Application of a 3D-printed ”fistula stent” in plugging enteroatmospheric fistula with open abdomen: A case report
2019-05-08ZiYanXuHuaJianRenJinJianHuangZongAnLiJianAnRen
Zi-Yan Xu, Hua-Jian Ren, Jin-Jian Huang, Zong-An Li, Jian-An Ren
Abstrac t BACKGROUND Open abdomen (OA) has been generally accepted for its magnificent superiority and effectiveness in patients with severe trauma, severe intra-abdominal infection, and abdominal compartment syndrome. In the meantime, OA calls for a mass of nursing and the subsequent enteroatomospheric fistula (EAF), which is one of the most common complications of OA therapy, remains a thorny challenge.CASE SUMMARY Our team applied thermoplastic polyurethane as a befitting material for producing a 3D-printed “fistula stent” in the management of an EAF patient,who was initially admitted to local hospital because of abdominal pain and distension and diagnosed with bowel obstruction. After a series of operations and OA therapy, the patient developed an EAF.CONCLUSION Application of this novel “fistula stent” resulted in a drastic reduction in the amount of lost enteric effluent and greatly accelerated rehabilitation processes.
Key words: 3D printing; Enteroatmospheric fistula; Open abdomen; Isolation technique;Case report
INTRODUCTION
The open abdomen (OA) therapy has been commonly recognized as a crucial treatment in settling severe trauma, severe intra-abdominal infection (sIAI) and abdominal compartment syndrome (ACS). The World Society of ACS has formulated a classification for OA in which frozen OA with fistula is classified as grade 4 (Table 1)[1]. Meanw hile, fistula, which is one of the most common complications of OA,remains a nightmare for all surgeons and intensive care unit doctors, and urgently needs to be solved[2].
Enteroatmospheric fistula (EAF) is a type of fistula different from enterocutaneous fistula (ECF). The occurrence rate of EAFs is reported to be 25% in patients receiving OA w ith an associated mortality rate of 42%[3]. Various reasons result in these openings between the atmosphere and the lumen within the gastrointestinal tract.Unlike ECF, EAF has neither connective fistulous tract or overlying soft tissue such as the skin, subcutaneous tissue, omentum majus and other gastrointestinal tract which lead s to high flow of enteric fistula effluent and makes it unlikely to reach spontaneous closure. In add ition to fluid and electrolyte imbalance, acid-base imbalance and gastrointestinal dysfunction, enteric fistula effluent from EAF can cause more disastrous effects[4]: enteric fistula effluent contaminates the exposed wound and exacerbates the infection condition which can lead to sepsis in severe cases. Besides, high and continuous output of digestive juices raises the difficulty in fistula closure, prolongs the length of hospital stay along with medical expense[3], and increases mortality tremendously.
To date, multiple techniques have been come up with to solve EAF such as silicone fistula plug[5], collapsible EAF isolation device[6], polyethylene glycol tube[7], and covered self-expending metal stent[8]. However, these techniques can only be used after the formation of frozen abdomen or the adhesion between the bowel and the abdominal wall. On the other hand, they can hardly reach accurate match with the trend of bowel or the shape of the orifice of the fistulous tract. Furthermore, after the labiate fistula is formed, the outcomes usually get worse[9]. In order to carry out enteral nutrition (EN) as early as possible and shorten the w aiting time before definitive gastrointestinal reconstruction, our team applied a 3D-printed “fistula stent”, which is a combination of 3D printing with traditional isolation technique, to control enteric fistula effluent at the early stage of OA.
CASE PRESENTATION
Patient information
A 33-year-old male patient complaining of abdominal pain and distension w as initially diagnosed as bowel obstruction and went through enterolysis and omentum majus biopsy (Table 2). Several days after the first surgery, the patient appeared a relapse of abd ominal p ain and received exploratory laparotomy and byp ass operation. Then, the p atient's physical condition turned to go dow nhill w ith intermittent fever and cachexia. Afterwards, the patient was referred to our clinic with sIAI, hypoalbuminemia, hydrothorax, poor incisions healing, and suspected fistula with intestinal juice flowing from the incision.
After the patient was admitted to our hospital, he presented with intermittent high fever (39 °C) and cachexia. Anti-infection therapy, acid suppression therapy, digestivejuices secretion suppression therapy, total parenteral nutrition (PN), and double-pipe d rainage w ere utilized to maintain the p atient's homeostasis. To relieve the symptoms, w e d ecided to carry out OA to locate the source of enteric fistula effluent and control the IAI. During the p roced ure of exploration, the fistula w as found situated at the place w here the side-to-side anastomosis w as carried out during the bypass operation (Figure 1A). The volume of enteric fistula effluent reached a peak of approximately 2500 m L/d, corrod ing surrounding tissue and incision continuously,thus making the orifice of the fistulous tract and incision unlikely to heal spontaneously. Afterw ard s, high-resolution computed tomograp hy and contrastmediated fistula angiography confirmed the existence of the EAF (Figure 1B), the area of which reached 8.5 cm2, thus making most other isolation techniques inapplicable and invalid[9].
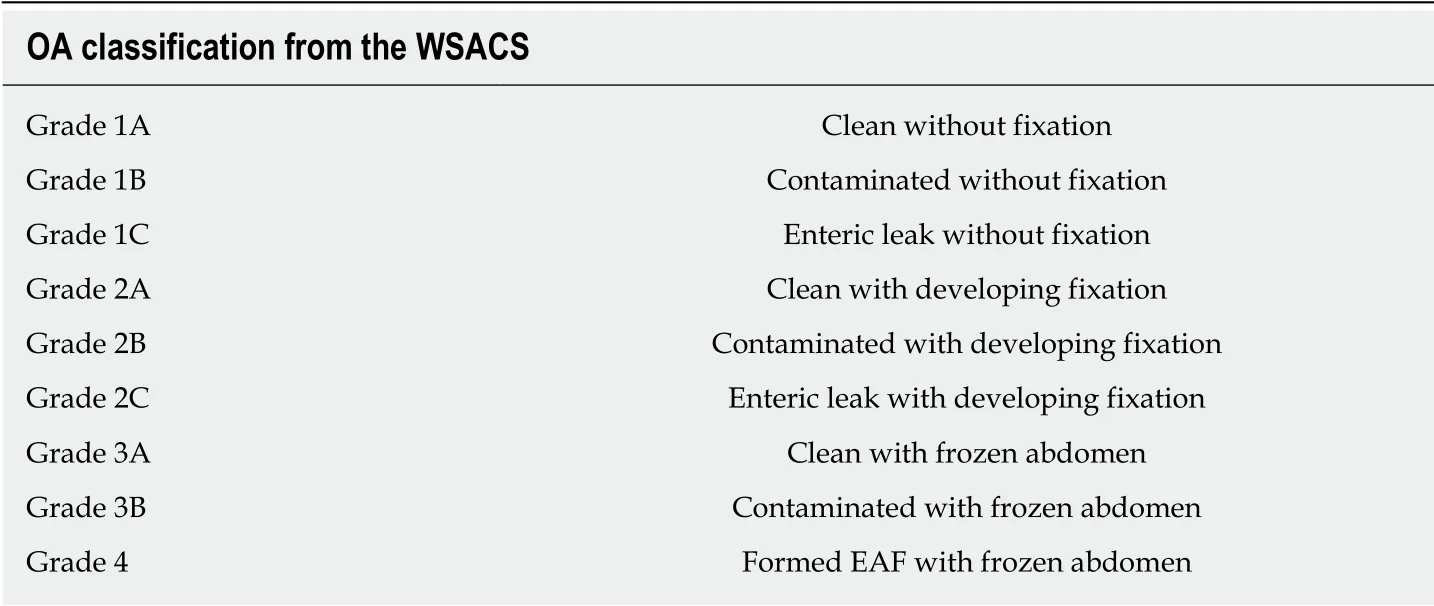
Table 1 Open abdomen classification from the World Society of the Abdominal Compartment Syndrome
Interventions
With the intention of blocking the enteric effluent to improve fistula healing, restoring EN, and carrying out definitive surgeries earlier, w e attempted to implant a “fistula stent” to plug the EAF. After investigating the anatomy of EAF from fistulography and measuring the actual inner diameter and tortuosity of the bowel w ith fingers, w e utilized Solid w ork softw are to build the model of a hollow and curving pipe stent w ith a small protuberance and integrated w all. The follow ing d ata w ere collected:inner diameter of 14 mm, thickness of pipe wall of 0.5 mm, length of 14 cm, and bend pipe instead of combined two straight ones. The w hole model w as saved in Standard Template Library (STL) form to be recognized by 3D printer (Figure 2A).
Thermoplastic polyurethane (TPU) is a synthetic and biocompatible material with strong tenacity and good flexibility, which has been applied widely in medical devices such as catheter and vascular graft. Our team has been investigating the properties and biocompatibility of TPU for years and the safety of TPU-made stent is assured[10].We collaborated w ith Nanjing Normal University and utilized Fused Deposition Mod eling (FDM), w hich is one of the most classic and mature techniques of 3D printing, to build the “fistula stent” from STL file (Figure 2B).
Nine days after carrying out OA, the stent w as implanted into the bowel through the orifice of the fistulous tract and proved to suit the trend of bowel ideally (Figure 3A-E). No obstruction w as observed around the orifice of the fistulous tract. Then,contrast-mediated fistula angiography was conducted to verify the effectiveness of the stent with the contrast agent flow ing past the stent w ithout obstruction (Figure 3F).The protuberance w as fixed w ith threads to avoid displacement d ue to peristalsis or postural changes (Figure 4). After the implantation, there was an obvious decrease in the amount of enteric fistula effluent and increase in stool frequency and capacity.Four d ays after implantation, w e restored the p atient's EN by nasal feeding, which w as started from 500 m L and increased to 1500 m L d uring the follow ing 3 d. No abnormal or subjective discomfort w as observed during the w hole process.
Several indexes of the patient were recorded in which w e regarded the amount of lost enteric effluent (Vloss) as the most significant one. In the d uration of therapy, Vlosswas equal to the amount of drainage fluid from the fistula orifice minus the amount of fistula irrigation fluid. EAF in this patient belonged to high flow fistula (> 500 m L/d)because the scope of the orifice of the fistulous tract occup ied nearly half of the intestinal w all (Figure 5A). Though it w as still higher than 400 m L/d, leakage ofenteric effluent was notably reduced after the implantation. With the increase in the amount of EN per day from 500 mL to 1500 mL, Vlossfloated upwards within a short time and gradually turn to decrease by degrees.
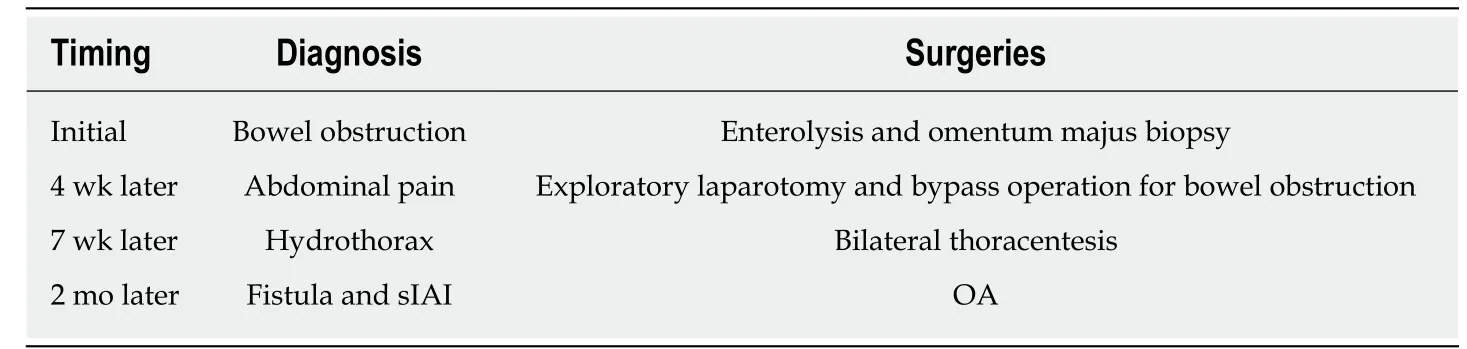
Table 2 Patient's previous surgeries
Outcomes
Seven days after implantation, no sign of pyrexia or obvious infection existed and the general condition of the patient w as improved with frozen abdomen formed steadily and d rainage unobstructed. Considering the good physical cond ition of the patient and the blocked EAF, skin grafting on the abd ominal w all w as cond ucted successfully. Timely blocking of EAF laid the foundation of the skin graft healing. Ten days after skin grafting, the abdomen had granulated around the EAF and the orifice of the fistulous tract had also decreased to a very small size (Figure 6). During the follow-up, w e observed that the orifice of the fistulous tract was enlarged d ue to the change in abd ominal incision and bod y p osition, so w e used butterfly-shap ed adhesive to strain the bilateral abdominal walls to control the Vlossfurther. The patient is receiving EN in good condition and waiting for definitive intestinal anastomosis and abdominal closure, in w hich the “fistula stent” w ill be draw n from the orifice of the fistulous tract.
FINAL DIAGNOSIS
EAF, sIAI, and hypoalbuminemia.
TREATMENT
Fistula stent; anti-infection therapy; total PN and double-pipe drainage.
OUTCOME AND FOLLOW-UP
The patient has passed the crisis and received skin grafting. The patient is receiving EN in good condition and w aiting for definitive intestinal anastomosis and abdominal closure.
DISCUSSION
What we introduce in this case report is a hollow and curving pipe stent with integrated wall, which is a combination of isolation technique with 3D printing[11], and can be applied to plug EAF at the very early stage of OA. Unlike silicone fistula plug reported by Ozer et al[5]or PEG tube reported by Miranda et al[7], 3D-printed “fistula stent” can be used to block EAF as soon as the EAF is discovered or formed. In other words, successfully plugging EAF from inside can transform some grade 4 OA to grade 1C or 2C in a sense, rather than simply blocking fistulous tract or ECF after the formation of frozen abdomen or skin grafting. We think that our report could start the train of thought for plugging EAF at the early stage to reduce the amount of lost enteric effluent as well as avoid water electrolyte imbalance, corrosion on wound surface, and IAI. Furthermore, “fistula stent” functions as a temporary tract to restore EN and improve bowel function, which can accelerate the process of rehabilitation. In addition, a stent which can block nearly all the orifice of the fistulous tract saves a lot of work of drainage and succus entericus reinfusion.
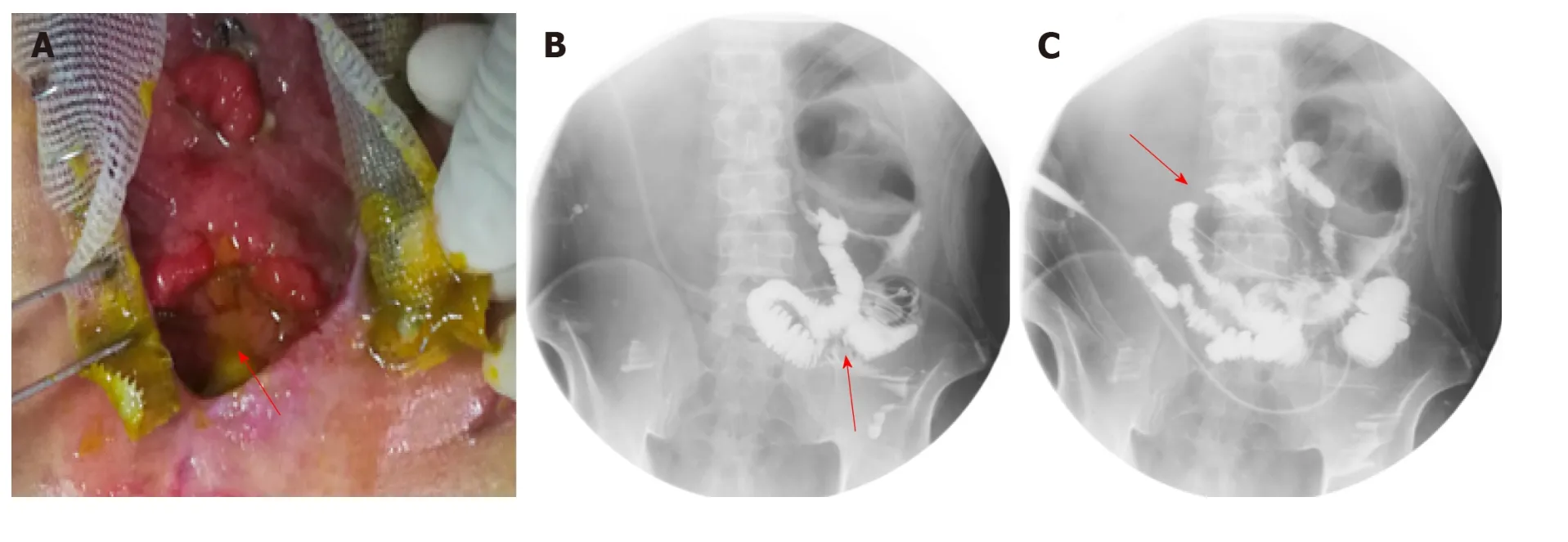
Figure 1 Clinical picture and imaging materials of the patient. A: The enteroatmospheric fistula (EAF) happened at side-to-side anastomosis was exposed and visible after open abdomen (red arrow); B: Contrast-mediated fistula angiography through the orifice of the fistulous tract (red arrow); C: The location of initial obstruction was shown (red arrow).
We regard our 3D-printed “fistula stent” as a strong and ideal supplement to negative pressure wound therapy, total PN, and somatostatin therapy, which are the key techniques to close an EAF[12]. And it has the p otential to be combined w ith advanced technique of bio-printed 3D human intestinal tissues[13]. How ever, several deficiencies exist w ith FDM based 3D-printed “fistula stent”. When adhesion between the bow el and the abdominal wall or the frozen abdomen formed, the size and shape of the orifice of the fistulous tract may change, thus causing the stent unfit w ith the bow el tract. On the other hand, the “fistula stent” has the risk of enlarging the EAF size, reducing the rate of spontaneous closure. Further, the sample size is still very limited and more w ell-designed trials are required to verify the effectiveness of 3Dprinted plugs.
CONCLUSION
EAFs in OA patients continue to be a challenge. Our 3D-printed “fistula stent” is presented to offer an effective and applicable method to block EAF at the early stage to simplify the management of OA with EAF, thus providing conditions for definitive surgeries.
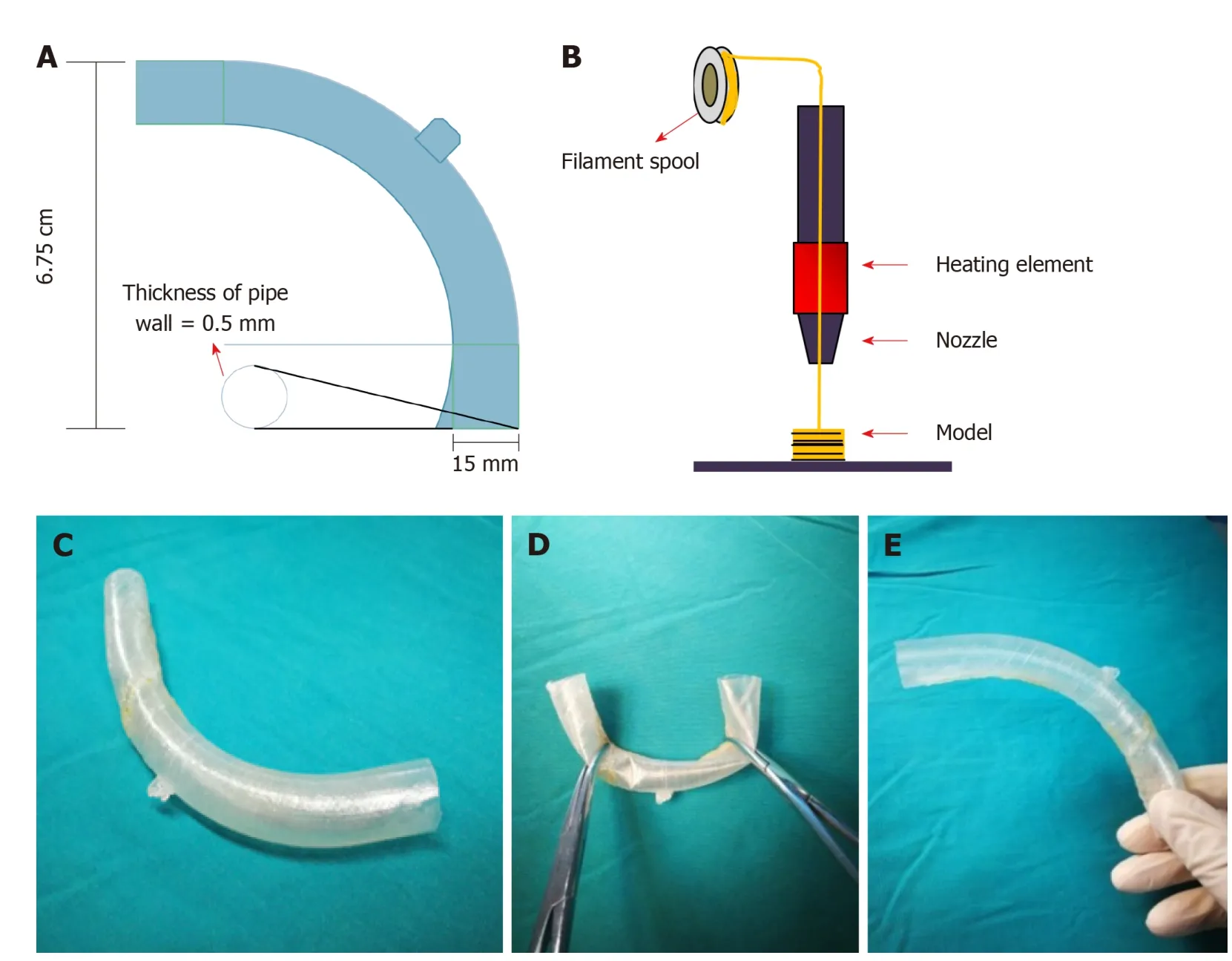
Figure 2 Manufacturing process of the “fistula stent”. A: The blueprint of “fistula stent” was built with Solidwork software and saved in Standard Template Library(STL) form; B: Fused Deposition Modeling is a process where thermoplastic filaments are driven with a motorized heated nozzle; C: Build model of a hollow and curving pipe stent with a small protuberance and integrated wall; D: Flexibility of the “fistula stent”; E: Shape memory of the “fistula stent” after application of an external force.
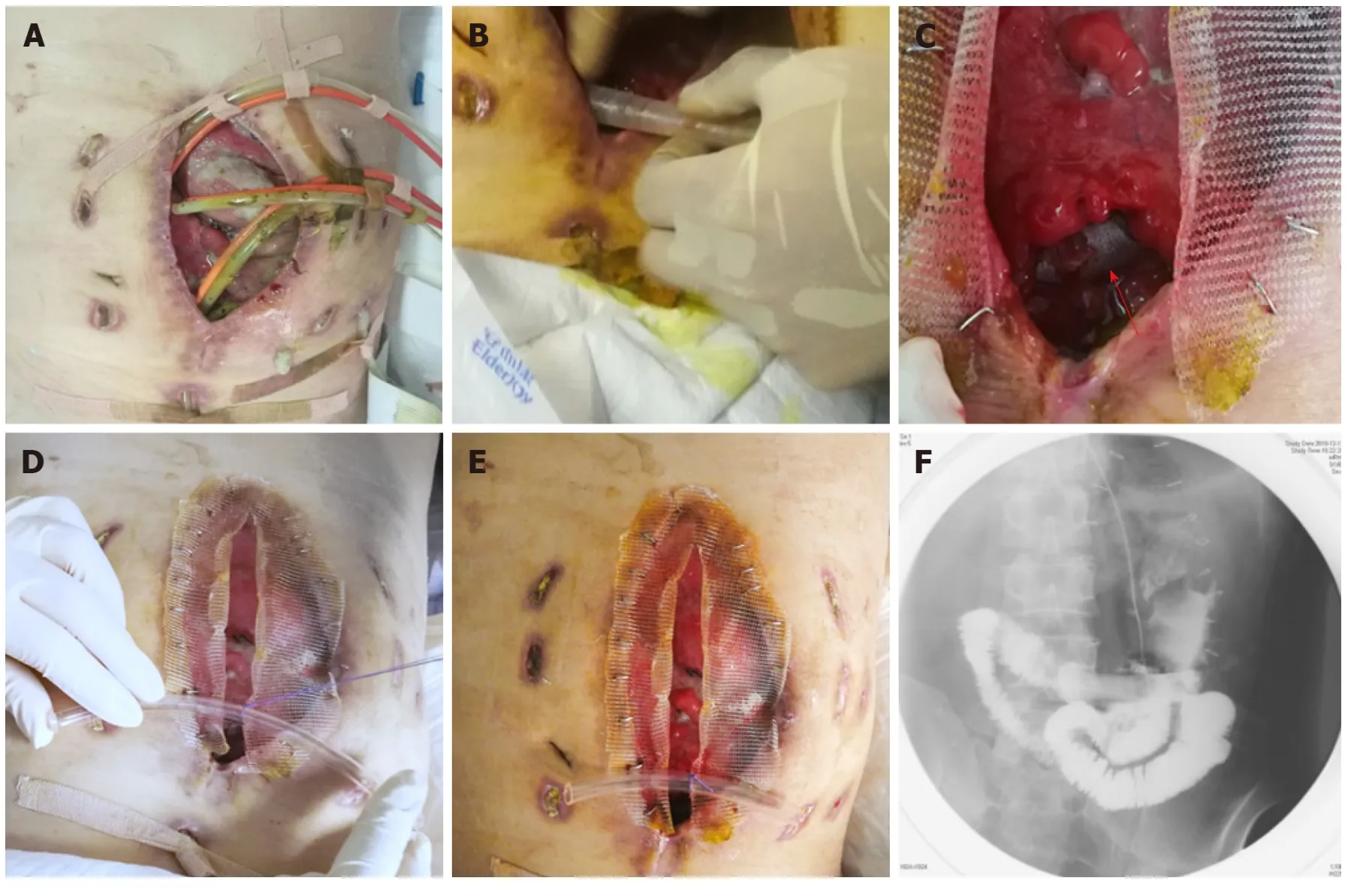
Figure 3 Process of the implantation and fixation of the “fistula stent”. A: Four double pipes were used to drainage enteric fistula effluent before implantation ; B:The process of implantation; C: After the implantation the stent can still be seen (red arrow); D: The process of fixation by suturing the small protuberance with a latex tube overhanging the abdominal wall; E: After the accomplishment of implantation, only one double pipe was needed for drainage; F: Contrast-mediated fistula angiography was conducted to verify the effectiveness of the stent and the contrast agent flowed past the stent without obstruction.
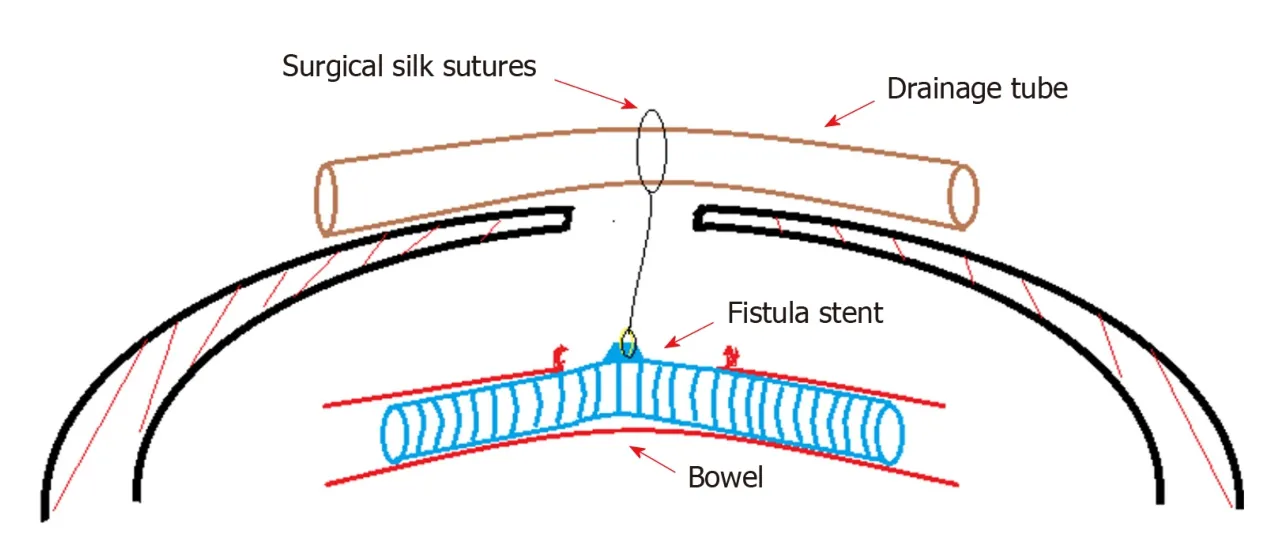
Figure 4 Concept graph of how is the “fistula stent” fixed in the bowel. The protuberance is connected with a latex drainage tube hanging over the abdominal wall in order to prevent the sliding due to peristalsis.
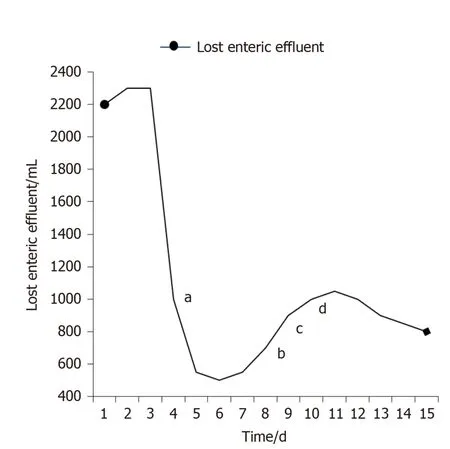
Figure 5 Recorded amount of lost enteric effluent.a The day of implantation; b Starting enteral nutrition (EN) of 500 mL; c EN of 1000 mL; d EN of 1500 mL.
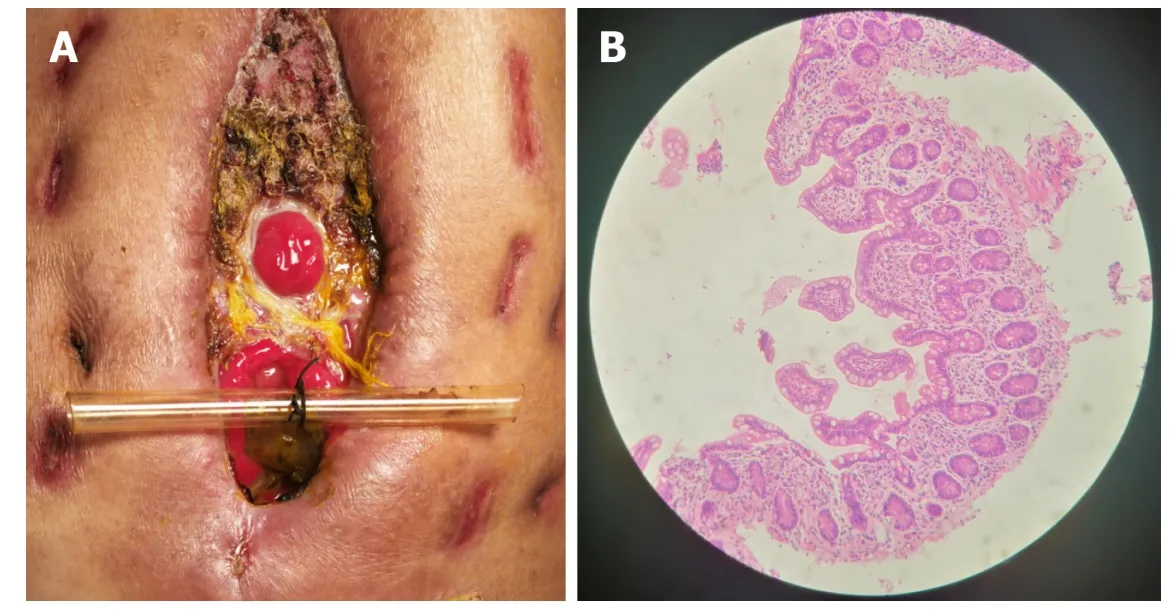
Figure 6 Follow-up after the implantation. A: After skin grafting, the abdomen had granulated around the enteroatmospheric fistula (EAF) and the orifice of the fistulous tract had also decreased to a very small size; B: Atrophic intestinal mucosa in the distal end of the EAF before restoring enteral nutrition.
杂志排行
World Journal of Gastroenterology的其它文章
- Precision surgical approach with lymph-node dissection in early gastric cancer
- Update on hepatocellular carcinoma: Pathologists' review
- Application of artificial intelligence in gastroenterology
- Clinical significance of programmed death 1/programmed death ligand 1 pathway in gastric neuroendocrine carcinomas
- Functional role of long non-coding RNA CASC19/miR-140-5p/CEMIP axis in colorectal cancer progression in vitro
- Seven-senescence-associated gene signature predicts overall survival for Asian patients with hepatocellular carcinoma
