatically the aggregation behavior of the proteins. The impact of the introduced conformational restraint was assayed in different conditions since α-syn is known to adopt a variety of structures in the aggregated state, depending on its microenvironment. These α-syn assemblies behave as strains, being able to propagate their structural imprints to endogenous α-syn. The existence of such strains might well account for the different pathologies seen in the synucleinopathies and even among individuals affected by Parkinson's disease (Bousset et al., 2013).In agreement with previous observations, Carija et al. (2019) observed that α-syn aggregation was influenced by pH and ionic strength, originating strains with distinct conformations, able of being internalized by human cells. Furthermore, the aggregation of wt α-syn and α-synCC in the same conditions was also different. α-SynCC aggregation was always lower than the one of wt α-syn, but the impact of the disulfide bridge was particularly significant at acidic pH (5.0). While wt α-syn showed the highest aggregation propensity at acidic pH, α-synCC did not aggregate at this pH, even after 14 days. Accordingly, human H4 cells incubated with α-synCC presented only small size intracellular inclusions, in contrast to the large aggregates observed for wt α-syn.The high aggregation of wt α-syn at acidic pH is due to a decrease in its net charge [isoelectric point (pI) = 4.67] and, consequently, in the electrostatic repulsions that help keeping the protein soluble. However,contrarily to what was assumed, this is not the only determinant and Carija et al. (2019) demonstrated that, even in this pro-aggregational conditions, the initial structure of the protein determines its ability to aggregate. This conformational control on the aggregating properties of the proteins was observed in all the assayed conditions, which indicates that is independent of the environment. This effect ultimately relies on the degree of exposure and flexibility of the hydrophobic and aggregation-prone NAC-domain in any given condition.
Remarkably, not only α-synCC was aggregation incompetent, but when mixed with wt α-syn it delayed its aggregation, in a concentration-dependent manner. At equimolar wt α-syn:α-synCC concentrations, the lag phase was approximately 2 hours longer, but delays were already observable at substoichiometric rates, suggesting that α-synCC binds to higher-order α-syn assemblies, like oligomers, the fibril surface or at the fibril ends. At long incubation times, all the samples eventually formed fibrils, but the morphology of the resulting aggregates was highly influenced by the presence of α-synCC, originating smaller and less ordered aggregates. Because α-syn interaction with membranes appears to be involved both in function and disease and the NAC domain participates in this process, Carija et al. (2019) tested if the aggregation of α-synCC in the presence of membranes was also compromised.They observed that α-synCC aggregation, which was already low in the absence of lipids, was strongly inhibited by its presence. Therefore, the authors concluded that having an accessible and flexible NAC domain is also essential for lipid mediated α-syn aggregation. Finally, they evaluated if the oligomers produced by wt α-syn and α-synCC were toxic for neuroblastoma cells. The oligomers were generated in conditions reported to form toxic species and, while this was true for wt α-syn oligomers,α-synCC oligomers did not affect cell viability. Since the morphological characteristics of the oligomers did not seem to explain this difference,as they were mostly similar, the authors proposed that the monomers interact through the NAC domain to form oligomers with a toxic structure, an observation that is also supported by other studies.
The data collected by Carija et al. (2019) converge to demonstrate that the Greek-key β-sheet motif does not exist in the early stages of aggregation and, thus, cannot be used for rationally designing therapeutic drugs or diagnostic tools, as it was previously proposed. On the other hand,the authors hypothesized that limiting the exposure of the NAC domain in soluble α-syn could be a good strategy to inhibit aggregation. In fact,it has been observed that naturally occurring interactions between the C-terminal part of the NAC domain and the C-terminal α-syn domain,as well as between the C-terminal and the N-terminal domains, protect monomeric α-syn from aggregation. When these interactions are perturbed, the NAC domain is exposed and aggregation proceeds (Bertoncini et al., 2005). The results of Carija et al. (2019) could explain why some molecules that inhibit α-syn aggregation were found to induce protein compaction. This is the case of epigallocatechin-3-gallate, which is known to inhibit α-syn fibrillogenesis while altering the course of the reaction towards the formation of off-pathway oligomers that are not toxic (Konijnenberg et al., 2016). In line with this hypothesis, the appearance of non-toxic oligomers was also observed with α-synCC (Carija et al., 2019). Interestingly, dopamine interaction with α-syn originates oligomers with significant toxicity and it was observed to promote α-syn extension (Konijnenberg et al., 2016). Another example where α-syn compaction and aggregation inhibition appear hand in hand is that of cyclized nordihydroguaiaretic acid analogs (Daniels et al., 2019). These molecules induce the compaction of monomeric α-syn in a concentration-dependent manner, reducing its aggregation. Remarkably, upon nordihydroguaiaretic acid treatment not only α-syn becomes resistant to aggregation, but it is able to prevent aggregation of non-incubated α-syn,similarly to what Carija et al. (2019) reported for α-synCC and wt α-syn(Figure 2). Promoting α-syn compaction was also proposed to explain why arginine hinders α-syn aggregation, both in vitro and in living cells(Ghosh et al., 2018). These evidences together with the study of Carija et al. (2019) we discuss here prompts the development of therapies aimed to reduce the exposure and flexibility of the NAC domain and opens new avenues for the treatment of Parkinson's disease.
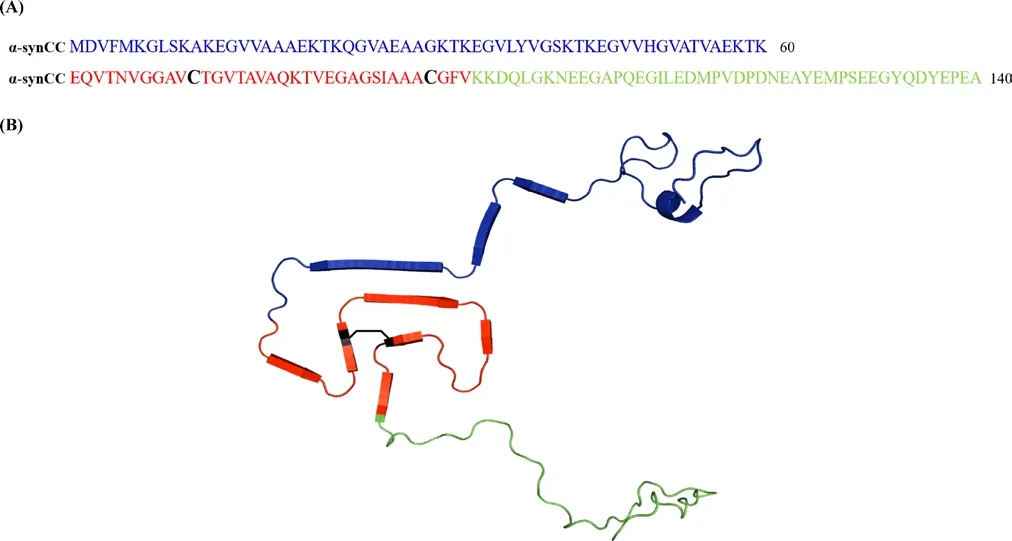
Figure 1 Sequence and structure of the disulfide bridge containing variant (α-synCC).(A) Primary sequence of α-synCC, evidencing the N-terminal domain(in blue), the non-amyloid β component (NAC) domain (in red) and the C-terminal domain (in green). The NAC domain contains the two mutated residues in positions 71 (Val to Cys) and 92 (Thr to Cys) (in black). (B) Cartoon representation of α-synCC three-dimensional structure with the different regions colored as before. The black line indicates the formation of the disulfide bond between Cys71 and Cys92.
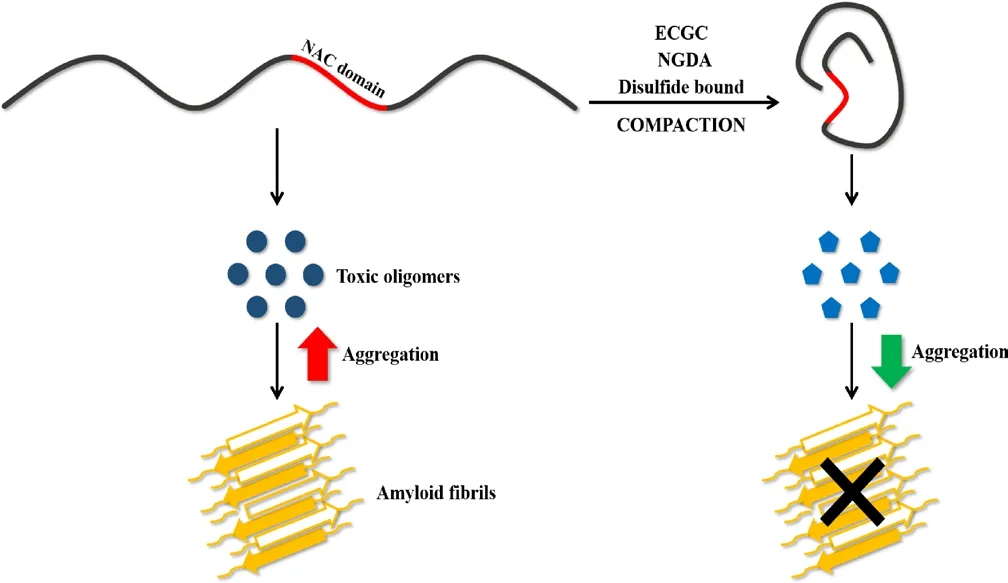
Figure 2 Schematic representation of the effect of α-synuclein (α-syn)compaction on its aggregation reaction.When the non-amyloid β component (NAC) domain (in red) is exposed and flexible, α-syn generates toxic oligomers that evolve into the formation of amyloid fibrils. α-syn compaction upon incubation with epigallocatechin-3-gallate (ECGC) or nordihydroguaiaretic acid(NGDA) or by introduction of an intramolecular disulfide bound leads to the production of non-toxic oligomers and a significant decrease in fibrillogenesis.
Francisca Pinheiro, Salvador Ventura*Institut de Biotecnologia i Biomedicina, Universitat Autònoma de Barcelona, Bellaterra, Spain; Departament de Bioquímica i Biologia Molecular, Universitat Autònoma de Barcelona, Bellaterra, Spain
*Correspondence to: Salvador Ventura, PhD,
salvador.ventura@uab.es.
orcid: 0000-0002-9652-6351 (Salvador Ventura)
Received: March 27, 2019
Accepted: May 5, 2019
doi: 10.4103/1673-5374.259608
Copyright license agreement:The Copyright License Agreement has been signed by both authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewers:Panteleimon Giannakopoulos, Hôpitaux Universitaires de Genève, Switzerland; Syed Faraz Kazim, Icahn School of Medicine at Mount Sinai, USA.
Additional file:Open peer review report 1.
杂志排行
中国神经再生研究(英文版)的其它文章
- Etomidate affects the anti-oxidant pathway to protect retinal ganglion cells after optic nerve transection
- Normal tension glaucoma: from the brain to the eye or the inverse?
- Mesenchymal stromal cell therapy for damaged retinal ganglion cells, is gold all that glitters?
- MicroRNAs as biomarkers of diabetic retinopathy and disease progression
- Diabetic neuropathy research: from mouse models to targets for treatment
- Potential therapeutic roles of retinoids for prevention of neuroinflammation and neurodegeneration in Alzheimer's disease