Clinical significance of programmed death 1/programmed death ligand 1 pathway in gastric neuroendocrine carcinomas
2019-05-08MinWeiYangXueLiangFuYongShengJiangXiaoJingChenLingYeTaoJianYuYangYanMiaoHuoWeiLiuJunFengZhangPeiFengLiuQiangLiuRongHuaZhiGangZhangYongWeiSunDeJunLiu
Min-Wei Yang, Xue-Liang Fu, Yong-Sheng Jiang, Xiao-Jing Chen, Ling-Ye Tao, Jian-Yu Yang, Yan-Miao Huo,Wei Liu, Jun-Feng Zhang, Pei-Feng Liu, Qiang Liu, Rong Hua, Zhi-Gang Zhang, Yong-Wei Sun, De-Jun Liu
Abstrac t BACKGROUND Recently, more and more studies have demonstrated the pivotal role of programmed death 1/programmed death ligand 1 (PD-1/PD-L1) pathway in the immune evasion of tumors from the host immune system. However, the role of PD-1/PD-L1 pathway in gastric neuroendocrine carcinomas (G-NECs) remains unknown.AIM To investigate the expression of PD-1/PD-L1 and role of PD-1/PD-L1 pathway in G-NECs, which occur rarely but are highly malignant and clinically defiant.METHODS We investigated the expression of PD-L1 on tumor cells and PD-1+, CD8+, and FOXP3+ T cell infiltration by immunohistochemistry in 43 resected G-NEC tissue specimens. The copy number alterations of PD-L1 were assessed by qRT-PCR.RESULTS Most of the G-NECs tumor cells exhibited a near-uniform expression pattern of PD-L1, while some showed a tumor-stromal interface enhanced pattern. Of the 43 approved by the Research Ethics Committee of Ren Ji Hospital.
Key words: Programmed death 1; Programmed death ligand 1; Gastric neuroendocrine carcinomas; Prognosis; Tumor infiltrating lymphocytes
INTRODUCTION
Neuroendocrine neoplasms (NENs), which used to be called neuroendocrine tumors,are benign or high-grade malignant. Meanwhile, they are pathologically and clinically heterogeneous rare tumors. In the past several years, the incidence of NENs has gradually increased over time[1,2], from 1.09/100000 in 1973 to 5.25/100000 in 2004 in the United States[3]. However, there was no improvement in outcomes because of the limited awareness of this disease[4,5]. As the most malignant subgroup, neuroendocrine carcinomas (NECs) are characterized by high-grade cytological atypia, apparent pleomorphism, extensive necrosis, and prominent mitotic activity[6]. As one of the most common type of NECs, gastric NECs (G-NECs) are poorly differentiated and high-grade malignant and might be either small-cell carcinomas or large-cell NECs in histology. Since the limited knowledge of the epidemiological and clinical characters for G-NECs, this disease deserves more attention.
Programmed d eath 1 (PD-1), first discovered in 1992 w hen searching genes responsible for programmed cell death by Ishida et al[7], is an immunoinhibitory receptor expressed by activated T cells, B cells, myeloid cells, and other antigenpresenting cells. Programmed death ligand 1 (PD-L1), a member of the B7 gene family, originally called B7H1 by Dong et al[8], was reported in 2000 by Freeman et al[9].It is an immunomodulatory glycoprotein, which cannot be detected in normal liver parenchyma, breast, colon, kidney, uterus, muscle, pancreas, or gastric tissue[10,11], but could be selectively expressed in various malignancies, such as esophageal cancer[12],gastric cancer[11], pancreatic cancer[13], colorectal cancer[14], breast cancer[15], thymoma[16],and thymic cancer[17].
The host immune functions play an important role in inhibiting the development of malignant tumors. And the induction of anti-tumor immune responses needs the host immune system to id entify the tumor antigen efficiently and to activate various T cells. Bind ing of PD-1 and PD-L1 inhibits p hosp hatid ylinositol 3-kinase/Akt,suppresses T-cell IL2 production, and then reduces T-cell proliferation and survival[18].Therefore, some malignant tumors can escape from immune-surveillance mechanisms by the exp ression of PD-L1[18]. Then, the blockad e of the PD-1/PD-L1 pathw ay has become an attractive target in cancer therapy. The success of immunotherapy in various malignancies such as ad vanced melanoma, non-small cell lung cancer, and renal cell carcinoma have suggested that immunotherap y can be a p romising alternative by blocking PD-1/PD-L1 signaling[19,20]. Until now, no study has evaluated the role of PD-1/PD-L1 in G-NECs.
CD8+tumor infiltrating lymp hocytes (TILs) are the key effector in antitumor immune response, and its p resence has been rep orted to be a favorable p rognostic factor in some malignancies, such as colorectal cancer[21], esophageal cancer[22,23], and breast cancer[24]. Meanw hile, it has been reported that the upregulation of PD-L1 on tumour cells may be d riven by the stimulation of CD8+T cells in melanoma[25,26].FOXP3, as a forkhead-family transcription factor, controls regulatory T cell (Treg)d evelop ment and function[27]. Tregs are a subset of T lymphocytes possessing the immunoregulatory capacity to suppress the proliferation and cytokine secretion of effector T lymphocytes. And previous stud ies have reported that the infiltration of FOXP3+Tregs is correlated w ith the upregulation of PD-L1 in gastric cancer[28], breast cancer[29], and colorectal cancer[14].
In this study, w e first detected PD-L1 expression in 43 G-NECs and analyzed the relationship betw een PD-L1 exp ression and p atients' p rognosis. Then, w e investigated PD-1+T cells, CD8+T cells, and FOXP3+Treg cells in NECs and their associations w ith clinicopathological parameters and PD-L1 expression. PD-L1 gene copy number alterations and its relationship to PD-L1 expression were also examined.
MATERIALS AND METHODS
Patients
This study examined 43 formalin-fixed, paraffin-embedd ed (FFPE) tissue samp les from patients w ith G-NECs treated at Ren Ji Hospital, School of Medicine, Shanghai Jiao Tong University, from 2007 to 2014. We diagnosed, graded, and staged G-NECs by the 2010 WHO classification[30]. Cases w ith mixed tumors w ere exclud ed. TNM staging w as d one accord ing to the 7th Ed ition of the AJCC Cancer TNM Classification[31]. None of these patients had received tumor-related treatment before. For all cases enrolled in this study, information on age, gender, tumor size, tumor location, T classification, lymph nod e metastasis, liver metastasis, pathological stage, pathology,treatment, and outcome was reviewed.
The overall survival (OS) time w as calculated from the date of surgery to death, or August 30, 2016, the ultimate follow-up deadline. Research Ethics Committee of Ren Ji Hospital approved this study and all participants signed informed consent forms.
Immunohistochemistry
Deparaffinage and rehydration of G-NEC tissues were first performed with xylene and graded alcohol, respectively. Activity of endogenous peroxidase was quenched with 3% hydrogen peroxide at room temperature for 15 min. Antigen retrieval was achieved at high temperature and high pressure. Blocking was done with 10% bull serum albumin for 30 min, then slides were incubated with antibodies against CD8(1:200, 17335-1-AP, Protein Tech, United States), FOXP3 (1:200, 22228-1-AP,Protein Tech), PD-1 (1:25, ab140950, Abcam, United Kindom), and PD-L1 (1:500,ab205921, Abcam) at 4 °C overnight. The next day, the slides were incubated with a corresponding peroxidase-labeled secondary antibody for 30 min at room temperature. Finally, positive staining was visualized with diaminobenzidine tetrahydrochloride (Maixin Biotech, China) and counterstained with hematoxylin.
The final score of PD-L1 expression w as assessed according to the percent of positive cell score × staining intensity score as follows: 0, 0-5%; 1, 6%-35%; 2, 36%-70%; 3, 71%-100%; 0, no staining; 1, weak staining; 2, moderate staining; 3, strong staining. The tissues with a final score < 4 were defined as PD-L1 low expression and those with a final score ≥ 4 were classified as PD-L1 high expression. The densities of PD-1+, CD8+, and FOXP3+cells were measured in four high power fields from each tumor by an experienced pathologist and the average density was calculated. Low infiltration of PD-1+, CD8+, and FOXP3+cells was defined as less than the median value.
Copy number assay of PD-L1
Tissue DNA w as extracted from FFPE tissue using QIAamp DNA FFPE Tissue Kit(QIAGEN, 56404, Germany). Copy number assay of PD-L1 w as performed by qRTPCR, CD274 TaqMan Cop y Number Assays (Hs03704252_cn, Ap plied Biosystems,United States) w as used to evaluate PD-L1 cop y number w hile Taq Man Cop y Number Reference Assay RNase P (Applied Biosystems 4403328, United States) w as used to normalize the results. TaqMan Genotyping Master Mix (Applied Biosystems 4381656, United States) w as used to perform the qRT-PCR on a 7500 real-time PCR system (Applied Biosystems, United States). Results w ere analyzed with Copy Caller Software (Applied Biosystems, United States).
Statistical analysis
Data are p resented as the mean ± SD. Statistical analyses and grap hical representations w ere performed using SPSS 22.0 softw are (IBM Corp., Armonk, NY, United States) and Graph Pad Prism 6 (San Diego, CA, United States) software, respectively.χ2test and Fisher's exact test w ere used to evaluate the correlation of PD-L1, PD-1,FOXP3, and CD8 with clinic-pathologic parameters in patients w ith G-NECs. Survival curves w ere evaluated using the Kaplan-Meier method and differences w ere analyzed by the log-rank test. Id entification of factors that had a significant inf luence on survival w as p erformed by univariate and multivariate Cox regression analyses.Comp arison betw een tw o group s w as p erformed by the stud ent's t-test or Mann-Whitney U test. P-values (tw o-sid ed) < 0.05 w ere consid ered statistically significant.
RESULTS
Clinicopathologic features of G-NECs
A total of 43 patients diagnosed with G-NECs were enrolled in this study and the clinicopathologic features are described in detail in Table 1. The cases included 35 males (81.4%) and 8 females (18.6%) with a median age of 62 years (range, 33-82 years). Nearly half of the tumors were located in cardia, w hile the others w ere distributed evenly in the corpus and the antrum of the stomach, w ith tw o cases located in the residual stomach anastomosis. The majority of patients were diagnosed with stage III disease (81.4%), two cases with stage II (4.65%), and six cases with stage IV (13.95%). For the patients with liver metastases, one of six received resection of both primary and metastatic tumors, while the others received palliative resection of primary tumor. None of the patients received neoadjuvant therapy prior to the surgical resection. The median OS was 31 mo (range, 1-90 mo).
Immunohistochemical characteristics of PD-L1, PD-1, CD8, and FOXP3 expression
PD-L1 was mainly expressed on both the membrane and cytoplasm of tumor cells(Figure 1C and F). Most of the G-NEC tissues d emonstrated a near-uniform expression pattern of PD-L1 (Figure 1E and F), while five specimens showed a tumorstromal interface enhanced pattern (Figure 1B). The expression score of PD-L1 was evaluated based on the product of ratio of positive cells (range, 0-3) and staining intensity (range, 0-3), as shown in Figure 1. We predefined the expression score less than 4 as low expression group while the others were high expression group, and 21(48.8%) of 43 cases were classified as high PD-L1 expression.
The expression of PD-1 was almost on the surface of immune cells in the stroma among tumor nests (Figure 2A), and the expression of CD8 and FOXP3 was mainly on both membrane and cytoplasm of immune cells in the stroma among tumor nests(Figure 2B and C). We defined the expression of PD-1, CD8, and FOXP3 by the number of positive immune cells. The median value of PD-1+ cells in a high power field was 12, and the median values of CD8+ and FOXP3+ cells were 22 and 102.
Association of immune marker staining and clinical parameters
Table 2 shows the correlation betw een immune markers and clinicopathologic characteristics. We found that the FOXP3+Treg cell infiltration was significantly associated with age (P = 0.019), gender (P = 0.015), and tumor location (P = 0.018). It demonstrated that female patients, patients with elder age, and patients with G-NECs at the corpus were more likely to have a high infiltration of Treg cells. Importantly,we also found that patients with high infiltration of PD-1 positive immune cells tended to have higher expression of PD-L1 (P = 0.009). The infiltration of CD8+cells was not associated with any clinical parameters or other immune markers. And all the five specimens with a tumor-stromal interface enhanced pattern of PD-L1 had high CD8+T cell infiltration.
Survival analysis of patients with G-NECs
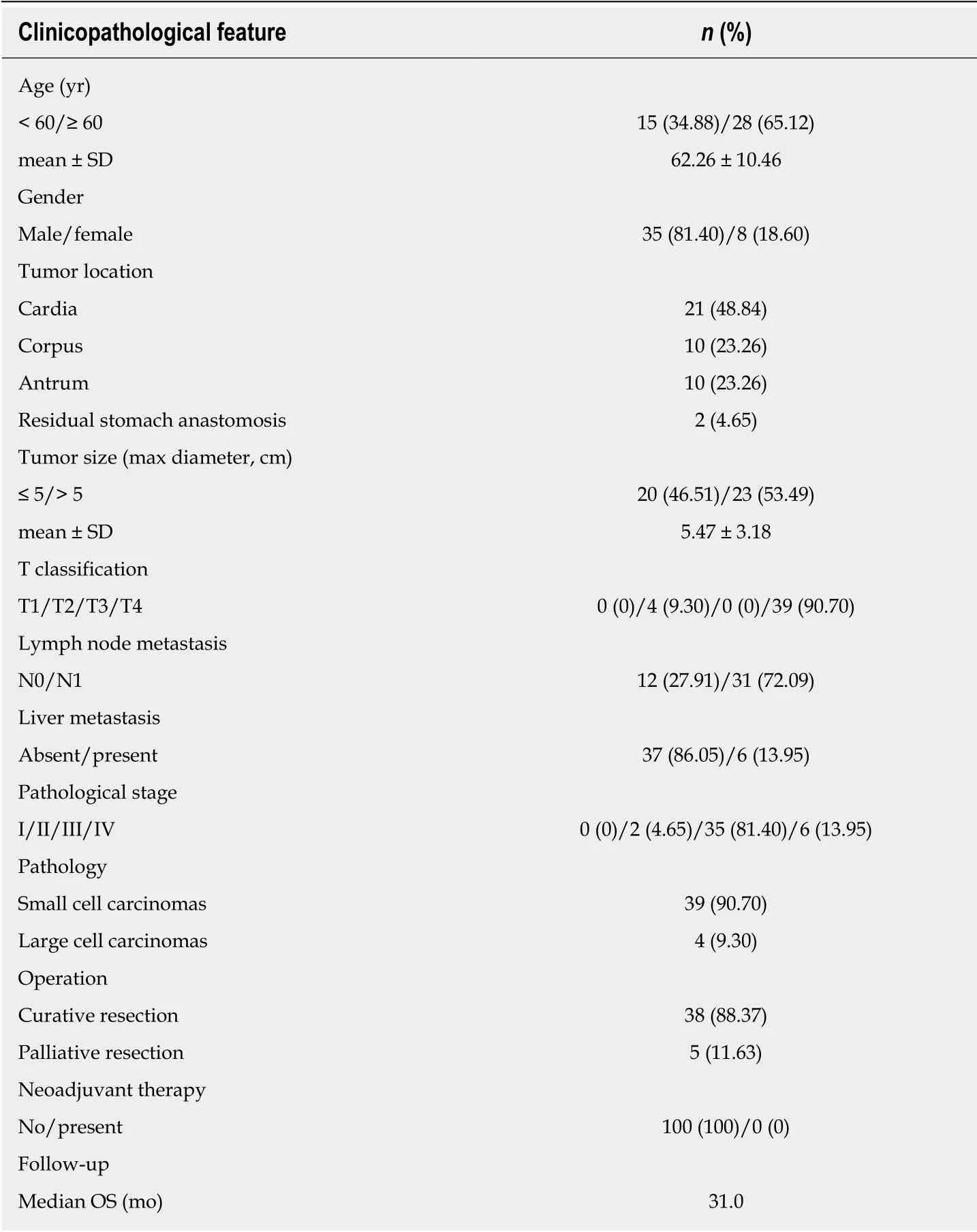
Table 1 Clinical characteristics of 43 patients with gastric neuroendocrine carcinomas
For Kap lan-Meier analysis, the OS of p atients w ith high exp ression of PD-L1 w as significantly shorter compared w ith patients w ith low PD-L1 expression (P = 0.016).We also found that p atients w ith high CD8+T cell infiltration may have a better clinical p rognosis, how ever, due to the limited patient cohort, the P-value d id not reach the statistical significance (P = 0.065). Univariate and multivariate analyses were performed to assess the impact of potential prognostic factors on OS. As show n in Table 3, corp us tumor [hazard ratio (HR) = 3.034, P = 0.032], liver metastasis (HR =3.515, P = 0.016), and PD-L1 expression (HR = 2.846, P = 0.021) w ere significantly associated w ith OS in univariate analysis, while multivariate analysis demonstrated that only liver metastasis (HR = 4.045, P = 0.031) and PD-L1 expression (HR = 3.646, P= 0.009) were independent prognostic factors. Based on the theory that PD-L1 played a role in immune evasion by suppressing the function of PD-1 positive immune cells,w e d ivid ed the p atients into tw o group s d ep end ing on the infiltration of PD-1 positive cells and performed the Kap lan-Meier analysis to explore the influence of PD-L1 expression on patients' survival. The results suggested that in the low PD-1+cell infiltration group, patients' survival w as not associated w ith PD-L1 expression statistically (Figure 3C, P = 0.242); w hile in the high PD-1+cells infiltration group,patients w ith high expression of PD-L1 had a significantly shorter survival (Figure 3D, P = 0.013).
Expression of PD-L1 is correlated with PD-L1 copy number in G-NECs
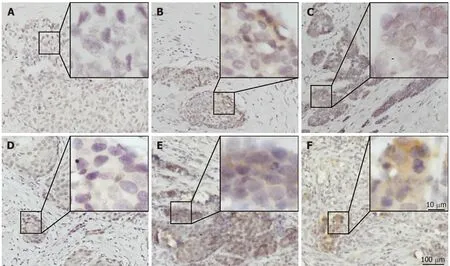
Figure 1 lmmunohistochemical staining for programmed death ligand 1 in human gastric neuroendocrine carcinomas. A: Representative case of negative programmed death ligand 1 (PD-L1) expression; B: Tumor-stromal interface enhanced expression of PD-L1; C: Membrane expression of PD-L1; D: Weak staining; E:Moderate staining; F: Strong staining. PD-L1: Programmed death ligand 1.
The G-NEC tissues demonstrated broad PD-L1 expression. Given the near-uniform expression pattern, we considered that genetic alteration may contribute to the PD-L1 expression. To address this issue, we analyzed the copy number variation in the tissue of G-NEC p atients. In a cohort of 19 G-NEC p atients, w e found that 6/19 d emonstrated a cop y number gain of PD-L1 comp ared to normal human tissue.Furthermore, we found that the copy number of PD-L1 was positively correlated with the expression of PD-L1 at the p rotein level (Figure 4). How ever, w e found no correlation between copy number gain and OS of patients (data not shown).
DISCUSSION
In this study, our data showed that more than half of G-NECs were diagnosed at an advanced stage (regional lymph node metastasis 72.09%, and liver metastasis 13.95%).And the median OS w as only 31 mo. Meanwhile, as a rare group of poorly differentiated tumors with high-grade malignancy, novel therapeutic approaches are lack because of its rarity, complexity, and heterogeneity. Now, cancer immunotherapy is considered to be a major treatment backbone in the following decade[32]. Overexpression of PD-L1 and PD-1 in tumor and tumor stromal cells has been reported in various types of cancers, and antibod ies targeting either PD-1 or PD-L1 have produced significant anti-tumor activity in several malignancies, such as non-smallcell lung cancer, melanoma, renal-cell cancer, bladder cancer, and head and neck cancer[20,33,34]. Therefore, there is an urgent need to define the significance of PD-1/PDL1 pathway in G-NECs, with the potential to provide a new promising therapeutic approach.
In a recent study, Kim et al[35]evaluated the expression of PD-L1 in 32 metastatic GEP-NENs including one gastric NEN. Meanwhile, Cavalcanti et al[36]detected the expression of PD-L1 in 57 GEP-NENs including ten gastric NENs. They found that the expression of PD-L1 was associated with higher WHO tumor grade (grade 3) and it can be used as a predictive and prognostic marker for survival. So far, these are the only reports about PD-1/PD-L1 in GEP-NENs, and there is no research about PD-1/PD-L1 in G-NECs.
In our study, we first investigated the expression of PD-L1 in 43 G-NECs and the relationship between PD-L1 expression and clinical data. We found that 21 (48.8%) of 43 patients can be classified as high PD-L1 expression, and these people had a significant worse prognosis. This result is consistent with many previous studies focusing on gastric cancer[11], esophageal cancer[12], pancreatic cancer[13], and breast cancer[29]. And w e also found that the expression of PD-L1 w as an ind epend ent prognostic factor in G-NECs independent of tumor size and N classification.
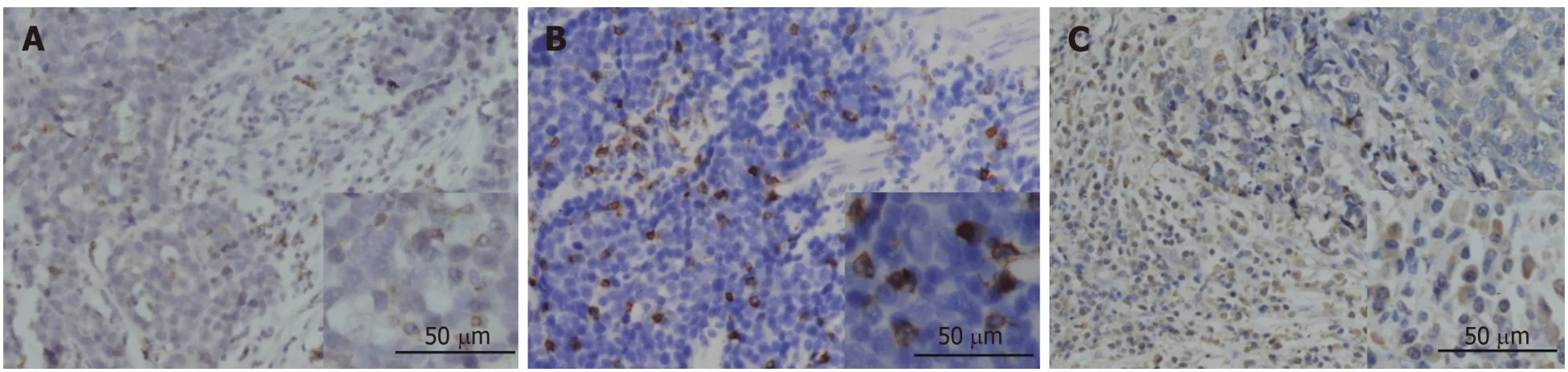
Figure 2 lmmunohistochemical staining for programmed death 1, CD8, and FOXP3 in human gastric neuroendocrine carcinomas. A: The programmed death 1 positive infiltrating lymphocytes; B: CD8 positive lymphocytes; C: FOXP3 positive Treg cells.
PD-1, the ligand of PD-L1, expressed by activated T cells, B cells, myeloid cells, and other antigen-presenting cells, w as also investigated in this research. According to previous stud ies, PD-1 expression in TILs is associated w ith PD-L1 exp ression in tumor cells[17,37], and increased number of PD-1+TILs w as also reported to be a significant predictor of poor survival[17,38,39]. In this study, we confirmed the positive association betw een PD-1+TIL number and tumor cell PD-L1 expression. Then, the subgroup analysis indicated that PD-L1 had a role in predicting the prognosis only in PD-1+group. These results supported that PD-L1 expressed by tumor cells interacts with PD-1+ TILs to suppress antitumor activity. However, in this study, we found no significant correlation betw een PD-1+TILs and survival.
In our stud y, PD-L1 cop y number analysis w as p erformed to investigate the mechanisms und erlying PD-L1 overexpression in G-NECs. We found that a large number of patients demonstrated a cop y number alteration and PD-L1 expression was significantly higher in cases with PD-L1 copy number gain, implying that PD-L1 gene alteration is a mechanism of PD-L1 overexpression, similar to a study in thymic cancer[17]. Meanw hile, w e noticed that survival analyses based on PD-L1 expression w ere inconsistent. Some recent research ind icated that PD-L1 exp ression w as associated w ith favorable OS in several malignancies, such as colorectal cancer,thymic cancer, breast cancer, and non-small cell lung cancer[14,17,40,41]. And in these stud ies, the authors d etected a significant p ositive correlation betw een PD-L1 exp ression and increased TILs, esp ecially CD8+TILs, the key effector in antitumor immune response. Moreover, in melanoma, it w as revaled that the up-regulation of immunosuppressive molecules PD-L1 and FOXP3+Tregs is driven by CD8+T cells[25],which means that the upregulated expression of PD-L1, PD-1, and FOXP3 might be a negative feedback mechanism follow ing CD8+T cell infiltration, to against activation of host antitumor immunity. Although the d iscrepancy betw een PD-L1 expression and p rognosis can be attributed to a d ifference in experiment method and tumor heterogeneity, w e determined CD8+TILs and FOXP3+Tregs in G-NECs.
As a glycoprotein, CD8 p lays an important role in cell-med iated immunity by binding to the major histocompatibility complex class I molecule together with the Tcell receptor to stimulate the cytotoxic effect of TILs on cancer cells[42,43]. And it has been rep orted as a favorable p rognostic factor in many malignancies[21-24]. In accordance with the previous research, w e found that patients w ith high CD8+T cell infiltration may have a better clinical p rognosis, although it d id not reach the statistical significance(P = 0.065). We found no association between PD-L1 expression and CD8+TILs, how ever, w e noticed that five sp ecimens w ith high CD8+ T cell infiltration exhibited a tumor-stromal interface enhanced expression pattern of PD-L1.And this might supp ort the correlation betw een tumor cell PD-L1 expression and CD8+T cell infiltration in the stroma. And there w as also no correlation betw een FOXP3+Tregs, CD8+TILs infiltration, and PD-L1 expression.
Finally, as a retrospective study, there are selection bias and some other limitations in our results. The small sample size of this research might result in some bias in the multivariable prognosis analysis, so larger sample studies are needed. In this research,we used IHC to investigate the expression of PD-L1, and our data might be limited by the lack of stand ard ization of IHC, for example sp ecificity and rep rod ucibility of antibodies, definition of optimal positivity cut-off, and interpretative subjectivity.
In conclusion, this stud y for the first time d emonstrated that the high PD-L1 expression by tumor cells w as associated with a poor prognosis in G-NECs, especially in the PD-1+subgroup. Although w e d id not find a significant correlation betw een CD8+TILs and PD-L1 expression, w e partly demonstrated the role of CD8+TILs as afavorable prognostic biomarker for G-NEC patients. Due to the coinstantaneous impact of copy number variation and TILs' stimulation on the expression of PD-L1,the mechanism of high expression of PD-L1 in G-NECs remains complicated. Most importantly, our findings provide important implications for the potential use of antibody therapies targeting the PD-1/PD-L1 signaling pathway in G-NECs.
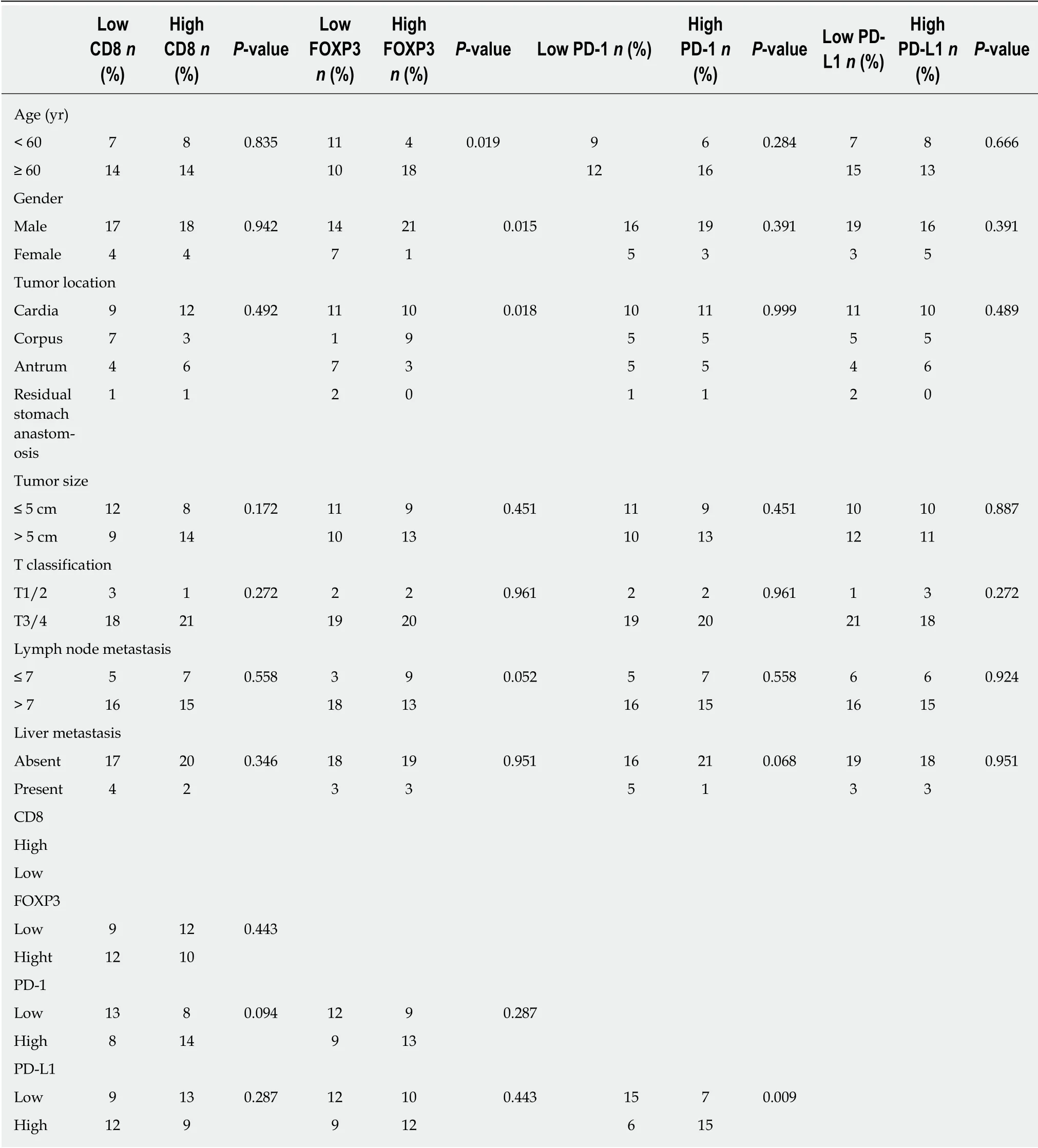
Table 2 Relationship between clinical characteristics and immune markers
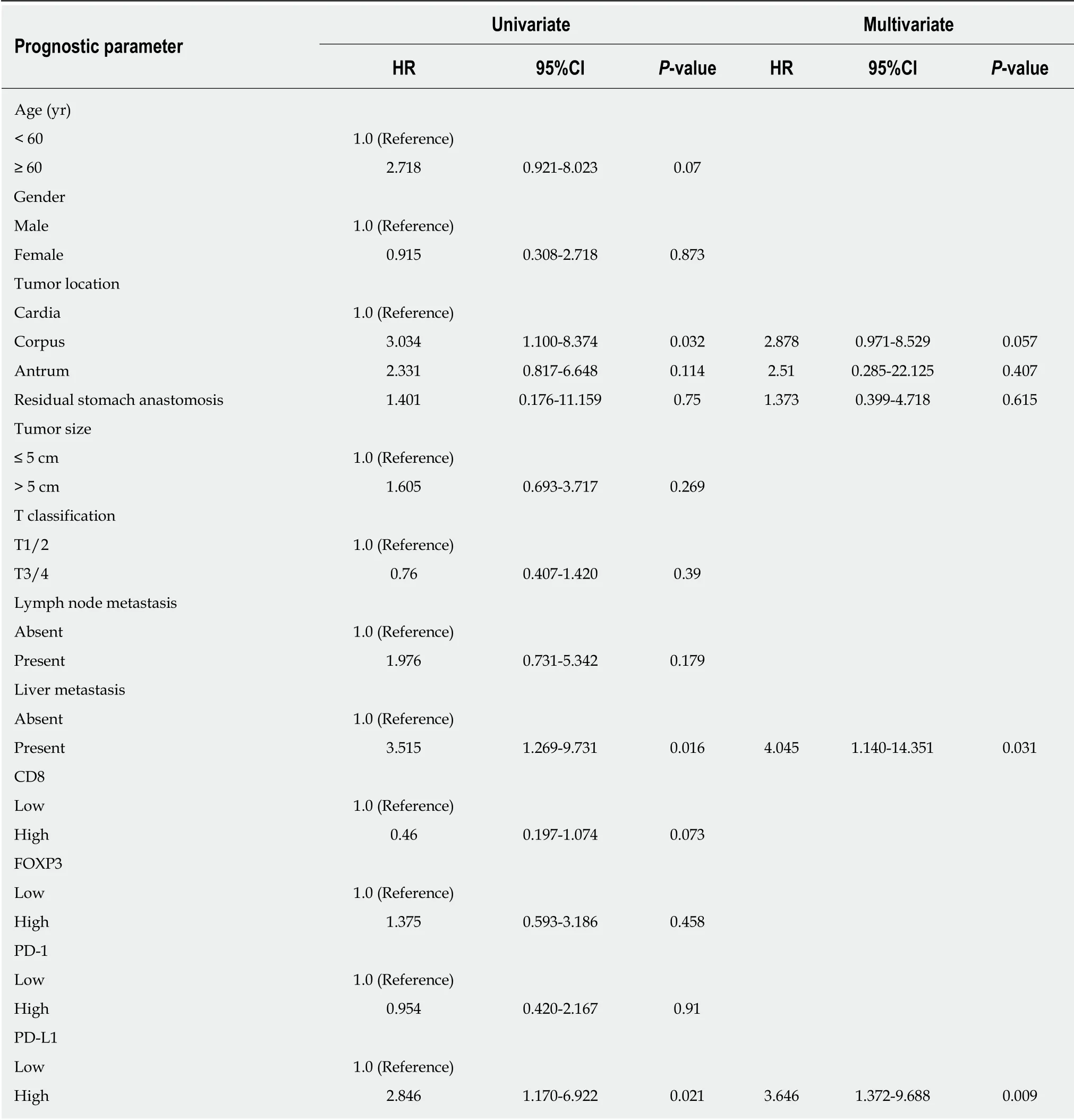
Table 3 Univariate and multivariate analyses of prognostic parameters for survival in the cohort patients with gastric neuroendocrine carcinomas
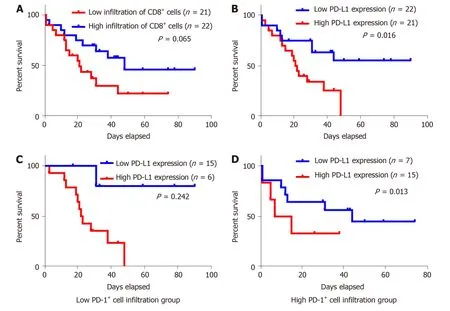
Figure 3 Kaplan-Meier survival analysis of the gastric neuroendocrine carcinomas patients. A: Patients with high infiltration of CD8+ lymphocytes tended to have a relative longer survival, but the difference was not statistically significant; B: Kaplan-Meier overall survival curves in patients with high and low expression of programmed death ligand 1 (PD-L1) in the whole population; C: Low PD-1+ cell infiltration group; D: High PD-1+ cell infiltration group. PD-L1: Programmed death ligand 1.

Figure 4 Programmed death ligand 1 copy number is associated with programmed death ligand 1 expression in gastric neuroendocrine carcinomas. A:Programmed death ligand 1 (PD-L1) copy number status varied in 19 gastric neuroendocrine carcinoma samples. The cases with copy number gain are shown in black; B: Cases with copy number gain showed significantly higher PD-L1 expression than those without. PD-L1: Programmed death ligand 1.
ARTICLE HIGHLIGHTS
Research background
Recently, more and more studies have demonstrated the pivotal role of programmed death 1/programmed death ligand 1 (PD-1/PD-L1) pathway in the immune evasion of tumors from the host immune system. However, the role of PD-1/PD-L1 pathway in gastric neuroendocrine carcinomas (G-NECs) remains unknown.
Research motivation
G-NECs are highly malignant, clinically defiant, and lack of effective treatment. Recent research has proved the role of treatment targeting PD-1/PD-L1 pathw ay in several other advanced cancers. This study for the first time demonstrated that PD-L1 can be expressed by G-NEC cancer cells and the high PD-L1 expression was associated w ith a poor prognosis. These findings provide important implications for the potential use of antibody therapies targeting the PD-1/PD-L1 signaling pathw ay in G-NECs.
Research objectives
We performed this research to investigate the expression of PD-1/PD-L1 and role of PD-1/PDL1 pathw ay in G-NECs.
Research methods
We investigated the expression of PD-L1 on tumor cells and PD-1+, CD8+, and FOXP3+T cell infiltration by immunohistochemistry in 43 resected G-NECs tissue specimens, while the copy number alterations of PD-L1 were assessed by qRT-PCR. Statistical analyses and graphical representations were performed using SPSS 22.0 softw are and GraphPad Prism 6 softw are,respectively. χ2test and Fisher's exact test were used to evaluate the correlation of PD-L1, PD-1,FOXP3, and CD8 with clinicopathologic parameters in patients with G-NECs. Survival curves were evaluated using the Kaplan-Meier method and differences were analyzed by the log-rank test. Identification of factors that had a significant inf luence on survival was performed by univariate and multivariate Cox regression analyses. Comparison betw een tw o groups was performed by the Student's t-test or Mann-Whitney U test.
Research results
We found that most of the G-NECs tumor cells exhibited a near-uniform expression pattern of PD-L1, while some showed a tumor-stromal interface enhanced pattern. Of the 43 G-NECs, 21(48.8%) were classified as a high PD-L1 expression group, and the high expression of PD-L1 was associated with poor overall survival (OS). The high expression of PD-L1 w as correlated with abund ant PD-1+tumor infiltrating lymphocytes (TILs) instead of CD8+TILs and FOXP3+regulatory T cells (Tregs). Our analysis also suggested that the infiltration of CD8+TILs tended to be a favorable factor for OS, although the difference did not reach the statistical significance (P =0.065). Meanw hile, PD-L1 was significantly overexpressed in cases w ith copy number gain as compared with those w ithout. However, as a retrospective study, the small sample size of this research might result in some bias in the multivariable prognosis analysis, so larger sample studies are needed.
Research conclusions
Our data demonstrated for the first time that the high expression of PD-L1 in G-NECs w as associated w ith a poor prognosis, w hile the high expression may be due to the copy number variation of PD-L1 gene or stimulation of TILs. These results provid e a basis for the immunotherapy targeting PD-1/PD-L1 pathw ay in G-NECs.
Research perspectives
By this study, w e found that PD-1/PD-L1 pathw ay is involved in G-NECs. In the follow ing, in vitro cell experiments and in vivo animal experiments are needed.
杂志排行
World Journal of Gastroenterology的其它文章
- Precision surgical approach with lymph-node dissection in early gastric cancer
- Update on hepatocellular carcinoma: Pathologists' review
- Application of artificial intelligence in gastroenterology
- Functional role of long non-coding RNA CASC19/miR-140-5p/CEMIP axis in colorectal cancer progression in vitro
- Seven-senescence-associated gene signature predicts overall survival for Asian patients with hepatocellular carcinoma
- Chronic functional constipation is strongly linked to vitamin D deficiency
