Predicting systemic spread in early colorectal cancer: Can we do better?
2019-07-10ScarletFionaBrockmoellerNicholasPaulWest
Scarlet Fiona Brockmoeller, Nicholas Paul West
Abstract Through the implementation of national bowel cancer screening programmes we have seen a three-fold increase in early pT1 colorectal cancers, but how these lesions should be managed is currently unclear. Local excision can be an attractive option, especially for fragile patients with multiple comorbidities, but it is only safe from an oncological point of view in the absence of lymph node metastasis. Patient risk stratification through careful analysis of histopathological features in local excision or polypectomy specimens should be performed according to national guidelines to avoid under- or over-treatment. Currently national guidelines vary in their recommendations as to which factors should be routinely reported and there is no established multivariate risk stratification model to determine which patients should be offered major resectional surgery.Conventional histopathological parameters such as tumour grading or lymphovascular invasion have been shown to be predictive of lymph node metastasis in a number of studies but the inter- and intra-observer variation in reporting is high. Newer parameters including tumour budding and poorly differentiated clusters have been shown to have great potential, but again some improvement in the inter-observer variation is required. With the implementation of digital pathology into clinical practice, quantitative parameters like depth/area of submucosal invasion and proportion of stroma can be routinely assessed. In this review we present the various histopathological risk factors for predicting systemic spread in pT1 colorectal cancer and introduce potential novel quantitative variables and multivariable risk models that could be used to better define the optimal treatment of this increasingly common disease.
Key words: Early colorectal cancer; Bowel cancer screening; Local resection; Major resection; Morphological risk factors; Conventional histopathology parameters; Novel histopathology parameters; Risk stratification models; Digital pathology Accepted: May 8, 2019 Article in press: May 8, 2019 Published online: June 21, 2019 P-Reviewer: Merkel S, Tandon RK S-Editor: Ma RY L-Editor: A E-Editor: Ma YJ
INTRODUCTION
Colorectal cancer (CRC) is one of the most common cancers worldwide[1,2]. It is the third most common cause of cancer death in the United Kingdom and the United States in both females and males[1]. Through the implementation of national population screening[3]like the United Kingdom National Health Service Bowel Cancer Screening Programme (NHS BCSP) we have observed a three-fold increase in early CRC (stage pT1) from 5% to 17%[4]. Early CRC is defined as the “invasion of neoplastic glandular epithelial cells through the muscularis mucosae into the submucosa of the bowel wall but not beyond”[5].
Currently it is unclear as to how pT1 CRC should be optimally managed. Major bowel resection can be performed, however, this is associated with a significant risk of post-operative mortality, especially in elderly patients, and also morbidity including permanent colostomy formation, sexual and genitourinary problems, and low anterior resection syndrome. Internationally, postoperative mortality rates vary markedly, largely depending on background comorbidity in the population. Local excision of the tumour and avoidance of major surgery is an attractive option for patients with rectal cancer or significant comorbidity, but this is only safe from an oncological viewpoint in the absence of lymph node metastasis (LNM)[6,7]. It is therefore important that when deciding whether major bowel surgery or local excision should be performed, the postoperative mortality and lymph node metastasis risk are accurately estimated to inform the decision. Approximately 10%-15% of all pT1 CRC have LNM at the time of primary diagnosis with pedunculated pT1 CRC having an even lower risk (3% to 7% in the Asian population). Despite this major bowel resection rates in pT1 CRC can be as high as 76%, meaning that many patients are potentially exposed to unnecessary risk[8-11].
When a local excision (including polypectomy) is performed for pT1 CRC, patient risk stratification is undertaken by histopathologists through careful analysis of the specimen to determine the risk of LNM. The detailed macroscopic and microscopic evaluation of the specimen produces a large amount of information to guide further treatment. Routine information that is generally collected internationally includes the type of tumour, differentiation grade, TNM stage, level of invasion, number of lymph nodes involved, lymphatic invasion status, venous invasion status, perineural invasion status and resection margin status[12]. Currently various national guidelines differ in their recommendations as to which histopathological factors should be reported and used to determine the risk of LNM and therefore use of major surgery,and there is no established multivariate risk stratification model. The classification of some factors is also not performed according to an international standard with various systems in use. This review will present and discuss the various histopathological risk factors that can be used to predict systemic spread in in pT1 CRC and introduce potential novel quantitative variables and multivariable risk models that could be used to better define the optimal treatment of this increasingly common disease.
LITERATURE SEARCH
Literature searching was performed in PubMed (https://www.ncbi.nlm.nih.gov/pubmed) for the following keywords: “lymph nodes”, “lymph node metastasis”,“T1” and “pT1” combined with “colorectal cancer”. Articles published between July 2004 and January 2019 were reviewed. In addition, manual cross-referencing was performed and further relevant papers published before 2007 identified through review articles. Inclusion criteria for studies included publication in English, use of at least 100 patients and availability of LNM status. Studies were excluded if neoadjuvant therapy was used due to the potential effect on tumour staging.
CONVENTIONAL HISTOPATHOLOGICAL PARAMETERS
Histological tumour type
The majority of CRCs are adenocarcinoma but specific histological variants including cribriform or micropapillary adenocarcinoma have been reported in case series to have a higher rate of LNM in early CRC[13], and may be used to indicate further treatment in margin negative local excisions[14]. Mucinous adenocarcinomas (> 50% of the tumour area composed of extracellular mucin), signet ring cell adenocarcinomas(> 50% of the area composed of signet ring cells) and medullary carcinomas are associated with deficient mismatch repair (dMMR), which has a better prognosis when compared to cases with proficient mismatch repair. Yet in the absence of deficient mismatch repair, these histological subtypes are associated with a poorer prognosis than conventional CRC and further treatment may be indicated. The importance of routine mismatch repair immunostaining is detailed below.
Tumour differentiation grading
Poor tumour differentiation has been shown in numerous studies, including metaanalyses, to be significantly associated with poorer survival[15-17]and prediction of LNM in early CRC[7,18]. Poor differentiation is primarily based on the architecture of the tumour and the hallmark is the absence of any tubular formation or irregularly folded, distorted and often small tubules. Tumour differentiation is a subjective parameter and grading may vary between assessors. Currently the WHO grading system proposes a four tier classification including: Grade 1 (well differentiated),grade 2 (moderately differentiated), grade 3 (poorly differentiated) and grade 4(undifferentiated)[5]. An improvement in inter-observer agreement can be achieved through compressing this system into two grades, i.e., well/moderate differentiation vs poor differentiation[19]. This has been routinely adopted in the United Kingdom on the basis that poor differentiation is an important high-risk feature in early CRC. With the implementation of digital pathology into routine clinical practice, tumour differentiation grading could be further improved by the implementation of automated algorithms to reduce the subjectivity of pathologist assessment[20-22].
For local excision specimens, there is currently uncertainty as to whether grading should be based on the predominant or worst area of differentiation, as most published studies have not specified how poor differentiation was defined. To avoid the risk of under-treatment in early CRC, the Royal College of Pathologists re-commend grading on the worst area in local excision specimens until further data are available[19]. In contrast, a major resection specimen is graded based on the predominant area of the tumour.
Whilst poor differentiation is generally accepted to be a poor prognostic feature, an exception in which it is associated with a more favourable stage-adjusted prognosis is in patients with dMMR, seen in 12%-15% of all cases[23,24]. Most dMMR cases are due to somatic epigenetic silencing of the MLH1 gene but a minority of cases are due to Lynch syndrome. From the current literature it is unclear if patients with poorly differentiated pT1 dMMR CRCs have a lower risk of LNM than poorly differentiated pT1 CRCs without dMMR. However, given the rarity of metastatic disease in dMMR CRC it is likely that poor differentiation is only an adverse factor in cases with proficient mismatch repair. Further evidence is required to confirm this hypothesis.Mismatch repair immunohistochemistry or alternative technologies including microsatellite instability testing should therefore be considered mandatory when determining LNM risk in local excision specimens.
Venous, lymphatic and perineural invasion
The presence of submucosal lymphatic invasion[7,17,25,26][relative risk (RR) = 5.2, 95%confidence interval (CI): 4.0-6.8[7]] and to a much lesser extent venous invasion (RR =2.2; 95%CI: 1.4 -3.2[7]) and perineural invasion[27]have been shown to be some of the strongest predictors of LNM in early CRC. It is therefore important to carefully assess for their presence and report these factors separately rather than stating the presence“lymphovascular invasion” for example. The location of the deepest point of involvement (either intramural or extramural) should be specified as this is also prognostic[28]. In the context of a local excision, the deepest point visible will usually be intramural. Lymphatic invasion is well recognised to be subjective with significant rates of inter-observer variation[29]. This can be caused by difficulties distinguishing lymphatics from venules, retraction artefacts, tumour budding and poorly differentiated clusters[29,30]. In cases of doubt, D2-40 immunohistochemistry can be helpful to confirm the presence of a lymphatic channel and elastin stains can be helpful to identify veins[25]. The use of such ancillary stains has been shown to significantly improve the inter-observer agreement[31].
Resection margin status
In polypectomy/local excision specimens, the status of the resection margin in conjunction with other high risk histopathology factors determines the risk of local recurrence. Tumours which are present at the resection margin or within the diathermised zone should be considered for further treatment regardless of high risk factors. In cases where the invasive tumour extends to the peripheral resection margin only, a repeat endoscopy and further local excision should be considered[19].
There is currently significant controversy about the degree of risk in cases where a tumour extends close to the deep resection margin (1 mm or less) but does not directly involve it. Within the NHS BCSP, the recently revised pathological reporting guidance has maintained 1 mm as the optimal cut-off to define margin involvement in order to reduce the risk of incomplete resection, despite the risk that this strategy will lead to a higher rate of major bowel surgery. Despite this guidance, in the absence of any other high-risk histopathology features, local re-excision may be a reasonable treatment option[11,32,33].
Level of submucosal invasion
Depending on the shape of the lesion (pedunculated or non-pedunculated), and the status of the muscularis mucosa (identifiable or non-identifiable), different classification systems may be used to define the level of submucosal invasion. A qualitative assessment of sessile lesions was initially proposed by Kudo et al[34]which separated the submucosa into thirds: sm1 (superficial); sm2 (middle); and sm3 (deep).Invasion into sm3 has been associated with a higher risk of LNM when compared to invasion confined to sm1/sm2 (RR = 3.6, 95%CI: 1.3-9.8)[7]. This method was further subsequently modified into a semi-quantitative system by Kikuchi (sm1: Invasion up to 0.2-0.3 mm; sm2: Intermediate invasion; sm3: Invasion near the muscularis propria)[35]. A third quantitative measurement with a clear cut-off defining the levels as sm1: Up to 0.5mm; sm2: 0.5-1.0 mm; sm3: Beyond 1.0 mm[36]. A second classification system was proposed by Haggitt for pT1 CRC with a polypoid shape, which assesses the depth of invasion into four levels, with level four invasion described as an adverse factor[37].
Several issues arise when attempting to apply these two classification systems in a routine clinical setting. Firstly, the received sample can become fragmented or suboptimally orientated on histological sections meaning that accurate assessment is not possible. To apply the Kudo system, the muscularis propria needs to be visible which is not usually present in local excisions (with the exception of full thickness transanal resection specimens). Without the muscularis propria indicating that the full thickness of the submucosa is included, accurate division of the submucosa into thirds is impossible. Haggitt classification can also be difficult to apply in poorly orientated specimens or polyps that are lacking a clear stalk. These limitations and difficulties show the need for better alternative measures that reply on quantitative parameters.
NOVEL HISTOPATHOLOGICAL PARAMETERS
Depth of submucosal invasion
Studies, in particular of Japanese populations, have identified the absolute depth of submucosal invasion as an important quantitative factor for predicting lymph node metastasis[38,39]. Ueno et al[11]proposed that the absolute depth of invasion beyond the muscularis mucosa and the width of the invasive tumour are more objective parameters than the conventional factors described above[11,17]. On univariate analysis,submucosal invasion ≥ 1 mm was predictive for LNM in pT1 CRC (RR = 5.2, 95%CI:1.8-15.4)[7].
The current guidelines from the Japanese Society for Cancer of the Colon and Rectum recommend major resection if the cancer has an involved deep margin or if one of the following high risk factors is present: poor differentiation, signet-ring cell carcinoma, mucinous carcinoma, depth of invasion > 1000 µm, vascular invasion or budding G2/G3[14]. Interestingly a study in the Japanese population found that submucosal invasion depth > 1000 µm alone would lead to approximately 80% of malignant polyps being treated with laparotomy[39]. This approach has not been routinely adopted in western populations as it would cause a significant increase in the major resection rate in patients with a significantly higher risk of post-operative mortality. In addition, there is only limited data on the prediction of LNM according to the absolute depth of submucosal invasion in western populations[40].
Area of submucosal invasion
Three dimensional histological reconstructions of the large intestinal submucosa has demonstrated that the number and size of blood and lymphatic vessels does not increase towards the base of the submucosa as expected[41,42]. There are a greater number of vessels in sm1 and the vessels are largest in sm2, suggesting that the absolute depth of submucosal invasion may not be the most important parameter.Pilot studies in a western populations have suggested that the width of the cancer(cut-off 11.5 mm; P = 0.001) and the area of submucosal invasion (cut-off 35 mm2; P <0.001) were significantly associated with the risk of LNM and showed superior prediction when compared to the depth of invasion[40]. Taken together, it is highly likely that the absolute area of submucosal invasion in sm1/sm2 where the majority of vessels are located is the best predictor of LNM. With the routine adoption of digital pathology, measuring the area of submucosal invasion is now readily feasible(Figure 1).
Tumour budding
The predictive value of tumour budding for LNM has been demonstrated for various cancers[43-46], but implementation into routine guidelines has been hindered by a lack of practical guidance on assessment. Multiple different systems are described in the literature leading to confusion over which system should be used[47]. Recently an international consensus group agreed the definition of budding as “a single cancer cell or a cell cluster of up to four tumour cells”[48]as well as detailing the practical steps that should be taken to evaluate this marker (Figure 2). This led to the implementation of tumour budding as an additional prognostic factor in the eighth edition of the Union for International Cancer Control's (UICC's) TNM classification.Tumour budding has been included in the College of American Pathologists (CAP)guidelines but not yet as a core factor in the UK guidelines until sufficient evidence of reproducibility and its role in patient risk stratification exists[19]. Nevertheless some studies have shown that the inter-observer variation in tumour budding is improved by the international consensus definition, although further studies in western populations are needed given that most of the large studies to date have been performed in Asian populations.
Poorly differentiated clusters
A new emerging risk factor for LNM in pT1 CRC is the presence of poorly differentiated clusters (PDC), which are defined as “malignant clusters with five or more cells lacking glandular differentiation”[49,50](Figure 2). Studies have shown that the presence of PDC is a strong predictor of LNM with greater reproducibility than tumour differentiation or budding in a study of 3,556 pT1 CRC [OR = 3.3 (95%CI: 2.6-4.1) P < 0.0001]. Further validation work is now required to confirm the importance of PDC in early CRC and define the optimal risk cut-offs in addition to clear practical guidance on the method of assessment[49].
Proportion of stroma
An emerging quantitative prognostic factor is the proportion of stroma within the overall tumour area[51]. It has been shown in a number of different cancers, including CRC, that a greater proportion of stroma is a poor prognostic factor[52-55]. The proportion of stroma can be subjectively estimated by the tumour stroma ratio[56]or accurately quantitated by cell density measurements[54]. It is thought that in CRC the high stroma group correlates with CMS4 consensus molecular subtyping, with both groups accounting for around 25% of cases with the poorest prognosis[57-59]. The prediction of LNM in pT1 CRC according to the proportion of stroma is as yet unknown but studies are ongoing.
Tumor immunology
The quantitation of the total number of tumour infiltrating lymphocytes has been shown to correlate with prognosis in CRC and other cancer types[60-62]. A study of 29 early stage CRC (stage I and II) showed that the combination of CD8(+) plus CD45RO(+) cells could be predictive for tumour recurrence and survival[63]. An increase in specific lymphocyte populations has been shown to strongly predict prognosis in CRC. The best-reported system in the recent literature is the Immunoscore®, which is generated on the basis of CD3 and CD8 expression in the tumour[64-66]. Automated algorithms to assess the number of lymphocytes are currently being explored. Again, the prediction of LNM in pT1 CRC according to tumour immunology is as yet unknown.
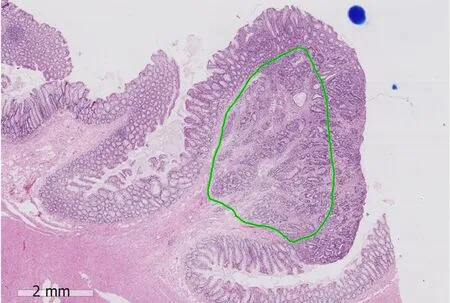
Figure 1 An example showing measurement of the area of submucosal invasion on a digital slide of a pT1 colorectal cancer (green area). The position of the destroyed muscularis mucosae has been estimated. The scale bar signifies 2 mm.
META-ANALYSIS OF HISTOPATHOLOGICAL PARAMETERS
A meta-analysis from 2011 included 17 studies with a total of 3621 patients and showed that the presence of lymphatic invasion (RR = 5.2, 95%CI: 4.0-6.8), high tumour budding (RR = 5.1, 95%CI: 3.6-7.3), submucosal invasion ≥ 1 mm (RR = 5.2,95%CI: 1.8-15.4), and poor differentiation (RR = 4.8, 95%CI: 3.3-6.9) were associated with a higher risk of LNM in pT1 CRC on univariate analysis[7]. Another fixed-effects meta-analysis included 76 studies and showed that lymphatic invasion (OR = 8.62)was the strongest factor, followed by tumour depth (pT2 vs pT1; OR = 2.62) and tumour differentiation (OR = 2.38) in predicting LNM[26]. In a subset analysis, poor differentiation at the invasive front (OR = 6.08) and tumour budding (OR = 5.82) were the most predictive in rectal cancer[26]. A meta-analysis from 2013 included 23 studies with 4510 patients and demonstrated similar findings, with a greater risk of LNM in pT1 CRC with a depth of submucosal invasion of > 1 mm (OR = 3.87, 95%CI: 1.50-10.00, P = 0.005), lymphovascular invasion (OR = 4.81, 95%CI: 3.14-7.37, P < 0.00001),poor differentiation (OR = 5.60, 95%CI: 2.90-10.82, P < 0.00001) or tumour budding(OR = 7.74, 95%CI: 4.47-13.39, P < 0.001)[17]. Finally, a meta-analysis in early CRC included 41 studies with 10137 patients and showed a strong association between the presence of tumour budding and risk of LNM in pT1 CRC (OR = 6.44; 95%CI: 5.26-7.87; P < 0.0001)[67].
MULTIVARIATE RISK STRATIFICATION MODELS
The univariate analyses described above have identified a number of conventional and novel histopathological risk factors for LNM in pT1 CRC. To date, very few studies have proposed multivariate risk prediction models and currently there is no single risk stratification model used internationally. Ueno et al[11]demonstrated that the best risk multivariate prediction model in a cohort of 251 cases included poor differentiation (OR = 2.9; 95%CI: 1.2-7.4; P = 0.023), vascular invasion (OR = 2.7;95%CI: 1.1-7.0; P = 0.039), tumour budding (OR = 3.7; 95%CI: 1.4-9.9; P = 0.008) and width of submucosal invasion (≥ 4000 μm)[11]. Another study of 140 cases used a logistic regression analysis and built an algorithm based on lymphatic invasion (OR =1.45, P < 0.05), absence of lymphocyte infiltration (OR = 16.6, P = 0.016), cribriformtype structural atypia (OR = 3.7; P < 0.05), venous invasion (OR = 3.26, P < 0.05), and depth of invasion (cut-off > 2 mm; OR = 1.45, P < 0.05)[68]. Lymphocyte infiltration was investigated in the invasive area of submucosal carcinoma and classified as either negative (no or little infiltration) or positive (follicular structures or infiltration by more lymphocytes than the number of tumour cells).
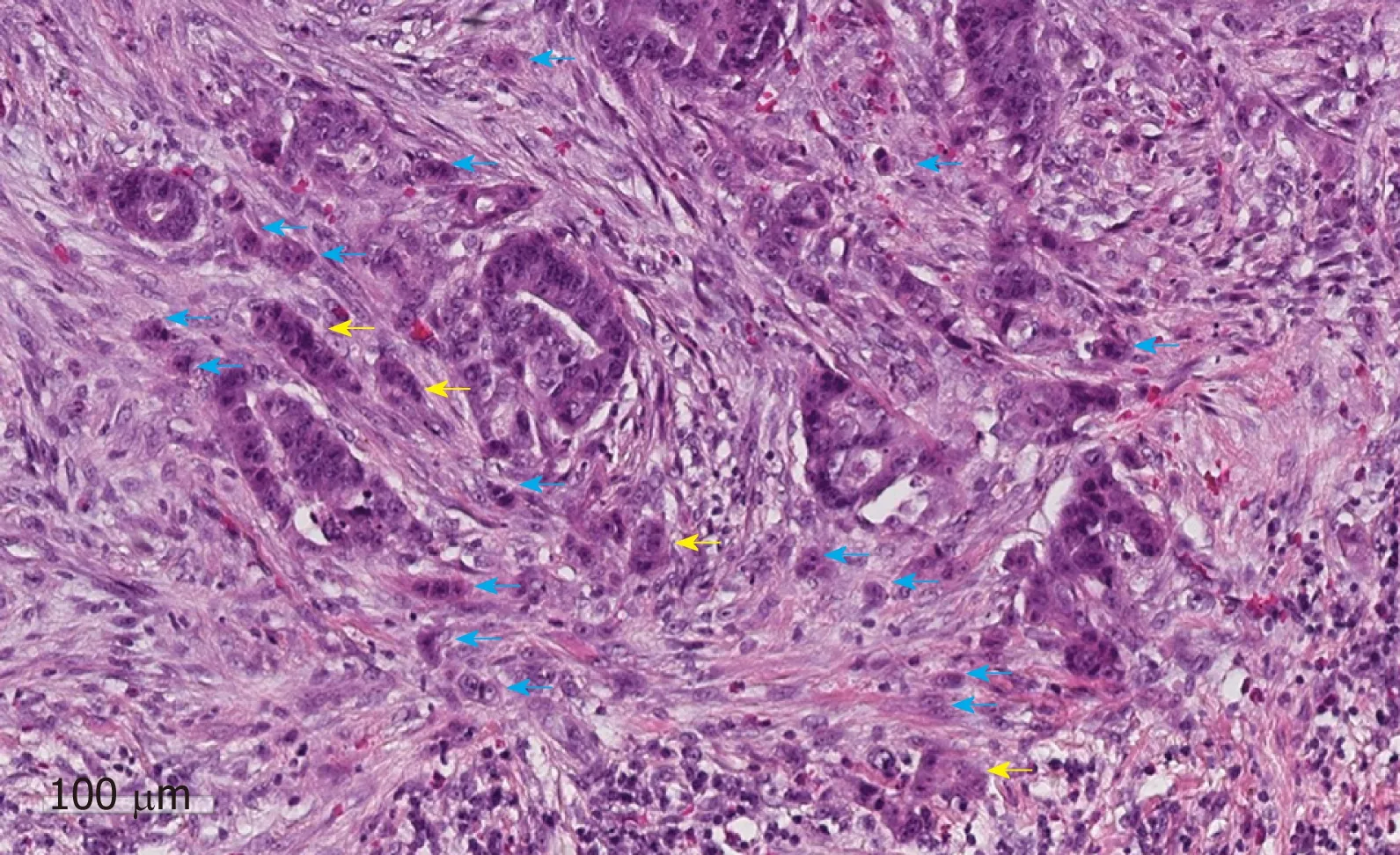
Figure 2 An example of tumour budding (blue arrows) and poorly differentiated clusters (yellow arrows) at the invasive edge of a pT1 colorectal cancer. The scale bar signifies 100 µm.
A larger retrospective study of 806 cases showed that independent predictors of LNM in multivariate analysis were: depth of submucosal invasion ≥ 1000 µm (OR =5.56; 95%CI: 2.14-19.10) and high-grade budding (OR = 3.14; 95%CI: 1.91-5.21). Highgrade budding was defined as five or more buds per high power field (0.95 mm2). The study proposed a three-tier risk classification system based on the depth of submucosal invasion and budding: high-risk with a depth of submucosal invasion ≥1000 µm and high-grade budding, intermediate-risk with a depth of submucosal invasion ≥ 1000 µm and low-grade budding, and low-risk with a depth of submucosal invasion < 1000 µm. Additional factors for used to subclassify the intermediate risk group further were lymphovascular invasion and differentiation grade[69].
A recently published study proposed a risk stratification model for pedunculated pT1 CRC (3% to 7% LNM rate in Asian populations)[9,10,39,69]and investigated the following six factors: tumour differentiation; submucosal invasion depth by Haggitt classification; lymphovascular invasion; tumour budding; PDCs; and the condition of the muscularis mucosae[70]. Tumour budding and PDC were defined and graded as above but assessed in a 0.785 mm2field (grade 1: 0-4; Grade 2: 5-9; Grade 3: 10 or more). The status of the muscularis mucosa was classified as Type A: Shattered but aligned muscularis mucosa or Type B: Incompletely or completely disrupted muscularis mucosae. The authors ultimately recommended a new model including the following four risk factors: lymphovascular invasion; Haggitt level 4 invasion;muscularis mucosae type B (incompletely or completely disrupted); high grade PDCs/tumour budding (both grade 2-3). High-grade tumour budding and highgrade PDC were grouped together as “any kind of positive budding”. The model had a sensitivity of 83.8% and specificity of 70.3% for predicting LNM (area under the curve value of 0.83), and would classify 32% of all cases in the high-risk group, 68% in the low risk group and miss 1.3% of all LNM.
Unfortunately the majority of these studies include retrospective series with small numbers of patients. Due to the 10%-15% rate of LNM in pT1 CRC, only a limited number of events are present leading to limited data in both Western and Asian populations. Well-designed prospective cohort studies are urgently needed to define robust international standards[49,69]. Ultimately a validated multivariate risk stratification model including both clinical and histopathological factors could be used to define prognosis in a similar way to that already developed for breast cancer.
DISCUSSION
In this review we have summarised the main conventional histopathological risk factors for LNM in pT1 CRC in addition to a number of novel factors that have recently been proposed. It is well recognised that parameters including lymphovascular invasion, poor differentiation, and depth of submucosal invasion are significantly correlated with an increased risk of LNM. Unfortunately, lymphovascular invasion and tumour differentiation grading show high levels of interobserver variation, which limits their clinical usefulness. Univariate markers for LNM in pT1 CRC should therefore be interpreted with caution when deciding whether to proceed with major bowel resection after local excision due to the significant risks of major morbidity and mortality.
With the routine implementation of digital pathology into clinical diagnostics[20-22],novel histopathological markers including the absolute depth of invasion, area of submucosal invasion and proportion of stroma can now be easily measured. Due to the quantitative nature of these parameters, it is likely that they are considerably more reproducible than the traditional subjective parameters. However, their evaluation can take a considerable amount of time to perform manually, hence there is a clear role for artificial intelligence, which is also likely to improve the reproducibility of con-ventional and novel risk factors.
Multivariate risk stratification models need to be developed and validated to optimise the management of pT1 CRC. In the future, artificial intelligence on digital pathology slides could be applied to compare different multivariate risk stratification models to identify the optimal way to accurately predict LNM[71]. This will should reduce the inaccuracy associated with relying on individual subjective markers and has the potential to further refine the group at highest risk of LNM and therefore reduce the overall number of patients exposed to the risks of major bowel resection.By combining the histopathological data with molecular data, patient data and treatment data, a personalised risk stratification model could be created with the aim of determining the optimal treatment pathway for individual patients[49].
CONCLUSION
In this review we present the various histopathological risk factors for predicting systemic spread in pT1 colorectal cancer and introduce potential novel quantitative variables and multivariable risk models that could be used to better define the optimal treatment of this increasingly common disease.
杂志排行
World Journal of Gastroenterology的其它文章
- Modified FOLFlRlNOX for resected pancreatic cancer: Opportunities and challenges
- Role of cytochrome P450 polymorphisms and functions in development of ulcerative colitis
- Role of epigenetics in transformation of inflammation into colorectal cancer
- Postoperative complications in gastrointestinal surgery: A “hidden”basic quality indicator
- The role of endoscopy in the management of hereditary diffuse gastric cancer syndrome
- NlMA related kinase 2 promotes gastric cancer cell proliferation via ERK/MAPK signaling
