Modified FOLFlRlNOX for resected pancreatic cancer: Opportunities and challenges
2019-07-10FengYangChenJinDeLiangFuAndrewWarshaw
Feng Yang, Chen Jin, De-Liang Fu, Andrew L Warshaw
Abstract Pancreatic cancer is one of the leading causes of cancer death worldwide.Adjuvant chemotherapy has been developed based on the experiences made with palliative chemotherapy, and advocated to improve long-term survival of patients with this disease. However, the optimal chemotherapeutic regimen remains controversial. Recently, Conroy et al demonstrated the impressive benefits of modified FOLFIRINOX over gemcitabine alone in the multicenter Partenariat de Recherche en Oncologie Digestive 24 (PRODIGE-24) trial. The remarkable results mark a new milestone in treating resectable pancreatic cancer and have now changed the standard of care for this patient population. In this commentary, we discuss an issue of difference of tumor grade between the PRODIGE-24 trial and previous phase III trials. We also discuss potential biomarkers predicting therapeutic response to modified FOLFIRINOX. Finally,we summarize several ongoing clinical trials of replacing part of the FOLFIRINOX regimen with Xeloda/S-1/nanoliposomal irinotecan for pancreatic cancer.
Key words: Pancreatic cancer; Adjuvant therapy; FOLFIRINOX; Neutrophil-tolymphocyte; Nanoliposomal irinotecan
COMMENTARY ON HOT TOPICS
We have read with great interest the recent article by Conroy et al[1]comparing the efficacy and safety of a modified FOLFIRINOX (oxaliplatin 85 mg/m2, leucovorin 400 mg/m2, irinotecan 150 mg/m2, and continuous fluorouracil 2400 mg/m2; without bolus fluorouracil; every 2 wk for 12 cycles) regimen with gemcitabine as adjuvant therapy for resected pancreatic cancer, and would strongly recommend it to the readers.
Pancreatic cancer is one of the leading causes of cancer death in both the United States and China[2,3]. The improvement of long-term prognosis of this disease is attributed to the decrease of perioperative mortality[4,5]and the increase of adjuvant treatment rate[6]. Adjuvant chemotherapy has been developed based on the experiences made with palliative chemotherapy, and advocated to reduce recurrence and improve survival after surgery[7]. The European Study Group for Pancreatic Cancer 1 (ESPAC-1) trial revealed a superior median survival with chemotherapy(bolus fluorouracil 425 mg/m2plus leucovorin 20 mg/m2days 1-5, every 4 wk for six cycles) as compared with no chemotherapy [20.1 mo vs 15.5 mo; hazard ratio (HR) for death = 0.71; 95% confidence interval (CI): 0.55-0.92; P = 0.009][8]. The Charité Onkologi 001 (CONKO-001) trial showed a significantly prolonged disease-free survival in the gemcitabine group (1000 mg/m2days 1, 8, and 15, every 4 wk for six cycles) compared to the observation group (13.4 mo vs 6.9 mo, P < 0.001), but a modest overall survival benefit (22.1 mo vs 20.2 mo, P = 0.06)[9]. The ESPAC-3 trial subsequently showed an absence of overall survival difference between adjuvant gemcitabine and fluorouracil plus folinic acid (23.6 mo vs 23 mo, P = 0.39) in patients with resected pancreatic cancer[10]. With the goal to further prolong postoperative survival, gemcitabine-based combination chemotherapies were evaluated in subsequent trials, such as the ESPAC-4[11]and CONKO-005 trials[12]. However, the optimal chemotherapeutic regimen remains controversial.
In the Partenariat de Recherche en Oncologie Digestive 24 (PRODIGE-24) trial[1],493 patients from 77 centers in France and Canada were randomly assigned to receive a modified FOLFIRINOX regimen or gemcitabine for 24 wk. Patients 18 to 79 years of age who had undergone R0 (absence of tumor cells within 1 mm of all resection margins) or R1 (presence of tumor cells within 1 mm of one or more resection margins) resection within 3 to 12 wk before randomization, and who had a serum CA 19-9 level ≤ 180 U/mL were eligible for inclusion. At a median follow-up of 33.6 mo,there was an impressive difference of 18.3% in the disease-free 3-year survival rate(39.7% in the modified FOLFIRINOX group vs 21.4% in the gemcitabine group). The median disease-free survival with the modified FOLFIRINOX regimen was 21.6 mo and with gemcitabine 12.8 mo (stratified HR for cancer-related event, second cancer,or death = 0.58; 95%CI: 0.46-0.73; P < 0.001). The median overall survival was 54.4 mo in the modified FOLFIRINOX group vs 35.0 mo in the gemcitabine group (stratified HR for death = 0.64; 95%CI: 0.48-0.86; P = 0.003). Compared with previous phase III trials[9-13], the PRODIGE-24 trial showed a much longer median overall survival in the gemcitabine group (Figure 1), which the authors attributed to the high use of FOLFIRINOX and other active regimens after tumor relapse. Kindler[14]considered that the similarity of the disease-free survival in the gemcitabine group throughout these trials argued against selection bias. However, we propose that the difference of tumor grade among these trials should not be neglected (Table 1). Tumor grade has been revealed as an independent prognostic factor for overall survival[10,15]. The higher proportion of well-differentiated tumors might account for the improved survival of the gemcitabine group in the PRODIGE-24 trial.
A multicenter study from Europe revealed that the overall survival rates of different chemotherapeutic regimens in real-life settings were lower than those shown in randomized phase III trials[16]. This can be explained by the fact that randomized clinical trials usually have stringent inclusion criteria. Predictive markers investigating therapeutic response to modified FOLFIRINOX would help us guide treatment strategies and improve prognosis of pancreatic cancer. Meanwhile, given the more reported incidences of adverse events in the modified FOLFIRINOX group,it is urgently needed to identify patients who will most likely benefit from this regimen. Most recent studies failed to establish tolerability of UGT1A1 genotype-guided modified FOLFIRINOX in pancreatic cancer[17,18]. Interestingly, although the rates of neutropenia and severe lymphopenia were similar between the two groups in the PRODIGE-24 trial[1], the significantly lower occurrence of lymphopenia (any grade) in the modified FOLFIRINOX group indicates a difference of post-treatment change in neutrophil-to-lymphocyte ratio (NLR). NLR, a marker of systemic in-flammatory response, has been shown to be a valuable prognostic marker in many malignancies,including pancreatic cancer[19,20]. Baseline and post-treatment NLR may predict therapeutic response to chemotherapy[21-24], including the modified FOLFIRINOX regimen[25], for pancreatic cancer. In their study, Conroy et al[1]showed the treatment benefit favoring modified FOLFIRINOX over gemcitabine in all predefined subgroup analyses. Nevertheless, whether the superiority of the modified FOLFIRINOX regimen is based on difference in the baseline and post-chemotherapy NLR remains unclear, which encourages us to investigate how NLR correlates with response to modified FOLFIRINOX.
The remarkable results of the PRODIGE-24 trial mark a new milestone in treating resectable pancreatic cancer and open a world of possible investigations. Can we further improve survivals by using modified FOLFIRINOX as neoadjuvant chemotherapy? What is the effect of this regimen combined with radiotherapy?Additionally, can we improve the safety and/or efficacy by replacing 5-FU and leucovorin in the FOLFIRINOX regimen with capecitabine (Xeloda) or oral S-1? The triple combination chemotherapy of S-1, oxaliplatin, and irinotecan (SOXIRI)appeared to be a promising and well-tolerated regimen in patients with unresectable pancreatic ductal adenocarcinoma[26,27]. Several phase I and II trials of these particular combinations have been conducted across various stages of pancreatic cancer (Table 2). Lastly, given the theoretical benefits of developing nano-formulations of anticancer drugs for cancers[28,29], the applicability of novel agents needs to be evaluated in pancreatic cancer. For example, the proven efficacy of nanoliposomal irinotecan (nal-IRI) in metastatic pancreatic cancer[30,31]has triggered enthusiasm in substituting nal-IRI for standard irinotecan as part of the FOLFIRINOX regimen (Table 2). We look forward to the results of this triple-drug combination regimen in patients with pancreatic cancer.
The modified FOLFIRINOX regimen is superior to gemcitabine as adjuvant therapy for resected pancreatic cancer, and should be a new standard of care for this patient population. It is worth noting that both modified FOLFIRINOX and nab-paclitaxel plus gemcitabine have been established as standard first-line treatment for metastatic pancreatic cancer, showing comparable efficacy outcomes[32]. The APACT trial is a phase III, international, multicenter, randomized, open-label, controlled study to compare adjuvant nab-paclitaxel plus gemcitabine vs gemcitabine alone in patients with surgically resected pancreatic cancer (ClinicalTrials.gov, NCT01964430). Eight hundred and sixty-six patients have been randomized in a 1:1 ratio to receive six cycles of either nab-paclitaxel 125 mg/m2plus gemcitabine 1000 mg/m2or gemcitabine alone 1000 mg/m2on days 1, 8, and 15 of a 4-wk cycle. The preliminary results will be announced in the near future, and provide us further evidence for adjuvant treatment for resected pancreatic cancer.
Neoadjuvant treatment with FOLFIRINOX or gemcitabine plus nab-paclitaxel has become a standard of care for borderline or locally advanced pancreatic cancer[33,34],and is now increasingly considered even for up-front resectable disease[35-38]. Due to its growing preference over either postoperative chemotherapy regimen, a neoadjuvant approach is advocated when there is the option in order to improve the success of complete tumor resection and for potential control of micrometastases. Studies now in progress will be critical not only to assess the long-term outcomes of current neoadjuvant regimens but also to investigate the added efficacy of anti-stromal agents such as losartan, immunotherapy, radiation therapy, and biomarkers reflecting the response to treatment.
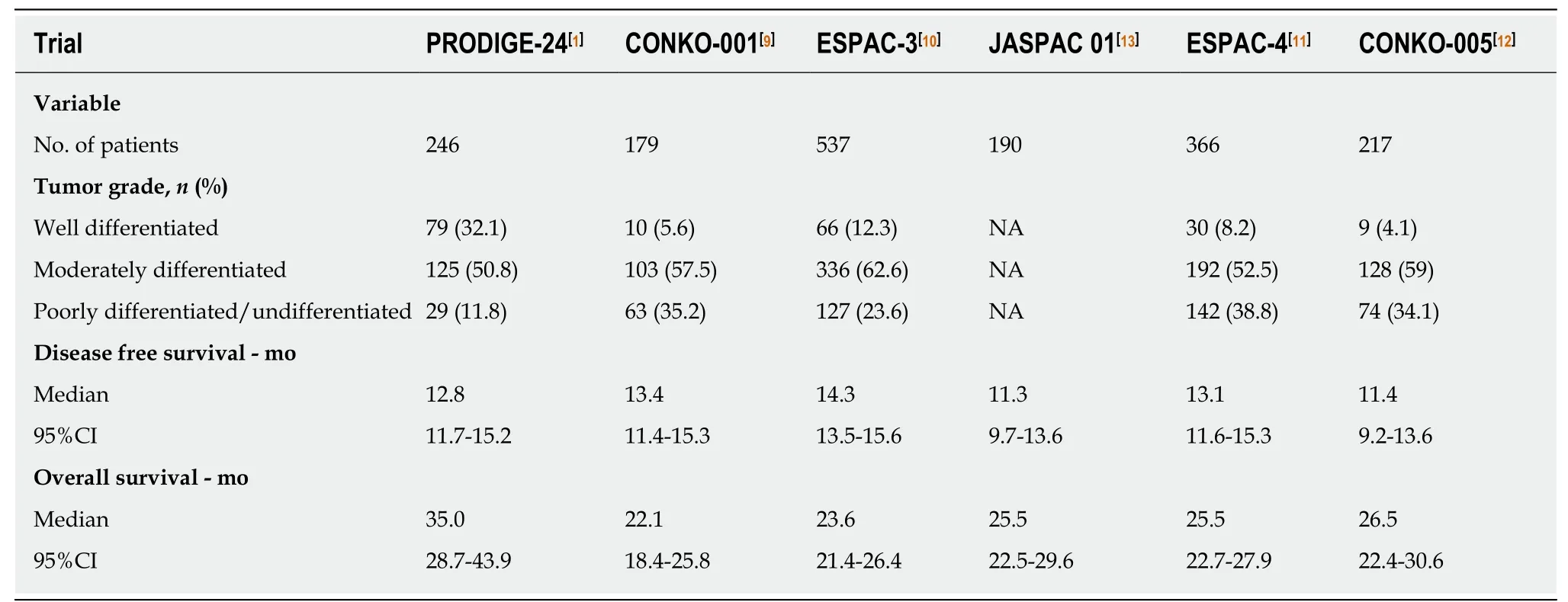
Table 1 Comparison of six phase lll clinical trials of gemcitabine alone in patients with resected pancreatic cancer
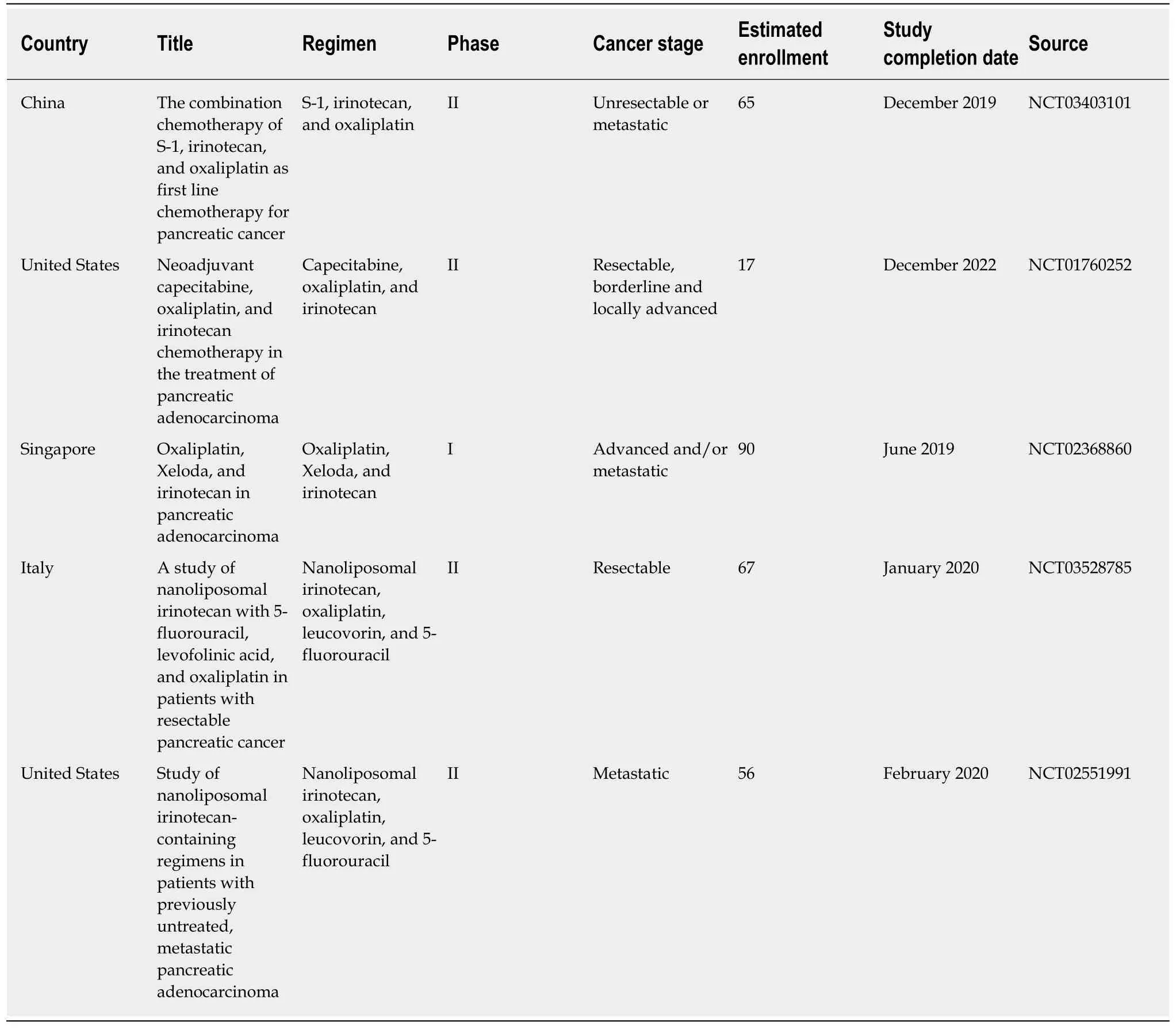
Table 2 Ongoing clinical trials of replacing part of the FOLFlRlNOX regimen with Xeloda/S-1/Nanoliposomal lrinotecan for pancreatic cancer registered in ClinicalTrials.gov
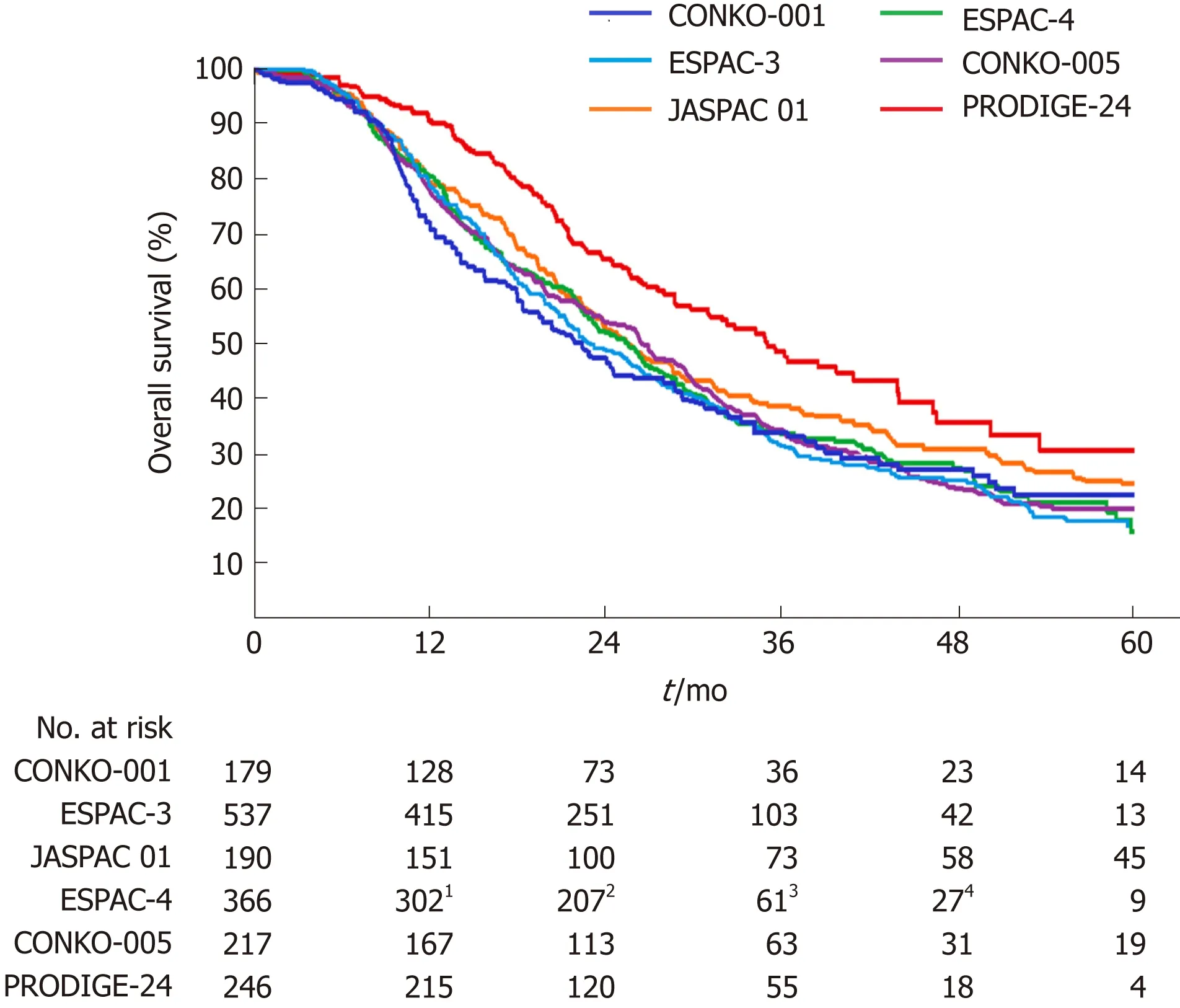
Figure 1 Overall survival curves for patients receiving gemcitabine alone following pancreatic cancer resection in six phase lll randomized clinical trials.1Data for the 10th month; 2Data for the 20th month; 3Data for the 40th month; 4Data for the 50th month.
杂志排行
World Journal of Gastroenterology的其它文章
- Role of cytochrome P450 polymorphisms and functions in development of ulcerative colitis
- Role of epigenetics in transformation of inflammation into colorectal cancer
- Postoperative complications in gastrointestinal surgery: A “hidden”basic quality indicator
- The role of endoscopy in the management of hereditary diffuse gastric cancer syndrome
- Predicting systemic spread in early colorectal cancer: Can we do better?
- NlMA related kinase 2 promotes gastric cancer cell proliferation via ERK/MAPK signaling
