Effects of Bifidobacterium infantis on cytokine-induced neutrophil chemoattractant and insulin-like growth factor-1 in the ileum of rats with endotoxin injury
2019-07-10WeiWangMeiSunYuLingZhengLiuYuSunShuQiangQu
Wei Wang, Mei Sun, Yu-Ling Zheng, Liu-Yu Sun, Shu-Qiang Qu
Abstract BACKGROUND The digestive tract is the maximal immunizing tissue in the body, and mucosal integrity and functional status of the gut is very important to maintain a healthy organism. Severe infection is one of the most common causes of gastrointestinal dysfunction, and the pathogenesis is closely related to endotoxemia and intestinal barrier injury. Bifidobacterium is one of the main probiotics in the human body that is involved in digestion, absorption, metabolism, nutrition, and immunity.Bifidobacterium plays an important role in maintaining the intestinal mucosal barrier integrity. This study investigated the protective mechanism of Bifidobacterium during ileal injury in rats.AIM To investigate the effects of Bifidobacterium on cytokine-induced neutrophil chemoattractant (CINC) and insulin-like growth factor 1 (IGF-1) in the ileum of rats with endotoxin injury.METHODS Preweaning rats were randomly divided into three groups: Control (group C),model (group E) and treatment (group T). Group E was intraperitoneally injected with lipopolysaccharide (LPS) to create an animal model of intestinal injury.Group T was intragastrically administered Bifidobacterium suspension 7 d before LPS. Group C was intraperitoneally injected with normal saline. The rats were killed at 2, 6 or 12 h after LPS or physiological saline injection to collect ileal Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0)license, which permits others to distribute, remix, adapt, build upon this work non-commercially,and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:http://creativecommons.org/licen ses/by-nc/4.0/Manuscript source: Unsolicited manuscript Received: November 21, 2018 Peer-review started: November 22,2018 First decision: December 12, 2018 Revised: March 12, 2019 Accepted: March 29, 2019 Article in press: March 30, 2019 Published online: June 21, 2019 P-Reviewer: Higuchi K, Jung DH,Pourshafie MR S-Editor: Yan JP L-Editor: Filipodia E-Editor: Ma YJ tissue samples. The expression of ileal CINC mRNA was evaluated by reverse transcription-polymerase chain reaction (RT-PCR), and expression of ileal IGF-1 protein and mRNA was detected by immunohistochemistry and RT-PCR,respectively.RESULTS The ileum of rats in Group C did not express CINC mRNA, ileums from Group E expressed high levels, which was then significantly decreased in Group T (F =23.947, P < 0.05). There was no significant difference in CINC mRNA expression at different times (F = 0.665, P > 0.05). There was a high level of IGF-1 brown granules in ileal crypts and epithelial cells in Group C, sparse staining in Group E, and dark, dense brown staining in Group T. There was a significant difference between Groups C and E and Groups E and T (P < 0.05). There was no significant difference in IGF-1 protein expression at different times (F = 1.269, P > 0.05). IGF-1 mRNA expression was significantly different among the three groups (P < 0.05),though not at different times (F = 0.086, P > 0.05).CONCLUSION Expression of CINC mRNA increased in the ileum of preweaning rats with endotoxin injury, and exogenous administration of Bifidobacterium reduced CINC mRNA expression. IGF-1 protein and mRNA expression decreased in the ileum of preweaning rats with endotoxin injury, and exogenous administration of Bifidobacterium prevented the decrease in IGF-1 expression. Bifidobacterium may increase IGF-1 expression and enhance intestinal immune barrier function in rats with endotoxin injury.
Key words:Bifidobacterium; Ileum; Cytokine-induced neutrophil chemoattractant; Insulinlike growth factor-1; Rats
INTRODUCTION
Severe infection is one of the most common causes of gastrointestinal dysfunction,and the pathogenesis is closely related to endotoxemia and intestinal barrier injury.Bifidobacterium is one of the main probiotics in the human body involved in digestion,absorption, metabolism, nutrition, and immunity to infection. In particular,Bifidobacterium plays an important role in maintaining intestinal mucosal barrier integrity[1]. Insulin-like growth factor 1 (IGF-1) is a polypeptide that is primarily secreted from the liver, but it is also expressed in other organs such as the intestine,making it part of the intestinal immune barrier[2]. This study investigated the protective mechanism of Bifidobacterium through ileal injury in preweaning rats.
MATERIALS AND METHODS
Materials
Bifidobacterium infantis (KLDS2.0002) suspension was purchased from the Key Laboratory of Dairy Science of Northeast Agricultural University. Escherichia coli (O55:B5) lipopolysaccharide (LPS) was purchased from Sigma. Immunohistochemical IGF-1 rabbit anti-rat IgG (primary antibody) was purchased from Wuhan Boshide Bioengineering Co. Ltd. (Wuhan, China), and horseradish-peroxidase-labeled IgG(secondary antibody) and concentrated DAB kits were purchased from Beijing Zhongshan Jinqiao Biotechnology Co. Ltd (Beijing, China). The primers for IGF-1,cytokine-induced neutrophil chemoattractant (CINC) and β-actin were designed and synthesized by Shanghai Shengneng Bocai Biological Technology Co. Ltd. A reverse transcription-polymerase chain reaction (RT-PCR) kit was purchased from Promega(Beijing, China).
Instruments
Instruments and equipment were obtained as follows: ultramicrotome (LKB, Sweden);GIS gel image processing system (Tanon, Shanghai, China); TC-XP gene amplification instrument (Hangzhou Bioer Technology Co. Ltd., Hangzhou, China); GE-100 gel electrophoresis system (Hangzhou Bioer Technology Co. Ltd., Hangzhou, China);Eclipse E800 Camera system (Nikon, Japan); CMIAS2001 Beihang Motic Image Analysis system (Beijing Mike Audi Image Technology Co. Ltd., Beijing, China);HFsafe 1200 biosafety cabinet (LiShen Scientific Instrument Co. Ltd., Shanghai,China); AE200s electronic analytical balance (Mettler Toledo Instruments Shanghai Co. Ltd., Shanghai, China); MDF-U53V 80 °C low temperature freezer (SANSY,Japan); Neofuge 23R desktop high speed refrigerated centrifuge (Shanghai, China);microsample feeder (Eppendorf AG, Germany); and XW-80A vortex mixer (Shanghai,China).
Animals
Healthy 18-day-old preweaning Wistar rats (31.16 ± 6.38 g) were provided by the Laboratory Animal Center of the Second Affiliated Hospital of Harbin Medical University. The rats were randomly divided into three groups: Control (group C),model (group E) and Bifidobacterium treatment (group T). The animals were sacrificed 2, 6 and 12 h after intraperitoneal injection of endotoxin (LPS) or normal saline, and ileal samples were collected. Each group had eight preweaning rats for each time point. Animals that died during the experiment were not included.
Bifidobacterium culture
TPY medium:10 g casein peptone, 2.5 g yeast extract powder, 5 g glucose, 5 g soy peptone, 1.0 g Tween 80, 0.5 g L-cysteine hydrochloric acid, 5 mL salt-mixture solution, and 995 mL distilled water, adjusted to pH 7.2, and sterilized at 121 °C for 20 min. Salt-mixture solution: 20.0 g K2HPO4, 5.0 g MgCl2∙6H2O, 2.5 g ZnSO4∙7H2O, 1.5 g CaCl2and 0.5 g FeC13, added to 100 mL distilled water.
Activation of freeze-dried strains:Before activation, cryopreserved strains were placed at room temperature for several hours. The ampoule was wiped with 70%alcohol in a sterile room, opened, and 0.2 mL TPY medium was added. The strains taken by sterilized platinum earrings were inoculated into TPY medium and onto agar plates. After anaerobic incubation for 48-72 h at 37 °C, three generations were continuously activated to enhance the viability of the strains.
Morphological observation:Platinum earrings were used to remove bacteria from a single colony or liquid culture and then smeared onto a microscope slide for Gram staining. The bacterial morphology was observed under the microscope. Generation of strains: After microscopic examination, strains with good morphology and no bacterial colonies in the solid medium were selected, and lines were drawn on TPY solid medium. At the same time, TPY liquid medium was added to bacteria to culture anaerobically at 37 °C for 48-72 h.
Measurement of viable bacteria:The 0.5 mL suspension was gradiently diluted 10 times with sterilized normal saline. The 0.2 mL suspension of the appropriate dilution gradient was extracted and placed on the TPY agar plate two or three times for each dilution gradient, and the suspension was uniformly spread out using a sterilized rod.After ventilation in the corridor of the anaerobic incubator, the suspension was transferred to an incubator for anaerobic cultivation at 37 °C for 48 h. Finally, bacterial colonies were counted after the strains were grown.
Preparation of animal models
Rats in groups E and T were intraperitoneally injected with 5 mg/kg LPS (5 mg/mL dissolved in normal saline). In group T, 0.5 mL Bifidobacterium suspension (2.0 × 109CFU/mL) was administered intragastrically twice daily 1 wk before LPS, until the end of the experiment. In group C, 1 mL/kg normal saline was intraperitoneally injected. The preweaning rats in each group were returned to their cage after treatment and continued to receive rat’s milk.
Specimen collection
After the animals were sacrificed, the abdomen was aseptically dissected, and the contents of the intestinal cavity were washed with ice-cold physiological saline. A 0.5-1 cm length of ileum 2-3 cm away from the ileocecal junction was removed and fixed in 4% paraformaldehyde dissolved in 0.1 M PBS. The tissue was embedded in paraffin and sectioned for immunohistochemical study. A 4-5 cm length of ileum 3-4 cm away from the ileocecal junction was removed and placed in an RNase-free Eppendorf tube and stored at - 80 °C prior to total RNA extraction and RT-PCR.
Immunohistochemical detection of IGF-1 protein in ileum
The SP method (peroxidase-labeled streptomycin, and streptavidin/peroxidase) was used for immunohistochemistry. Paraffin sections were dewaxed and washed twice with 0.01 M PBS at pH 7.4 for 5 min. The sections were incubated in recently prepared 3% H2O2(in 80% methanol) at room temperature for 5-10 min to eliminate endogenous peroxidase activity and washed three times in PBS for 5 min. Antigen retrieval was performed with 1 mM EDTA (pH 9.0) under high temperature and high pressure for 10 min. When returning to room temperature, sections were washed with distilled water, then washed three times with PBS for 5 min, covered with 10% normal goat serum (PBS diluted) and incubated at room temperature for 20 min to reduce non-specific staining. Excess liquid was discarded. A total of 50 μL rabbit anti-rat IGF-1 antibody (1:100) was incubated at 4 °C overnight or 37 °C for 1 hour, followed by three washes in PBS for 5 min each. Then, 50 μL biotin-labeled secondary antibody was incubated at 37 °C for 10-30 min, and washed three times with PBS for 5 min.Finally, 50 μL horseradish-peroxidase-labeled streptavidin was incubated at room temperature for 20 min and washed three times in PBS for 5 min. The sections were stained with newly prepared diaminobenzidine color developing agent at room temperature for 5-10 min, with the degree of dying being monitored under microscope, then washed with distilled water for 5 min. The sections were stained again with hematoxylin for 2 min, and washed with distilled water for 5 min. They were differentiated by 0.1% hydrochloric acid alcohol, and washed in flowing water for 30 min. The stained sections were dehydrated in graded series of ethanol, cleared,and sealed with gum. For the negative controls, PBS was used in place of the rabbit anti-rat IGF-1 primary antibody. The other steps were the same as above, excluding non-specific staining.
Homogeneous brown staining was considered positive. For immunohistochemical analysis, five to eight stained sections were selected for each time point. Five nonoverlapping views were randomly selected under light microscopy (× 400), and the optical density of each view was measured by Nikon Eclipse E800 Image Acquisition System and Beihang Motic Pathological Image Analysis System.
RT-PCR for detection of IGF-1 and CINC mRNA in ileum
For total RNA extraction, ileal tissues preserved at -80 °C were ground in liquid nitrogen under aseptic conditions, then poured into an Eppendorf tube. TRIzol Reagent (50-200 mg tissue/mL TRIzol) was added to the ileal tissues for 5 min at room temperature to facilitate dissociation of nucleic acid-protein complexes. Protein and DNA were removed by addition of 0.2 mL chloroform, and the supernatant was extracted by rapid vibration and centrifugation at 12000 rpm at 4 °C for 15 min.
RNA was precipitated by addition of 0.5 mL isopropanol. After mixing and incubating at room temperature for 10 min, the samples were centrifuged at 12000 rpm at 4 °C for 15 min. The supernatant was discarded, and the precipitate was resuspended in 1 mL 75% ethanol (prepared in 0.1% DEPC water) and centrifuged at 7500 rpm at 4 °C for 5 min. The supernatant was discarded and dried for 5-10 min.The RNA was dissolved in RNase-free DEPC-treated water to a volume of 20 mL,mixed, and stored at - 20 °C.
For reverse transcription into cDNA, 2 μL RNA was added to the total reaction of 20 μL. The reverse transcription reaction solution was prepared as follows: 2 μL 10 ×buffer, 4 μL MgCl2(25 mM), 2 μL dNTP (10 mM), 0.5 μL RNase, 0.7 μL AMV (22 U/μL), 1 μL oligo-dT 15 (50 μM), 2 μL total RNA and 7.8 μL enzyme-free water. The reverse transcription reaction was carried out at 42 °C for 15 min, 95 °C for 15 min,and 0-5 °C for 5 min. The cDNA concentration was determined by ultraviolet spectrophotometry.
Amplification of CINC, IGF-1 and β-actin was as follows: CINC, primer sequence:5′-TGA GCT GCG CAG TCA GTG CCT GCA-3′, antisense primer sequence: 5′-ACA CCC TTT AGC ATC TTT TGG ACA-3′. The length of product was 390 bp. IGF-1,primer sequence: 5′-GCT GCC ACT TGG ATC GCT ATT C-3′; antisense primer sequence: 5′-CGT CCC GGG TCG TTT ACA CA-3′. The length of product was 300 bp.β-actin, primer sequence: 5′-CAT CTG CTG GAA GGT GGA CA-3′; antisense primer sequence: 5′-GAG AGG GAA ATC GTG CGT GAC-3′. The length of product was 452 bp.
The total reaction system volume was 25 μL, with 2 μg cDNA (the volume of cDNA was calculated according to the cDNA concentration of each sample); each reaction system was brought to 25 μL with enzyme-free water. Other specific components and volumes were as follows: 1.0 μL MgCl2(50 mM), 2.5 μL 10 × buffer, 0.5 μL dNTPs (10 mM), and 0.5 μL Taq polymerase (5 U/μL). The amplification conditions for CINC were: 94 °C for 5 min; 35 cycles of 94 °C for 30 s, 55 °C for 30 s and 72 °C for 30 s; then 72 °C for 7 min. The amplification conditions for IGF-1 were: 94 °C for 5 min; 35 cycles of 94 °C for 30 s, 55 °C for 30 s and 72 °C for 30 s; then 72 °C for 7 min. The amplification conditions for β-actin were: 94 °C for 5 min; 30 cycles of 94 °C for 30 s,56 °C for 40 s and 72 °C for 22 s; then 72 °C for 7 min.
For semi-quantitative analysis of PCR products, PCR reaction products (3-5 μL)were mixed with ethidium bromide and subjected to 2% agarose gel electrophoresis.The PCR products were quantified by ImageJ analysis, and β-actin was the internal reference for RNA detection. The relative expression of genes = optical density of tested genes/intrinsic optical density.
Statistical analysis
Statistical analyses were performed using SPSS version 21.0 software. The results were expressed as mean ± SD. The variance analysis of factorial design was used for statistical analysis. P < 0.05 was considered statistically significant.
RESULTS
CINC mRNA expression in ileal tissue
CINC mRNA was not expressed in group C at any time examined. There was a significant difference between groups T and E (F = 23.947, P < 0.05). There was no significant difference in expression within a group at different time points (F = 0.665,P > 0.05) (Figures 1 and 2).
IGF-1 protein expression in ileal tissue
There was some IGF-1 expression in group C at every time point examined. There were more brown granules in the intestinal crypts and epithelial cells compared to the other groups. The brown staining became faint and sparse in group E. The brown staining became dark and dense in group T. There was no significant difference in staining at different time points (F = 1.269, P > 0.05), although there were significant differences among groups (F = 32.463, P < 0.05). Further analysis showed significant differences between groups C and E and groups E and T (P < 0.05) (Figures 3-7).
IGF-1 mRNA expression in ileal tissue
IGF-1 mRNA was highly expressed in group C. There was no significant difference in expression at different times (F = 0.086, P > 0.05), although there were significant differences between groups (F = 46.670, P < 0.05). Further analysis revealed that there was a significant difference between groups C and E, groups C and T, and groups E and T (P < 0.05) (Figure 8).
DISCUSSION
Gastrointestinal dysfunction plays an important role in the development of multiple organ dysfunction syndrome (MODS). The intestine is one of the target organs injured in MODS, and it plays an important role in initiating MODS, in which intestinal mucosal barrier damage is a key factor. Bacterial or endotoxin translocation is closely related to excessive growth of opportunistic pathogens in the intestine, weakened intestinal immunity, and intestinal mucosal damage[3]. The intestinal flora is a key component of the mechanical, immune and biological barriers of the intestinal mucosa, as it plays a key role in preventing bacterial or endotoxin translocation.
Bifidobacterium is one of the main components of the intestinal mucosal barrier and is one of the main probiotics in the human body. It is involved in digestion,absorption, nutrition, metabolism, anti-infective immunity, and especially in maintaining the integrity of the intestinal mucosal barrier. The digestive tract is the largest bacterial habitat in the body. The micro-ecological stability of the intestine can be destroyed by fasting, antacids or antibiotics in critically ill patients, which can disrupt the stability of the intestinal micro-ecology. Consequently, intestinal flora imbalance is the primary cause of bacterial translocation and intestinal infection. It has been reported that Bifidobacterium has an effect on rotavirus enteritis[4]and can prevent necrotizing enterocolitis in premature rats[5,6], where it has been shown to have a therapeutic effect on necrotizing enterocolitis[7,8]. Bifidobacterium also improves immunity and the inflammatory response in weaning rats with colitis[9], and there has been an increased research focus on the effects of Bifidobacterium on metabolic syndrome[10-15]. In recent years, Bifidobacterium has been used to prevent cardiac damage[16], and its effect on the immune state in early life has been studied[17].However, the intestinal protective mechanism of Bifidobacterium has not been entirely elucidated.
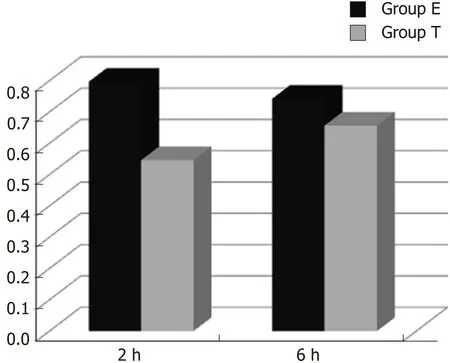
Figure 1 Optical density ratio of cytokine-induced neutrophil chemoattractant mRNA/β-actin mRNA in ileal tissue (mean ± SD).
In inflammatory reactions, neutrophils in the blood must be activated after entering tissue spaces through capillary walls, where they then exert their biological effects.Neutrophil chemotaxis is mediated by CINC, a murine chemotactic cytokine similar to human interleukin-8. CINC-1 expression is increased in rat models of ulcerative colitis[18], intestinal and lung ischemia/reperfusion models and endotoxin injury models[19,20]. CINC-1 expression in the lung tissue of rats is increased by endotoxin[20,21]and chronic intermittent hypoxia[22].
In the present study, there was no significant CINC mRNA expression in the ileum of group C rats at any time point examined. CINC mRNA expression was high at 2 and 6 h in group E, which meant that the model of intestinal injury was successful.CINC mRNA expression in the ileum of rats in group T was significantly decreased compared to group E (P < 0.05), which indicated that Bifidobacterium relieved ileal inflammation and protected the rat intestine.
IGF-1 is a multifunctional cellular regulatory factor that is mainly secreted by the liver. Thus, the liver is the main source of circulating IGF-1, but IGF-1 is also expressed in other tissues including the intestine, condylar cartilage cells in rats[23], T lymphocytes in mice dendritic epithelia[24], brain tissue of rats[25,26], placenta of pregnant mice[27-29], and colonic smooth muscle cells in diabetic rats[27]. IGF-1 plays a role in cell growth[30-32], differentiation[33,34]and metabolism[35]. One recent study focused on continuous IGF-1 expression in the intestine in a rat model of short bowel syndrome[36]. Other studies have shown that exogenous IGF-1 improved intestinal barrier function in rats with cirrhosis[37]or acute necrotizing pancreatitis[38], where survival rates have been significantly improved by minimal invasive interventions[39-43]. How IGF-1 is expressed during intestinal infection and how Bifidobacterium affects that expression have not been reported.
In this study, IGF-1 protein and mRNA expression in the ileum decreased after intraperitoneal injection of endotoxin, as reported previously[44]. Intragastric administration of Bifidobacterium increased IGF-1 protein and mRNA expression,indicating that IGF-1 plays an important role in the recovery of ileal mucosal damage.Bifidobacterium may enhance the immunological barrier function of the intestine by inhibiting local inflammatory responses and increasing IGF-1 expression.many brown-staining granules in intestinal crypt and epithelial cells (× 400).
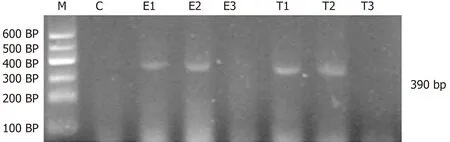
Figure 2 Cytokine-induced neutrophil chemoattractant mRNA expression in ileal tissue. M: DNA marker; C: Group C; E1-3: Group E 2 h, 6 h, and 12 h,respectively; T1-3: Group T 2 h, 6 h, and 12 h, respectively; 390 bp: Expected size of cytokine-induced neutrophil chemoattractant RT-PCR product.
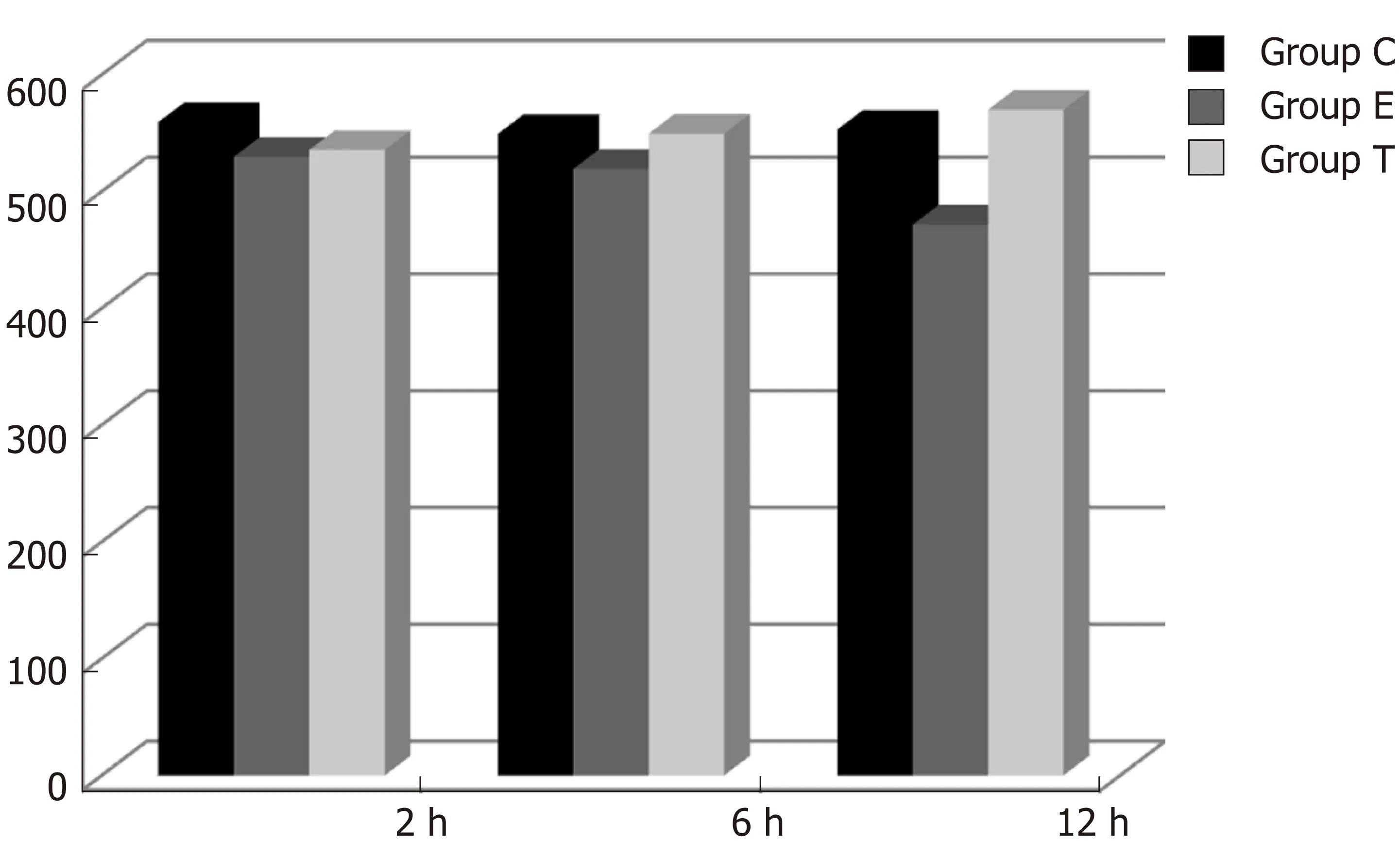
Figure 3 lmmunohistochemical density of insulin-like growth factor 1 in the ileum (mean ± SD).
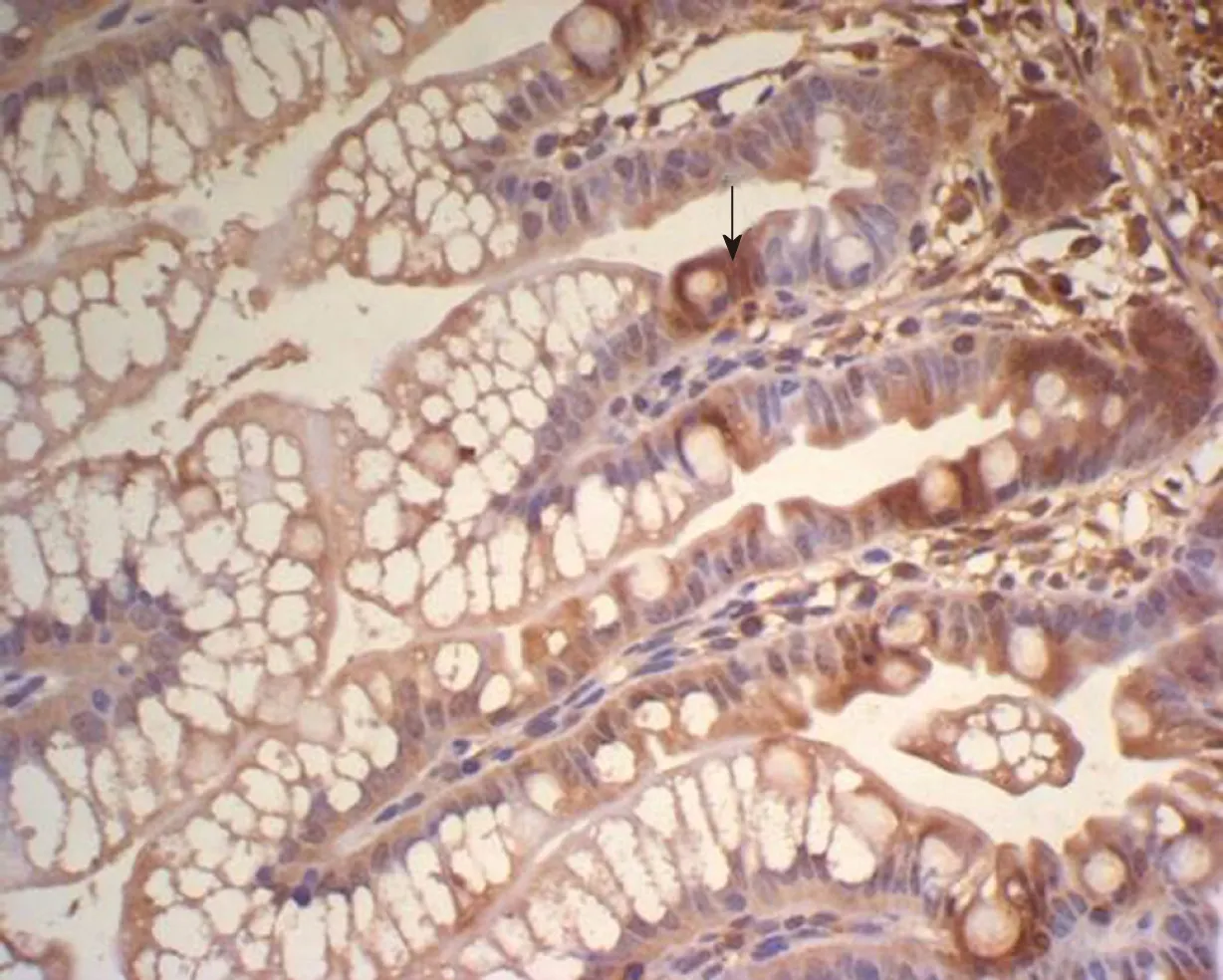
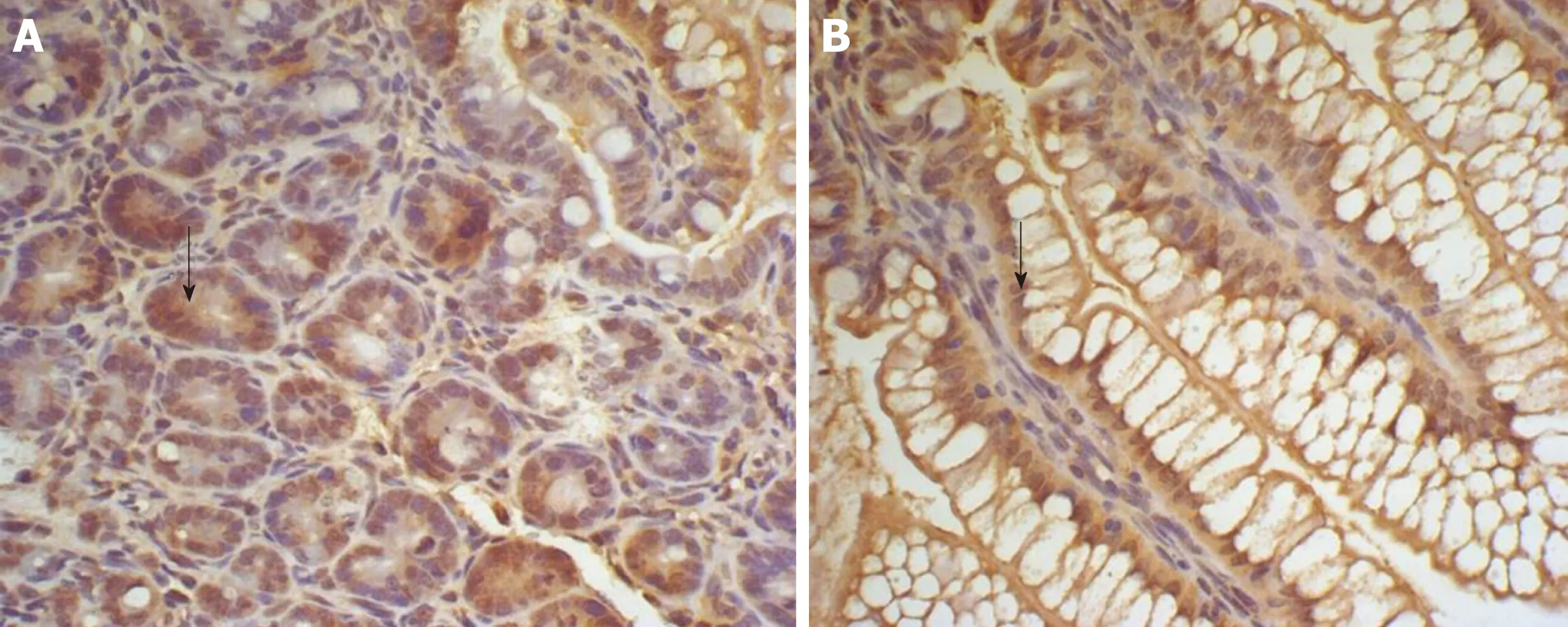
Figure 5 Expression of insulin-like growth factor 1 in the ileum of Group E and T at 2 h. A: Expression of insulin-like growth factor 1 in the ileum of group E at 2 h, showing many brown-staining granules in the intestinal epithelial cells (× 400); B: Expression of insulin-like growth factor 1 in the ileum of group T at 2 h, showing
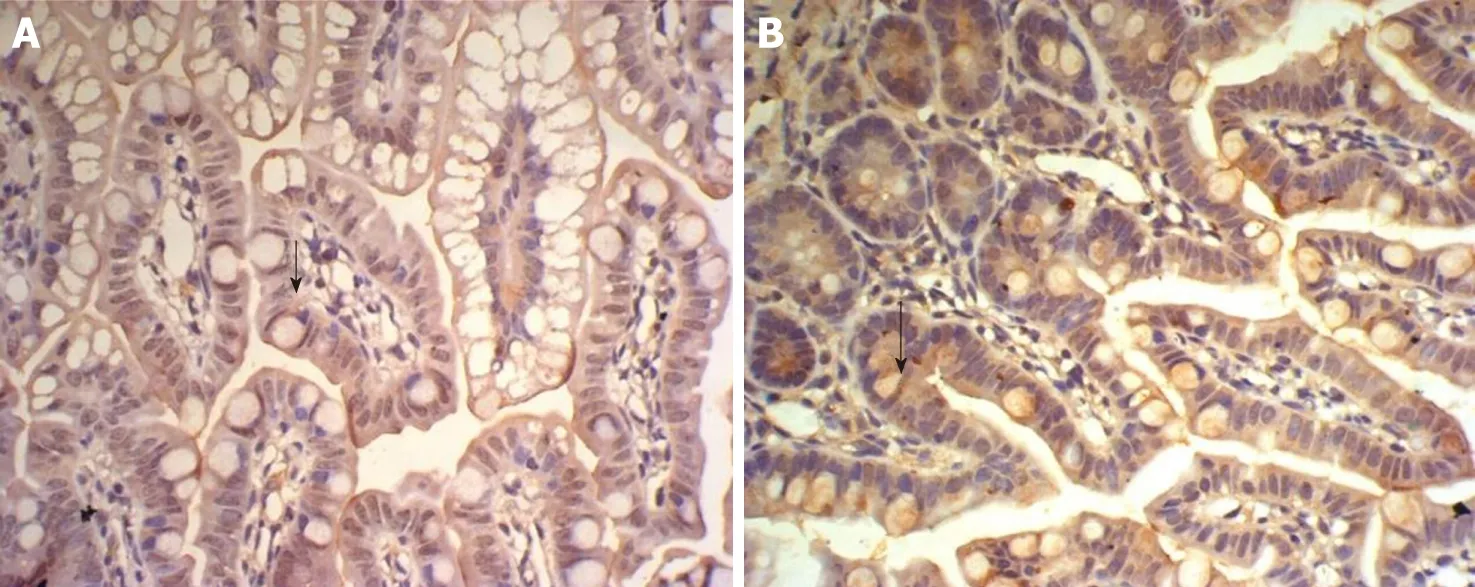
Figure 6 Expression of insulin-like growth factor 1 in the ileum of group E and T at 6 h. A: Expression of insulin-like growth factor 1 in the ileum of group E at 6 h, showing faint brown-staining granules in the intestinal epithelial cells (× 400); B: Expression of insulin-like growth factor 1 in the ileum of group T at 6 h, showing many densely distributed brown-staining granules in the intestinal crypt and epithelial cells (× 400).
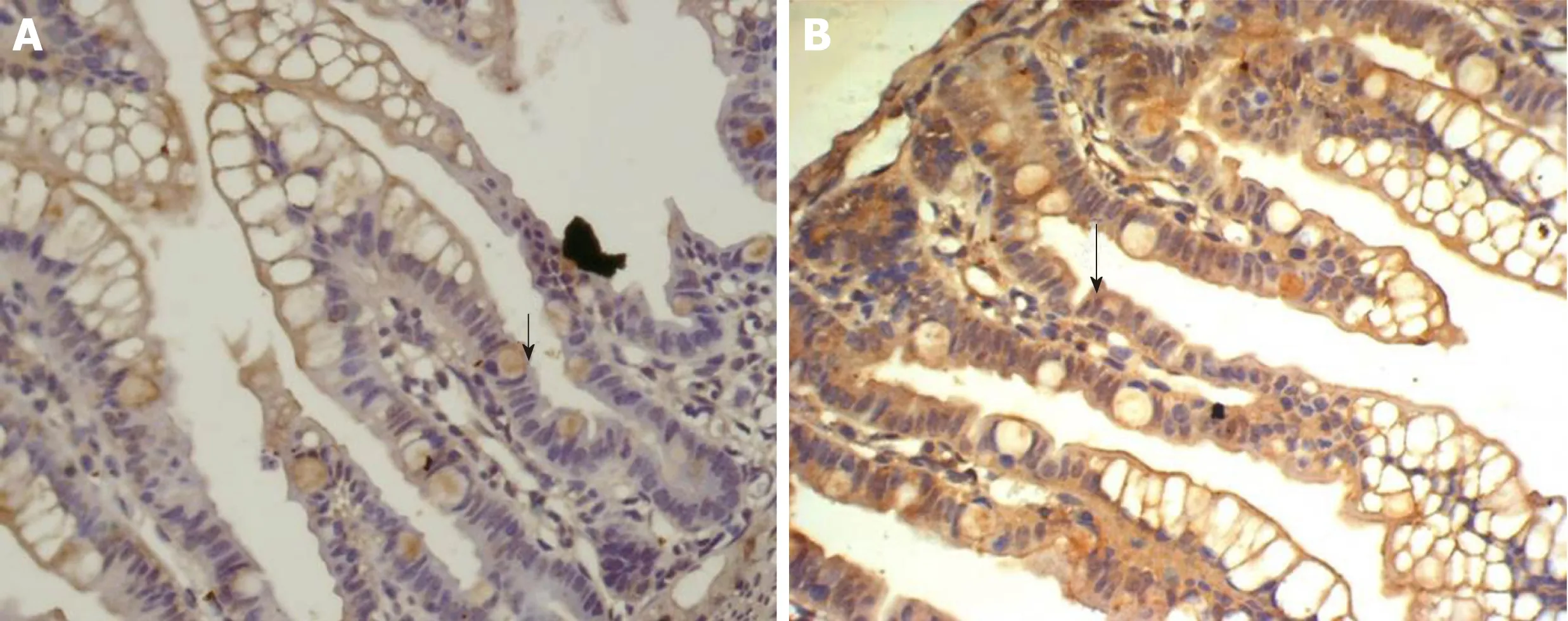
Figure 7 Expression of insulin-like growth factor 1 in the ileum of group E and T at 12 h. A: Expression of insulin-like growth factor 1 in the ileum of the group E at 12 h, showing faint and sparse brown-staining granules in the intestinal epithelial cells (× 400); B: Expression of insulin-like growth factor 1 in the ileum of group T at 12 h, showing diffuse and dense distribution of brown-staining granules in intestinal crypt and epithelial cells (× 400).
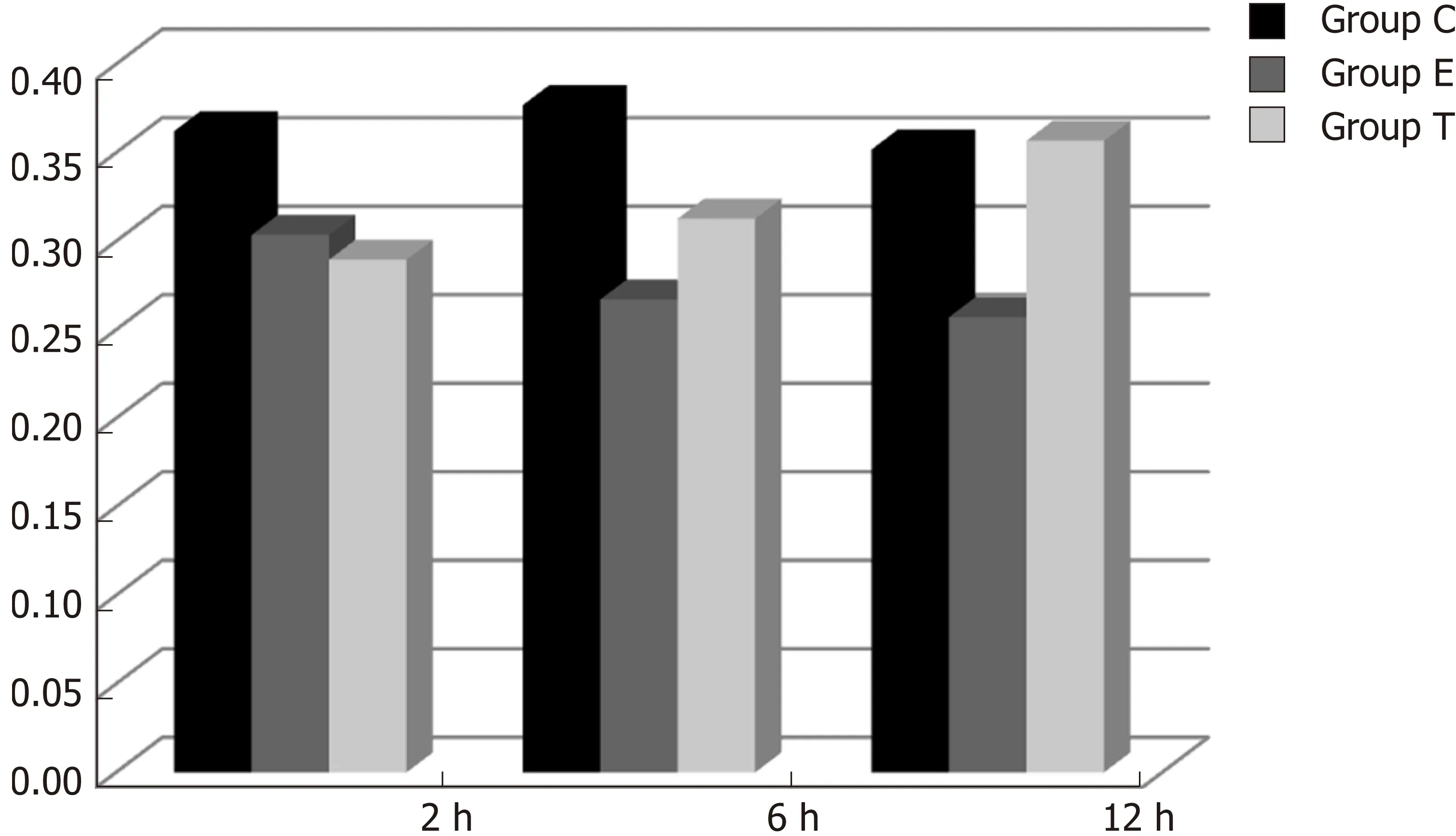
Figure 8 Optical density ratio of insulin-like growth factor 1 mRNA/β-actin mRNA in the ileum (mean ± SD).
ARTICLE HIGHLIGHTS
Research background
Severe infection is one of the most common causes of gastrointestinal dysfunction, and its pathogenesis is closely related to endotoxemia and intestinal barrier injury.
Research motivation
Bifidobacterium plays an important role in maintaining the integrity of the intestinal mucosal barrier.
Research objectives
This study investigated the protective mechanism of Bifidobacterium during ileal injury in rats.
Research methods
Using endotoxin injured rat models, ileal cytokine-induced neutrophil chemoattractant (CINC)mRNA expression was evaluated by reverse transcription-polymerase chain reaction (RT-PCR),and expression of ileal insulin-like growth factor 1 (IGF-1) protein and mRNA was detected by immunohistochemistry and RT-PCR, respectively.
Research results
There was a significant difference in CINC mRNA expression between the different groups (P <0.05). There was a significant difference in IGF-1 brown granule expression among the different groups (P < 0.05), and expression of IGF-1 mRNA significantly differed among the three groups(P < 0.05)
Research conclusions
Bifidobacterium may increase IGF-1 expression and enhance intestinal immune barrier function in rats with endotoxin injury.
Research perspectives
This study can provide a new therapeutic tool and theoretical support for gastrointestinal dysfunction.
杂志排行
World Journal of Gastroenterology的其它文章
- Modified FOLFlRlNOX for resected pancreatic cancer: Opportunities and challenges
- Role of cytochrome P450 polymorphisms and functions in development of ulcerative colitis
- Role of epigenetics in transformation of inflammation into colorectal cancer
- Postoperative complications in gastrointestinal surgery: A “hidden”basic quality indicator
- The role of endoscopy in the management of hereditary diffuse gastric cancer syndrome
- Predicting systemic spread in early colorectal cancer: Can we do better?
