Lesson Eighty-nine Noninvasive clues for diagnosing ventricular tachycardia mechanism
2019-06-11
The electrophysiologic mechanisms responsible for ventricular tachycardia(VT)fall in one of 3 categories:abnormal automaticity;triggered activity;and reentry.
Abnormal automaticity
Automaticity is the property of cardiac cells to generate spontaneous action potentials and is the result of diastolic depolarization caused by a net inward current during phase 4 of the action potential.Normal automaticity isa property ofthe sinoatrialand atrioventricularnodesand dependsmainly on 2 phenomena:diastolic activation of If(funny current),a mixed Na-K inward current,which unlike most voltagesensitive currents,is activated by hyperpolarization rather than depolarization;and release of calcium from the sarcoplasmic reticulum into the cytosol.The calcium,in turn,activates the Na+-Ca2+exchanger,resulting in a net influx of sodium ions.
Ventricularmyocardialcells do notdisplay spontaneous diastolic depolarization or automaticity under normal conditions,but abnormal automaticity may occur under pathological conditions when the resting membrane potential becomes less negative.This may be consequence of a decrease in IK1 or an enhanced calcium release from the sarcoplasmic reticulum.Similar to normal automaticity,abnormal automaticity is enhanced by β-adrenergic agonists and by reduction of external potassium.
Examples ofabnormalautomaticity include accelerated idioventricular rhythm in the setting of acute ischemia,myocarditis or cocaine intoxication
Triggered activity
It refers to action potential formation resulting from oscillations in membrane potential that are dependent on the preceding action potential.When the amplitude of one ofthese afterdepolarizations reaches certain threshold,voltage-gated ion channels are activated,generating an action potential.Triggered activity can occur in the form of early or delayed afterdepolarizations.Early afterdepolarizations(EADs)occur during phase 2 or 3 of the cardiac action potential and are more manifest at slower heart rates,whereas delayed afterdepolarizations(DADs)occur during phase 4 of the action potential,after full repolarization,and are more dependent on faster heart rates.
Examples of EADs include drug and electrolyte-induced Torsades de Pointes1and some forms of polymorphic VT due to congenital long QT syndrome.DADs are responsible for the majority of outflow tractVTs,catecholaminergic polymorphic ventricular tachycardia (CPVT) and ventricular arrhythmias associated with digitalis toxicity.
Reentry
It is the most common mechanism of VT.It involves continuous repetitive propagation of an impulse around an area of anatomical or functional conduction block.The following 3 criteria were originally proposed by Mines for identification of reentry:1)unidirectional block must occur;2)a region of slow conduction with return of the excitatory wave to its point of origin;and 3)interruption of the reentrant circuit at any points should terminate the tachycardia.The substrate for reentry requires the presence of 2 pathways with different electrophysiologic properties separated by a central area of block (anatomical or functional).When an impulse encounters the central obstacle,unidirectional block occurs in one of the pathways and slow conduction occurs through the other pathway,creating a circus movement.For reentry to occur,the conduction within the unblocked pathway must be slow enough so the previously blocked pathway can recover its excitability by the time the reentrant wavefront returns.In other words,the anatomical length of the circuit should equal or exceed the reentrant wavelength.
Reentrantarrhythmias can be reproducibly initiated and terminated by programmed stimulation.They can also interact with pacing and demonstrate the hallmark features of resetting and entrainment with fusion.
Examples of reentry include:1)scar-related VT in patients with structurally abnormal hearts due to ischemic or nonischemic cardiomyopathies;2)bundle branch reentry,which is typically seen in patients with infrahisian conduction disease and involves antegrade conduction overtherightbundleand retrograde conduction over the left bundle (or vice versa);3)idiopathic left ventricular tachycardia(also known as fascicular VT,Belhassen VT or verapamil-sensitive VT),in which the macro-reentrant circuit involves the left posterior fascicle(or less commonly the left anterior fascicle)and abnormal slowly conducting Purkinje fibers;and 4)Phase 2 reentry associated with VAs in Brugada syndrome.
Sinus rhythm ECG
Baseline sinus rhythm 12-lead ECG may be helpful by indicating disease processes known to be associated with specific VT mechanisms.The presence of Q waves consistent with prior myocardial infarction indicates the substrate for scar-related reentry,especially if the VT morphology is consistent with an exit from the region of the infarct.Epsilon waves in the right precordial leads,especially in the setting of a left bundle-branch block VT,is a marker of arrhythmogenic right ventricular cardiomyopathy/dysplasia(ARVC/D)and suggestsreentryinvolvingnon-ischemicscar localized to the right ventricle.Any evidence for His-Purkinje system disease as indexed by QRS widening,especially in the setting ofa dilated cardiomyopathy,can predispose to bundle branch reentrant VT.A Brugada pattern is associated with phase 2 reentry,a special type of reentry proposed to be caused by heterogeneity in action potential distribution between the epicardium and endocardium.
Twelve-lead morphology of the ventricular tachycardia
Outflow tract VT and premature ventricular contractions(PVCs)characterized by large monophasic R waves in the inferior leads,especially in patients with structurally normal heart,can confidently be attributed to DAD-induced triggered activity.However,reentrant VTs associated with nonischemic cardiomyopathies can frequently originate near the perivalvular region.As such,these tachycardias will mimic morphology of outflow tract VT due to a triggered mechanism.The presence ofmultiple VT morphologies and the identification ofa region oflow bipolarvoltage surrounding these valvularstructuressupportthe diagnosis ofnonischemic cardiomyopathy and a probable reentrant VT mechanism.
A QRS morphology during VT typical for LBBB is suggestive of bundle branch reentrant VT.This typically occurs in the context of dilated cardiomyopathy with underlying His-Purkinje system disease.The VT is typically rapid and often presents with presyncope,syncope or cardiac arrest.
A situation commonly encountered in patients with structural heart disease is the presence of more than one VT morphology.Paired VTs with similar cycle length but opposite axis,especially in the context of an ischemic etiology,points to macro-reentry involving a large circuit(Fig.1).For example,2 different VTs with LBBB pattern and left axis,one of them with a basal exit and another with apical axis,suggests macro-reentry involving a septal substrate.The presence of multiple LBBB VT morphologies with late precordial transition should suggest the possibility of ARVC.
A particular and rare ECG pattern is bidirectional VT,defined as a tachycardia showing beat-to-beat alternation in the QRS axis.The most common causes of bidirectional VT are digitalis toxicity and CPVT,and the proposed mechanismis DAD-mediated triggered activity in anatomically separate parts of the conduction system.
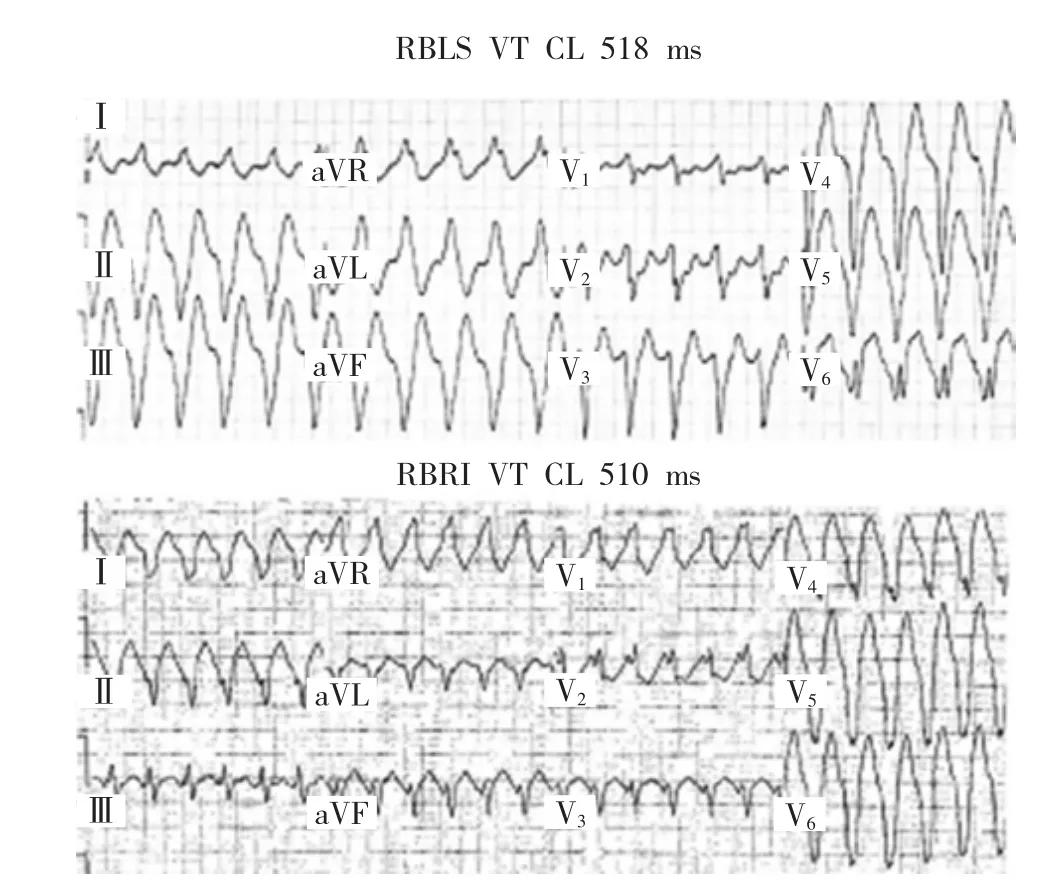
Figure 1 Paired VT morphologies in a patient with ischemic cardiomyopathy.The superior panel shows a right bundle left superior(RBLS)axis VTwith a CL of 518ms and the inferior panel shows a right bundle right inferior (RBRI) axis VT with a CL of 510ms. This is manifestation of a large macro-reentrant circuit with exit sites mapped to the septal and lateral aspects of the ischemic scar.
Tachycardia onset and termination
Two different patterns of VT initiation have been described:VT preceded by ventricular ectopy(single or multiple)of different morphology than that of the tachycardia;and VT not preceded by ventricular ectopy(sudden onset).As previously mentioned,a hallmark of reentrant VTs is reproducible initiation with ventricular programmed stimulation.This type of initiation can also be seen in triggered VTs,but not in automatic VTs.Spontaneous PVCs are a noninvasive correlate of ventricular extrastimuli and,thus,initiation with a PVC of different morphology than that of the tachycardia suggests a reentrant mechanism(fig.2).This in contrast with automatic and triggered VTs,which typically start with a beat similar to the ensuing beats of tachycardia.However,it must be said that sudden onset does not exclude reentry.In summary,consistent initiation with a PVC morphologically distinct to the VT typically will indicate reentry.On the other side,sudden initiation can be seen with any VT mechanism.
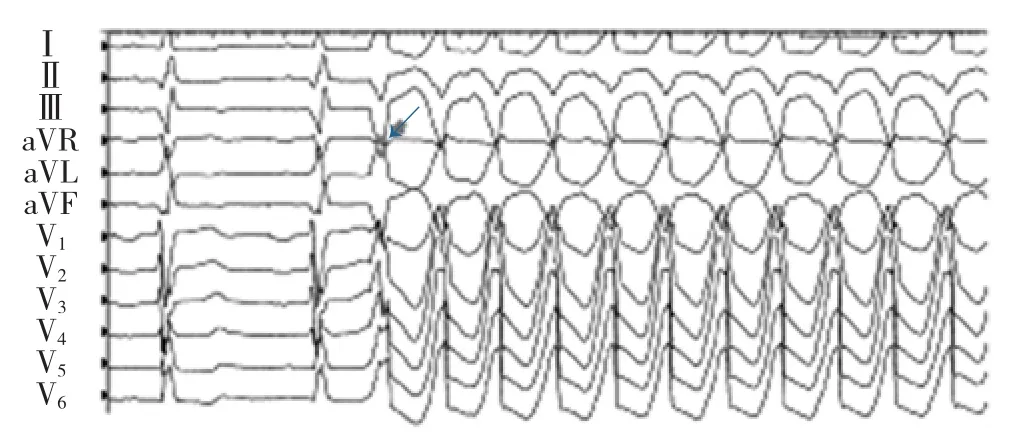
Figure 2 Examples of VT initiation with a PVC of different morphology than the ensuing beats of tachycardia(arrow).
The prototype of automatic VT is accelerated idioventricular tachycardia(AIVT),which is most often observed in the setting of acute myocardial infarction and reperfusion,but it can also be seen in acute myocarditis, hypertensive heart disease, digitalis intoxication and cocaine intoxication.It often begins as a late-coupled ventricular beat at a rate just faster than the preceding sinus rate.
A progressive increase in rate with tachycardia onset(warm-up)and/or progressive deceleration before tachycardia termination(cool-down)are also suggestive of an automatic mechanism.
Response to pharmacologic agents
Probably the most useful and specific response to pharmacological intervention is the effect of adenosine on VT due to triggered activity.The cellular basis for triggered activity due to DAD is intracellular calcium overload,which induces a Ca2+-dependent depolarizing current (transient inward current or Iti),mainly given by activation of the Na+-Ca2+exchanger.The transient inward sodium current gives rise to DADs,which,if of sufficient amplitude,triggers a new action potential that may result in tachycardia.Catecholamines,through stimulation ofthe β-adrenergicreceptor,causes activation of adenylyl cyclase (AC),increase of cAMP,activation of protein kinase A and phosphorylation of the L-type Ca channel, ryanodine receptor2, and phospholamban3.This results in increased intracellular calcium levels and enhanced activity of the Na+-Ca2+exchanger.Adenosine exerts an inhibitory effect on AC and cAMP,reversing intracellular calcium overload.Given that adenosine has no antiarrhythmic effect in reentrant VT and only transiently suppresses(but does not terminate) automatic VT,termination of VT in response to adenosine is considered diagnostic of cAMP-mediated triggered activity.
词 汇
sarcoplasmic adj.肌质的
cytosol n.细胞溶质
afterdepolarization n.后除极
catecholaminergic adj.儿茶酚胺活性的,儿茶酚胺能的
excitatory adj.显示激动的,有刺激性的,兴奋的
infrahisian adv.在希氏束下
hallmark n.&vt.标记,标志;给 打上烙印,给 标识
correlate n.&vt.相关物,相关联的人;与 相关,相互联系,
注 释
1.Torsades de Pointes指尖端扭转型室性心动过速,是一种特殊的室性快速心律失常,多见于QT间期延长及低血钾患者,常呈慢心率依赖。
2.ryanodine receptor可音译为雷诺丁受体,是一种大流量钙离子释放通道,介导细胞内内质网和肌浆网的钙离子释放,在众多生物学功能中起关键作用。
3.phospholamban是一种受磷蛋白,是心肌收缩的关键性调节蛋白,能可逆性抑制肌浆网上的钙离子泵功能,导致心肌收缩力下降,并可引起扩张型心肌病。
参考译文
第89课 室性心动过速机制的无创诊断线束
室性心动过速的电生理机制不外乎以下3种:自律性异常、触发活动和折返。
自律性异常
自律性是心肌细胞产生自发动作电位的特性,是动作电位四相期间内向净电流导致舒张期除极的结果。正常自律性是窦房结和房室结的特性,主要基于两种现象:If(funny电流)的舒张期激动,一种混合型钠-钾内向电流,这不同于多数电压敏感的电流,是由超极化而非除极激动激活,和从内质网释放的钙进入胞内,随后钙激活钠-钙交换器,导致钠离子净内流。
正常情况下心室肌细胞并不发生舒张期除极或无自律性。但在病理情况下,当静息膜电位负值减小时可发生异常自律性。这可能是IK1减少或从肌质网释放的钙增加的结果。与正常自律性相似,beta激动剂和胞外钾减少可促进异常自律性。
自律性异常包括急性心肌缺血、心肌炎或可卡因中毒情况下的加速性室性自主节律。
触发活动
动作电位形成来自膜电位振荡,依赖于前面的动作电位。当这些后除极中有振幅达到一定阈值时,电压门控离子通道激活,产生动作电位。触发活动可发生于早后除极或延迟后除极。早后除极发生于心脏动作电位的2相或3相,多见于心率较慢时,而延迟后除极发生于动作电位4相,即完全复极后,多呈较快心率依赖。
早后除极的例子有药物和电解质诱发的尖端扭转型室性心动过速和某些与先天性长QT间期综合征相关的多形性室性心动过速。延迟后除极是多数流出道室性心动过速、儿茶酚胺类多形性室性心动过速和地高辛中毒相关室性心动过速的发病机制。
折返
这是室性心动过速最常见的机制。涉及冲动围绕解剖区域或功能传导阻滞区连续重复传导。Mines最初提出鉴别折返的下述3条标准:(1)必须存在单向阻滞;(2)经缓慢传导区激动波回到其起点:(3)折返环的任一点中断将终止心动过速。折返基质需存在两条电生理特性不同的通路,并由(解剖或功能)阻滞中心区分隔开。当脉冲遇到中心屏障时,一条通路发生单向阻滞,而另一条通路呈缓慢传导,产生循环运动。要发生折返,单向阻滞通路上的传导必须足够慢,以保证折返波回来时其前阻滞的通路已恢复兴奋性。换言之,环路的解剖长度应等于或大于折返波长。
折返型心律失常可通过程控刺激反复诱发和中止。他们可以与起搏相互影响,证实重置和融合拖带的标志性特性。
折返的例子有:(1)因缺血性或非缺血性心肌病导致心脏结构异常患者的疤痕相关室性心动过速;(2)束支折返,典型的见于希氏束以下传导病变的患者,涉及经右束支前传和经左束支逆传(或反之);(3)特发性左室心动过速(也称分支型室性心动过速、Belhassen室性心动过速、异博定敏感型室性心动过速),其大折返环涉及左后分支(左前分支不常见);(4)与Brugada综合征室性心律失常相关的2相折返。
窦性节律心电图
基础窦性节律12导联心电图有助于提示与特殊室性心动过速机制相关的疾病过程。与以往心肌梗死相一致的Q波提示瘢痕相关的折返,特别当室性心动过速的形态与梗死区出口相一致时。右胸导联Epsilon波,特别在左束支阻滞图形室性心动过速时,是致心律失常右室心肌病/心肌发育不良的标志,提示折返涉及位于右心室的非缺血疤痕。希浦系统病变迹象如QRS波群增宽,特别在扩张型心肌病,倾向于束支折返室性心动过速。Brugada图形与2相折返有关,是一种特殊的折返,认为由心外膜与心内膜之间动作电位不均质引起。
室性心动过速的12导联形态
流出道室性心动过速和期前收缩特征表现为下壁导联大的单向R波,特别是心脏结构正常的患者,可以确信为延迟后除极诱发的触发活动所致。然而,与非缺血心肌病相关的折返型室性心动过速常起源于瓣周附近。因为如此,这些心动过速形态类似于由触发机制引起的流出道室性心动过速。室性心动过速呈多种形态和瓣膜结构周边的低双极电压区支持非缺血性心肌病的诊断和可能的折返机制。
室性心动过速QRS波群呈典型的LBBB形态提示束支型折返室性心动过速。这通常发生于扩张型心肌病合并希氏束系统疾病者。这种室性心动过速典型的是速率快,常以先兆晕厥、晕厥和心跳骤停而就诊。 .
结构性心脏病患者常见的情况是出现一种以上的室性心动过速形态。成对室性心动过速周长相似而极性相反,尤其存在缺血病因时,提示涉及大环的巨折返(图1)。例如,2种不同的LBBB形态且电轴左偏的室性心动过速,一种呈基底部出口电轴,而另一种呈心尖出口电轴,提示涉及间隔基质的巨折返。表现为多种LBBB室性心动过速形态且胸导联移行晚提示致心律失常心肌病/心肌发育不良可能。
双向性室性心动过速是一种特殊而罕见的心电图类型。心动过速显示QRS波群电轴每搏交替。双向性室性心动过速的最常见原因是地高辛中毒和儿茶酚胺类心动过速,其可能的机制为解剖上隔离的传导系统部分延迟后除极介导的触发活动。
心动过速的发生与中止
已描述过两种室性心动过速发作类型:一种为室性心动过速前有与其形态不同(一种或多种)的室性期前收缩;另一种室性心动过速前无心室异位波动(突发)。如以前所述,折返型室性心动过速可由心室程序刺激反复诱发。这种类型发作也见于触发型室性心动过速,但不见于自律性室性心动过速。自发性室性期前搏动相当于一种非侵入性的室性期外刺激,因此,由与室性心动过速形态不同的室性期前搏动诱发的提示属折返机制(图2)。这与自律性和触发的室性心动过速不同,这两种室性心动过速的起始搏动与其后心动过速的搏动相似。然而,有必要说的是突发并不能排除折返。总之,始终由不同于室性心动过速的室性期前搏动诱发的室性心动过速通常是折返。相反,突发的可见于任何室性心动过速机制。
自律性室性心动过速的原型是加速的室性自主心动过速,这最常见于急性心肌梗死和再灌注过程,但也可见于心肌炎、高血压性心脏病、地高辛中毒和可卡因中毒。这常始于一速率略快于其前窦性节律的长联律间期室性搏动。
心动过速开始时速率逐渐增加(温热)和中止前速率逐渐下降(冷却)也支持自律性机制。
对药物的反应
腺苷对触发活动引起的室性心动过速的影响可能是对药物干预最有用和特异的反应。与延迟后除极相关的触发活动的细胞基础是胞内钙过负荷,这诱发钙离子依赖除极电流(短暂内向电流或Iti),主要通过激活钠-钙(Na+-Ca2+)交换器。短暂的内向钠电流引发延迟后除极,这在振幅足够高时触发一新的动作电位而导致心动过速。儿茶酚胺通过刺激β肾上腺素能受体,激活腺苷环化酶(AC),增加cAMP,激活蛋白激酶A和使L型钙通道、ryanodine受体和phospholamban磷酸化。这可引起胞内钙水平提高并促进Na+-Ca2+交换器的活化。腺苷抑制AC和cAMP,逆转胞内钙过负荷。鉴于腺苷对折返型室性心动过速无抗心律失常作用,对自律性室性心动过速只是短暂抑制(不能中止),因此,腺苷中止的室性心动过速应考虑为cAMP介导的触发活动。
图1缺血性心肌病患者成对室性心动过速形态。上图显示右束支左上电轴室性心动过速,周长518ms,下图显示右束支右下电轴室性心动过速,周长510ms。这是一种大的巨折返环表现,标测到出口部位位于缺血性疤痕的间隔面和外侧面。
图2不同于室性心动过速形态的室性期前搏动诱发室性心动过速图例(箭头所示)。