Therapeutic implications of advanced age at time of spinal cord injury
2019-07-18AndrewN.Stewart,JohnC.Gensel,BeiZhang
A recent demographic shift towards increased age at time of spinal cord injury (SCI), as well as decreased functional recovery following SCI in older populations, create the need to investigate how age effects SCI pathology and repair (Scivoletto et al., 2003).While decreased neuroplasticity or physical strength with age may contribute to functional deficits, work from our lab and others have identified exacerbated acute inflammatory events as contributors to age-dependent secondary injury. Specifically, our recent paper identified that increased production of reactive oxygen species(ROS) from macrophage nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (NOX) with age exacerbates secondary injury after SCI (Zhang et al., 2019). Collectively, we identified ROS as instrumental in worsening functional outcomes following aged SCI and demonstrated that the therapeutic efficacy of ROS based treatments is age-dependent. This short review focuses on the role of age on worsening functional outcomes following SCI and provides a mechanistic overview into how age exaggerates secondary injury.
Clinically, increased age at time of SCI is associated with decreased functional recovery as measured using The American Spinal Injury Association scale and functional independence measure,as well as decreased bowel and bladder control (Scivoletto et al.,2003). However, despite these decreases in functional recovery, aged individuals suffer milder traumatic events such as slip-and-fall accidents when compared to spinal cord trauma in younger individuals that often arise from high velocity impacts including sporting and vehicle collisions or violence. Differing primary mechanisms of injury between aged and young demographics complicate comparative studies and contribute to controversy regarding the role of age in exacerbating mechanisms of secondary injury.
Studies in animal models of SCI can address clinical limitations by controlling for primary injury severities across different ages.Rodent models of SCI have validated an age-dependent decline in functional recovery consistent with clinical reports. Decreases in neural plasticity and regenerative potential likely contribute to worse recovery with age, and these neuron-intrinsic contributors to age-related deficits in recovery after SCI have been recently reviewed (Geoffroy et al., 2017). Briefly, Geoffroy et al. (2017) examined the contribution of neuron-intrinsic vs. -extrinsic influences on SCI recovery and regeneration with age using phosphatase and tensin homolog (PTEN) deletion to augment the growth state of neurons. When PTEN was deleted from young and aged mice,damaged axons maintained an intrinsic regenerative potential but were only able to regenerate across spinal lesions in young mice.This was attributed to a corresponding age-dependent increase in astrocyte and microglial densities at the lesion boundaries, which suggests that increased inflammation with age exerts inhibitory neuron-extrinsic influences to regenerative potential (Geoffroy et al., 2016). This age-dependent influence of inflammation on axon growth may be associated with macrophage senescence. Indeed, age alters microglia/macrophage growth factor and cytokine expression(Ritzel et al., 2015), which likely diminishes the tissue remodeling and axon growth potential of activated macrophages. Macrophage senescence also reduces phagocytic function (Ritzel et al., 2015)potentially reducing myelin debris clearance thereby sustaining an environment inhibitory to axon growth.
Our recent work further identifies that an increased inflammatory response with age exacerbates secondary injury and contributes to worse functional outcomes. Specifically, SCI in 14-month-old vs. 4-month-old mice results in increased ROS production from macrophages as a consequence of upregulated NOX (Zhang et al.,2016). The increased ROS production coincides with an increased accumulation of oxidized dihydroethidium, an exogenous superoxide tracer, and 4-hydroxy-2-nonenol, the downstream indicator of toxic ROS-derived lipid peroxidation, at 1-week post-SCI (Zhang et al., 2016). Similar results were also reported in 12-month-old vs.3-month-old rats (von Leden et al., 2017), thus implicating macrophage ROS production as an important contributor to age-dependent secondary injury.
This correlation between increased NOX-derived ROS and worse outcomes following aged SCI led us to investigate the contribution of NOX-derived ROS on age-dependent increases in secondary injury. To accomplish this, we administered the NOX inhibitor, apocynin, to treat young (4-month-old) and middle-aged(14-month-old) SCI mice beginning 1 hour after SCI. Apocynin is one of the most widely used small molecule inhibitors of NOX activity and is utilized to study a number of NOX-related diseases.Although the mechanism of action for apocynin is not fully known,it involves blocking the binding of cytosolic p47phox to the membrane NOX complex (Stefanska and Pawliczak, 2008). When the cytosolic and membrane bound complex is formed, ROS is produced as the result of NOX-mediated electron transport across the membrane.
Treating middle-aged and young mice with apocynin elicited significant locomotor improvements and reduced lesion volumes only in middle-aged mice (Zhang et al., 2019). Interestingly,reduced ROS production was also found only in middle-aged apocynin-treated mice, and only in monocyte-derived (F4/80+,P2Y12
-), but not microglial-derived (F4/80+, P2Y12+), macrophages.This finding implicates monocyte-derived macrophages as the major source of NOX-derived ROS following aged SCI. Unexpectedly,treatment with apocynin also reduced total monocyte-derived macrophage accumulation in middle-aged spinal cords, further implicating NOX as both pro-inflammatory and damaging to neurons in aged SCI conditions. Why apocynin exerted anti-inflammatory effects only following middle-aged SCI remains unknown. However,because NOX is essential for monocytes to differentiate into mature macrophages (Xu et al., 2016), it may be likely that apocynin both limits macrophage differentiation, thereby reducing total inflammation, as well as reduces ROS production from already differentiated macrophages. Further studies that use NOX-knockout mice combined with bone-marrow chimeric techniques may provide insights to better understand the age-specific treatment effects of apocynin.
Decreased ROS-mediated lipid peroxidation through reduced macrophage ROS production likely explains some of the protective effects of apocynin in middle-aged SCI animals. However,apocynin is not only a NOX-specific inhibitor but it also acts as ROS scavenger and can inhibit Rho kinases (Stefanska and Pawliczak, 2008). These multi-modal properties of apocynin could increase anti-oxidant defenses through ROS scavenging and may exert anti-inflammatory effects through rho kinase inhibition. As previously reported, age also decreases the anti-oxidant capacity of macrophages. Specifically, levels of reduced glutathione, a principal intracellular non-enzymatic antioxidant, decrease in macrophages with age (Vida et al., 2017). This decrease in redox balance can promote and sustain pro-inflammatory macrophage activation through a feed-forward mechanism involving ROS activation of nuclear factor-kappaB, resulting in increased expression of NOX and other pro-inflammatory cytokines (Anrather et al., 2006). Collectively,these alterations in redox balance and signaling confirm an age-dependent sensitivity of macrophages to ROS and subsequently to therapies targeting ROS such as apocynin.
Increases in ROS production and decreases in ROS defense are recognized and well-established in the general context of age;however, implications for these mechanisms of aging on exacerbating secondary injury after neurotrauma are only recently being acknowledged. An important implication of our findings is the potential for age at time of SCI to predict treatment efficacy when using immunomodulatory or ROS-based therapies. To date only one treatment, methylprednisolone, has been used in the clinic to treat SCI, but even this has fallen under scrutiny due to concerns regarding efficacy and adverse consequences (Hall, 2016). Along with other SCI clinical trails, the National Acute Spinal Cord Injury Studies (NASCIS) responsible for obtaining evidence of efficacy for methylprednisolone did not consider age as a contributing variable to the observed treatment effects. Our work emphasizes the importance for considering age in clinical trail design, and for evaluating age as a covariant to predict therapeutic success, particularly when evaluating immunomodulatory or ROS based therapies for SCI.Cumulative work reviewed above suggests a causal link between the aging macrophage on both reducing recovery from SCI and exacerbating secondary injury through upregulation of NOX-derived ROS (Figure 1).
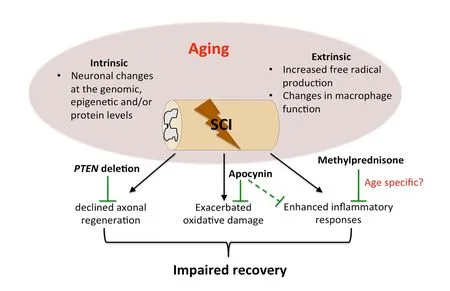
Figure 1 Age-dependent mediators and treatments for spinal cord injury (SCI).Both neuron intrinsic and extrinsic changes during aging contribute to declined axonal regeneration, exacerbated oxidative damage, and enhanced inflammation after SCI, which lead to worse functional recovery in aged individuals. Phosphatase and tensin homolog (PTEN)deletion targeting intrinsic neuronal signaling pathways counteracts the age-dependent decline of axonal regeneration albeit with reduced effects. Apocynin treatment,targeting the neuronal extrinsic factors of oxidative damage and inflammation, ameliorates age-related exacerbations of reactive oxygen species and may modify inflammatory responses through decreasing macrophage infiltration. Moreover, the efficacy of clinical therapies for SCI such as Methylprednisolone, may be confounded by age. Images were based upon primary findings in Geoffroy et al. (2016) and Zhang et al. (2019).
Andrew N. Stewart, John C. Gensel, Bei Zhang*Spinal Cord and Brain Injury Research Center, Department of Physiology, University of Kentucky College of Medicine, Lexington,KY, USA (Stewart AN, Gensel JC)College of Public Health, Shaanxi University of Chinese Medicine,Xianyang, Shaanxi Province, China (Zhang B)
*Correspondence to: Bei Zhang, PhD, cady.zhang@hotmail.com.
orcid: 0000-0001-6943-1552 (Bei Zhang)
Received: February 12, 2019
Accepted: April 29, 2019
doi: 10.4103/1673-5374.259606
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check:Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewer: Emily Burnside, King's College London, UK.
Additional file:Open peer review report 1.
杂志排行
中国神经再生研究(英文版)的其它文章
- Etomidate affects the anti-oxidant pathway to protect retinal ganglion cells after optic nerve transection
- Normal tension glaucoma: from the brain to the eye or the inverse?
- Mesenchymal stromal cell therapy for damaged retinal ganglion cells, is gold all that glitters?
- MicroRNAs as biomarkers of diabetic retinopathy and disease progression
- Diabetic neuropathy research: from mouse models to targets for treatment
- Potential therapeutic roles of retinoids for prevention of neuroinflammation and neurodegeneration in Alzheimer's disease