冬凌草的化学成分研究
2019-05-15李珊珊张琦李鑫许婧郭远强
李珊珊,张琦,李鑫,许婧,郭远强
(1.哈尔滨商业大学药学院,黑龙江 哈尔滨 150076; 2.南开大学药学院,天津 300350)
冬凌草 [Rabdosiarubescens(Hemsl.) Hara]为唇形科香茶菜属植物,学名碎米桠,主要分布于河南及黄河流域以南[1]。冬凌草味甘苦、性微寒,具有清热解毒、消炎止痛及抗肿瘤等作用[2]。临床上主要用于治疗咽喉肿痛、扁桃体炎、蛇虫咬伤及食道癌等疾病[3]。近年来,对冬凌草的化学成分研究表明,其主要成分为二萜类化合物,另外还含有一些黄酮、三萜、生物碱、多糖和挥发性成分[4-6]。为了充分开发冬凌草的药用植物资源,本实验对冬凌草的化学成分及其抗肿瘤活性进行了研究,从中分离鉴定了6个化合物,分别为:rabdosianin A(1)、parvifoline G(2)、rabdoternin C(3)、betulin(4)、betulinic acid(5)、古柯二醇(6),结构见图1,其中1~3为二萜类化合物,4~6为三萜类化合物。经文献检索,化合物6为首次从香茶菜属中分离得到,化合物1、2、4、5为首次从冬凌草中分离得到。
1 仪器与材料
1.1 仪器 半制备高效液相色谱仪 (北京创新通恒科技有限公司,LC 3000),色谱柱为YMC-Pack ODS-AM(250 mm × 20 mm);德国Bruker AV-400型核磁共振波谱仪。
1.2 材料 柱层析硅胶(100~200目,烟台江友硅胶开发有限公司);ODS 中压柱色谱填料(50 μm,日本 YMC公司);显色试剂为10%的硫酸乙醇溶液;有机溶剂购于天津化学试剂公司,均为分析纯,水为蒸馏水。冬凌草药材于2017年购于河北安国药材市场,由南开大学药学院郭远强教授鉴定。药材标本保存于南开大学药学院天然药物研究室。
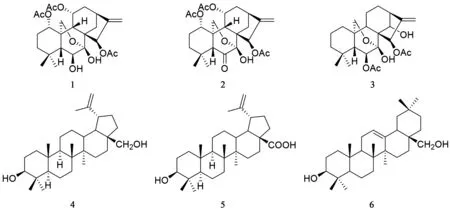
图1 化合物1~6的结构
2 提取和分离
干燥的冬凌草(12.0 kg),用甲醇加热回流提取3次(首次2 h,后两次各1.5 h),合并甲醇提取液,过滤并减压浓缩得浸膏1.0 kg。浸膏混悬于水中,经乙酸乙酯萃取,直至萃取到乙酸乙酯层颜色清浅,合并浓缩得乙酸乙酯萃取物205.5 g。乙酸乙酯萃取物经硅胶柱色谱分离,石油醚-丙酮梯度洗脱(100∶0~100∶25),薄层色谱(TLC)检测并合并得到6个粗分组分Fr.1~Fr.6。组分Fr.5经中压液相色谱(MPLC)(60%~90% 甲醇/水)梯度洗脱,得到8个亚组分Fr.5-1~Fr.5-8,亚组分Fr.5-4经半制备高效液相色谱 (HPLC)(70% 甲醇/水)分离纯化,得到化合物1(7.1 mg),亚组分Fr.5-6经半制备高效液相色谱 (HPLC)(83% 甲醇/水)分离纯化,得到化合物3(55.9 mg)。组分Fr.4经同上分离方法得到化合物2(6.9 mg)。组分Fr.2得到化合物4(5.6 mg)、化合物5(4.4 mg)、化合物6(4.2 mg)。
3 结构鉴定
3.1 化合物1 白色固体。1H-NMR (CDCl3,400 MHz)δ:4.87 (1H,m,H-1),1.76 (1H,m,H-2),1.33 (1H,m,H-2),1.37 (1H,m,H-3),1.48 (1H,m,H-3),1.68 (1H,m,H-5),3.95 (1H,d,J=8.1 Hz,H-6),2.14 (1H,d,J=3.4 Hz,H-9),4.82 (1H,m,H-11),1.63 (1H,m,H-12),2.32 (1H,dd,J=9.3,16.4 Hz,H-12),2.67 (1H,m,H-13),1.62 (1H,m,H-14),2.48 (1H,d,J=12.3 Hz,H-14),5.62 (1H,t,J=2.5 Hz,H-15),5.04 (1H,d,J=2.3 Hz,H-17),4.86 (1H,brs,H-17),1.08 (3H,s,H-18),1.13 (3H,s,H-19),4.12 (1H,d,J=9.0 Hz,H-20),4.50 (1H,dd,J=1.8,9.1 Hz,H-20),2.09 (3H,s,H-2′),2.11 (3H,s,H-2″),1.94 (3H,s,H-2‴)。13C-NMR (CDCl3,100 MHz)δ:76.7 (C-1),25.2 (C-2),38.7 (C-3),33.2 (C-4),53.1 (C-5),74.0 (C-6),96.3 (C-7),50.4 (C-8),48.2 (C-9),40.5 (C-10),69.5 (C-11),39.8 (C-12),34.9 (C-13),26.6 (C-14),74.6 (C-15),156.6 (C-16),109.5 (C-17),33.7 (C-18),22.6 (C-19),65.5 (C-20),171.4 (C-1′),22.2 (C-2′),170.6 (C-1″),21.9 (C-2″),169.9 (C-1‴),21.8 (C-2‴)。以上数据与文献[7]报道的化合物rabdosianin A的数据基本一致,因此鉴定化合物1为rabdosianin A。
3.2 化合物2 无色晶体(甲醇)。1H-NMR (CDCl3,400 MHz)δ:4.94 (1H,m,H-1),1.76 (1H,m,H-2),1.80 (1H,m,H-2),1.43 (1H,m,H-3),1.52 (1H,m,H-3),2.43 (1H,s,H-5),2.08 (1H,m,H-9),4.91 (1H,m,H-11),1.71 (1H,m,H-12),2.35 (1H,dd,J=8.8,15.6 Hz,H-12),2.78 (1H,dd,J=4.7,8.9 Hz,H-13),1.49 (1H,m,H-14),2.56 (1H,d,J=12.3 Hz,H-14),5.41 (1H,t,J=2.9 Hz,H-15),5.08 (1H,dd,J=1.3,1.6 Hz,H-17,4.83 (1H,brs,H-17),1.40 (3H,s,H-18),1.08 (3H,s,H-19),4.82 (1H,d,J=8.7 Hz,H-20),4.24 (1H,d,J=9.2 Hz,H-20),2.13 (3H,s,H-2′),1.96 (3H,s,H-2″),2.04 (3H,s,H-2‴)。13C-NMR (CDCl3,100 MHz)δ:75.8 (C-1),24.9 (C-2),38.8 (C-3),34.4 (C-4),61.8 (C-5),206.2 (C-6),92.2 (C-7),48.4 (C-8),47.1 (C-9),42.8 (C-10),69.4 (C-11),41.0 (C-12),35.0 (C-13),24.9 (C-14),76.5 (C-15),153.9 (C-16),110.1 (C-17),34.4 (C-18),21.8 (C-19),65.4 (C-20),170.5 (C-1′),22.2 (C-2′),169.7 (C-1″),21.7 (C-2″),169.4 (C-1‴),20.7 (C-2‴)。以上数据与文献[8]报道的化合物parvifoline G的数据基本一致,因此鉴定化合物2为parvifoline G。
3.3 化合物3 白色粉末。1H-NMR (CDCl3,400 MHz)δ:1.42 (1H,m,H-1),1.08 (1H,m,H-1),1.26 (1H,m,H-2),1.50 (1H,m,H-2),1.46 (1H,m,H-3),1.12 (1H,m,H-3),1.59 (1H,d,J=8.1 Hz,H-5),5.28 (1H,d,J=7.6 Hz,H-6),2.30 (1H,m,H-9),1.46 (1H,m,H-11),1.29 (1H,m,H-11),2.38 (1H,m,H-12),1.54 (1H,m,H-12),2.58 (1H,d,J=9.3 Hz,H-13),4.61 (1H,s,H-14),6.04 (1H,s,H-15),5.09 (1H,s,H-17),5.16 (1H,s,H-17),0.83 (3H,s,H-18),1.13 (3H,s,H-19),4.15 (1H,d,J=9.6 Hz,H-20),3.84 (1H,d,J=9.9 Hz,H-20),2.20 (3H,s,H-2′),2.04 (3H,s,H-2″)。13C-NMR (CDCl3,100 MHz)δ:31.5 (C-1),14.8 (C-2),41.2 (C-3),33.4 (C-4),53.3 (C-5),74.1 (C-6),97.7 (C-7),51.9 (C-8),46.5 (C-9),35.8 (C-10),18.5 (C-11),31.2 (C-12),44.4 (C-13),75.2 (C-14),73.2 (C-15),156.9 (C-16),111.7 (C-17),33.0 (C-18),22.5 (C-19),66.9 (C-20),172.3 (C-1′),22.0 (C-2′),170.8 (C-1″),21.2 (C-2″)。以上数据与文献[3]报道的化合物rabdoternin C的数据基本一致,因此鉴定化合物3为rabdoternin C。
3.4 化合物4 白色固体。1H-NMR (CDCl3,400 MHz)δ:3.19 (1H,dd,J=5.2,11.2 Hz,H-3),0.69 (1H,d,J=9.4 Hz,H-5),1.26 (1H,m,H-9),1.60 (1H,m,H-13),2.38 (1H,m,H-18),1.59 (1H,m,H-19),0.97 (3H,s,H-23),0.76 (3H,s,H-24),0.82 (3H,s,H-25),1.02 (3H,s,H-26),0.98 (3H,s,H-27),3.33 (1H,d,J=10.4 Hz,H-28),3.80 (1H,d,J=10.8Hz,H-28),4.58 (1H,s,H-29),4.68 (1H,d,J=2.0 Hz,H-29),1.68 (3H,s,H-30)。13C-NMR (CDCl3,100 MHz)δ:38.9 (C-1),27.4 (C-2),79.0 (C-3),38.7 (C-4),55.3 (C-5),18.3 (C-6),34.2 (C-7),40.9 (C-8),50.4 (C-9),37.3 (C-10),20.8 (C-11),25.2 (C-12),37.1 (C-13),42.7 (C-14),27.0 (C-15),29.2 (C-16),47.8 (C-17),47.8 (C-18),48.7 (C-19),150.5 (C-20),29.7 (C-21),34.0 (C-22),28.0 (C-23),15.4 (C-24),16.1 (C-25),16.0 (C-26),14.8 (C-27),60.5 (C-28),109.7 (C-29),19.1 (C-30)。以上数据与文献[9]报道的化合物betulin的数据基本一致,因此鉴定化合物4为betulin。
3.5 化合物5 白色固体。1H-NMR (CDCl3,400 MHz)δ:3.19 (1H,dd,J=4.8,11.1 Hz,H-3),0.68 (1H,d,J=9.4 Hz,H-5),1.26 (1H,m,H-9),2.18 (1H,m,H-13),1.63 (1H,m,H-18),2.99 (1H,m,H-19),0.96 (3H,s,H-23),0.75 (3H,s,H-24),0.82 (3H,s,H-25),0.93 (3H,s,H-26),0.98 (3H,s,H-27),4.60 (1H,s,H-29),4.74 (1H,s,H-29),1.69 (3H,s,H-30)。13C-NMR (CDCl3,100 MHz)δ:38.9 (C-1),27.4 (C-2),79.0 (C-3),38.7 (C-4),55.3 (C-5),18.3 (C-6),34.3 (C-7),40.7 (C-8),50.5 (C-9),37.2 (C-10),20.8 (C-11),25.5 (C-12),38.4 (C-13),42.4 (C-14),30.5 (C-15),32.1 (C-16),56.3 (C-17),46.9 (C-18),49.2 (C-19),150.4 (C-20),29.7 (C-21),37.0 (C-22),28.0 (C-23),15.4 (C-24),16.1 (C-25),16.0 (C-26),14.7 (C-27),180.5 (C-28),109.7 (C-29),19.4 (C-30)。以上数据与文献[9]报道的化合物betulic acid的数据基本一致,因此鉴定化合物5为betulic acid。
3.6 化合物6 白色固体。1H-NMR (CDCl3,400 MHz)δ:3.23 (1H,m,H-3),0.75 (1H,m,H-5),1.53 (1H,m,H-9),5.19 (1H,t,J=3.7 Hz,H-12),1.98 (1H,m,H-18),1.00 (3H,s,H-23),0.78 (3H,s,H-24),0.93 (3H,s,H-25),0.94 (3H,s,H-26),1.17 (3H,s,H-27),3.21 (1H,m,H-28),3.56 (1H,d,J=11.0 Hz,H-28),0.89 (3H,s,H-29),0.87 (3H,s,H-30)。13C-NMR (CDCl3,100 MHz)δ:38.6 (C-1),27.2 (C-2),79.0 (C-3),38.8 (C-4),55.1 (C-5),18.3 (C-6),32.6 (C-7),39.8 (C-8),47.6 (C-9),36.9 (C-10),23.5 (C-11),122.4 (C-12),144.2 (C-13),41.8 (C-14),25.5 (C-15),22.0 (C-16),47.6 (C-17),42.3 (C-18),46.4 (C-19),31.0 (C-20),34.0 (C-21),31.0 (C-22),28.1 (C-23),15.6 (C-24),15.5 (C-25),16.7 (C-26),26.0 (C-27),69.8 (C-28),33.2 (C-29),23.6 (C-30)。以上数据与文献[10]报道的化合物古柯二醇的数据基本一致,因此鉴定化合物6为古柯二醇。
4 体外抗肿瘤活性
采用噻唑蓝(MTT)法,以依托泊苷为阳性对照,检测化合物1~6对人白血病细胞K562的体外抗肿瘤活性。实验结果表明,阳性对照依托泊苷的IC50值为2.57 μmol·L-1,化合物5表现出高于阳性对照的抑制作用,其IC50值为1.45 μmol·L-1,其余化合物均无活性。
