Endoscopic response to tumor necrosis factor inhibitors predicts long term benefits in Crohn's disease
2019-05-08IgnacioAlfaroMariaCarmeMasamuntNuriaPlanellAliciapezGarcJesCastroMartaGallegoRebecaBarasteguiAngelGinerAlejandroVaraAzucenaSalasElenaRicartJuliPanIngridOrd
Ignacio Alfaro, Maria Carme Masamunt, Nuria Planell, Alicia López-García, Jesús Castro, Marta Gallego,Rebeca Barastegui, Angel Giner, Alejandro Vara, Azucena Salas, Elena Ricart, Julián Panés, Ingrid Ordás
Abstrac t BACKGROUND Identifying predictors of therapeutic response is the cornerstone of personalized medicine.AIM To identify predictors of long-term mucosal healing (MH) in patients with Crohn's disease (CD) treated with tumor necrosis factor α (TNF-α) inhibitors.METHODS Prospective single center study. Consecutive patients with clinically active CD requiring treatment with a TNF-α inhibitor were included. A baseline segmental CD Endoscopic Index of Severity (CDEIS) ≥ 10 in at least one segment or the presence of ulcerations were required for inclusion. Clinical, biological and endoscopic data were obtained at baseline, weeks 14 and 46. Endoscopic response(ER) was defined as a decrease ≥ 50% from baseline CDEIS and MH as partial CDEIS ≤ 5 in all segments.RESULTS Of 62 patients were included. At baseline, median CD Activity Index and CDEIS were 201 and 6.7, respectively with a significant reduction after one year of treatment (53 and 3.0 respectively, P < 0.001). At week 14, 56% of patients achieved ER and 34% MH. At week 46, the corresponding percentages were 52%and 44%. Baseline disease characteristics or biomarkers did not predict MH. A decrease from baseline CDEIS at week 14 of at least 80% was the best predictor of MH at week 46 (59% sensitivity and 91% specificity; area under the curve =0.778).guardian provided informed w ritten consent about personal and medical data collection prior to study enrolment.
Key words: Crohn's disease; Endoscopy; Mucosal healing; Crohn's Disease Endoscopic Index of Severity; Tumor necrosis factor
INTRODUCTION
Crohn's disease (CD) is a chronic inflammatory bowel disease (IBD) characterized by a wide spectrum of disease phenotypes that typically alternates periods of active inflammation and remission. Persistent inflammation leads to the accumulation of intestinal damage and loss of function in the affected intestinal segments, resulting in disability in many patients[1]. The main treatment goal in CD is to achieve healing of inflammatory lesions to prevent damage progression by introducing highly effective therapies early during the course of the disease. Tumor necrosis factor α (TNF-α)inhibitors are one of the most effective therapies for induction and maintenance of remission in patients with CD[2].
Response to anti-TNF therapy is heterogeneous. In patients with CD several predictors of clinical response have been described, includ ing age[3,4], d isease duration[5], inflammatory phenotype[6], and smoking status[6-10], but the effect size of these factors is small and not sufficient to guid e clinical practice. Furthermore,predictors of endoscopic healing remain to be definitively established. In the field of biomarkers, normalization of C reactive protein (CRP) and calprotectin has been associated w ith mucosal healing (MH)[6,11]. With regard to endoscopic lesions,achievement of MH after three months of treatment with a TNF-α inhibitor has been identified as a predictor of long-term endoscopic response[12,13]. However, to date, no observational prosp ective studies have been specifically designed to identify predictors of MH, which should be the therapeutic target[14].
The availability of new treatment agents with mechanisms of action different from TNF-α inhibition, has brought about the need for identifying predictors of efficacy, in particular pred ictors of MH, in ord er to individualize treatment strategies and facilitate a more personalized medicine. The aim of this study w as to identify predictors of MH after one year of treatment with TNF-α inhibitors.
MATERIALS AND METHODS
Study design and patients
This is a prospective observational single center study. Recruitment of patients was performed from November 2012 until May 2016. The study was approved by the local ethics committee of Hospital Clinic de Barcelona on November 22 of 2012, and was performed accord ing to the good clinical practice guidelines of the European Med icines Agency (CMPM/ICH/135/95, July 2002). All patients provid ed w ritten informed consent before inclusion. Eligibility criteria w ere p atients w ith clinically active luminal CD requiring treatment w ith an anti-TNF for ind uction of remission.At baseline, all p atients und erw ent a colonoscop y for assessment of end oscopic disease activity. A baseline segmental CD Endoscopic Index of Severity (CDEIS) ≥ 10 in at least one segment, or the p resences of ulcerations, w as required for inclusion.Exclusion criteria were formal contraindication for anti-TNF therapy, intolerance or contraindication for undergoing an ileocolonoscopy, absence of endoscopic activity as described above or a patient's refusal to participate.
Anti-TNF treatment
The ind uction therap y for infliximab w as 5 mg/kg at w eeks 0, 2 and 6; for ad alimumab 160 mg and 80 mg at w eeks 0 and 2, resp ectively, followed by 40 mg every other w eek. For maintenance, 5 mg/kg infliximab w as ad ministered every 8 weeks and 40 mg of adalimumab every other week. Intensification of treatment was performed reactively w hen loss of clinical response was d ocumented in association with objective evidence of active disease (increased fecal calprotectin or
CRP or p resence of end oscop ic lesions) and d rug levels below recommend ed threshold s (infliximab < 3 μg/m L, ad alimumab < 7 μg/m L). If remission w as documented by endoscopy, no modification of treatment was performed.
Clinical and biomarker data
Clinical evaluation was performed at baseline, and at weeks 6, 14, 30 and 46. At each visit the CD Activity Index (CDAI) was determined. Blood and fecal samples were also collected at each visit for assessment of biomarkers (CRP and calprotectin).Monitoring for adverse events and use of concomitant medications was registered at each visit, including unscheduled visits performed if the patient developed symptoms betw een scheduled visits. Drug levels and anti-drug antibodies were analyzed at baseline, week 14, week 46, and in case of loss of clinical response.
Endoscopic examination
Ileocolonoscopy was performed at baseline, week 14 and week 46. All examinations were performed under anesthesia by an endoscopist experienced in the assessment of IBD (ER, IA, IO). Quantification of endoscopic disease activity was based on the CDEIS, with measurement of segmental and global scores. Investigators evaluating the endoscopic lesions were blinded to the patient's symptoms. Endoscopic response was defined as a decrease of at least 50% from baseline in the global CDEIS and MH as segmental CDEIS ≤ 5 in all segments, which implies the disappearance of mucosal ulcerations.
Statistical analysis
A formal sample-size calculation w as not performed. Precision of the predictors id entified is provided by 95% confidence intervals. Quantitative variables are expressed as mean. Percentages are given for discrete variables. Analyses w ere performed according to the intention-to-treat principle includ ing all recruited patients. Patients with missing data were classified as non-responders for clinical and end oscopic outcomes (Non-response imputation). Missing quantitative data at different time points was imputed using the last observation carried forward. For identification of clinical, biological and endoscopic predictors of endoscopic response,logistic regression analysis was performed. A receiver operating characteristic (ROC)curve was constructed to determine the best cut-off value for predicting MH after one year of treatment. Spearman rank-order correlation coefficient was performed to identify association between variables. Statistical significance was set at P < 0.05 for all tests. Statistical analysis was performed using the statistical package SPSS V.23. The Statistical methods of this study were review by one of the authors (Ingrid Ordas).
RESULTS
From 100 potentially eligible patients with clinically active disease, 62 were finally included. Thirty eight patients were excluded for the following reasons: colonoscopy could not reach the affected area (n = 14), absence or mild endoscopic activity with all segmental CDEIS < 10 (n = 8), patient´s refusal to participate (n = 7), spontaneous patient's improvement w ithout need of anti-TNF treatment initiation (n = 7) or because anti-TNF treatment was initiated for complex perianal disease without significant luminal activity (n = 2).
Seven patients dropped out from the study, three of them during induction and four during the maintenance period. In 5 cases because surgery was required, in one patient treatment w as sw itched to another anti-TNF due to immunogenicity w ith secondary loss of response and in one case treatment was stopped due to an adverse event (infusion reaction). All seven cases were imputed as non-responders. Fifty-nine patients (95.2%) comp leted the 14 w k ind uction p eriod. Of these, 53 und erw ent end oscopic evaluation. Fifty-six patients (90.3%) completed one year of follow up of w hom forty-seven und erw ent end oscop ic evaluation (Figure 1). End oscop ic assessment w as not p erformed in some patients at w eeks 14 or 46 d ue to p atient's refusal; all of them were considered as non-responders.
Demograp hic and baseline disease characteristics are summarized in Table 1. A majority of patients received combination therapy (86%). The proportion of patients achieving MH at w eek 46 und er IFX and ADA w ere similar (46% vs 42%), the subsequent analysis was therefore performed in the pooled population.
Clinical, biological, pharmacokinetic, and end oscopic data at baseline and during follow up are presented in Table 2. At baseline, median CDAI was 201; treatment with anti-TNF resulted in a significant decrease in CDAI to 60 (P < 0.001) at week 14 and to 53 at w eek 46 (P < 0.001). Changes in biomarkers are summarized in Table 2.Calprotectin levels decreased progressively w ith significant d ifferences relative to baseline at w eeks 14 and 46. CRP value also d ecreased during follow-up reaching statistical significance at w eek 46 in the w hole stud y pop ulation and also in the subgroup of patients w ith elevated CRP (> 1.0 mg/d L) at baseline (n = 26; 41%).Hemoglobin and albumin concentrations significantly increased at w eeks 14 and 46 relative to baseline (P < 0.05).
Median infliximab and adalimumab concentrations at week 14 were 3.1 μg/mL and 8.9 μg/m L, respectively. After one year of treatment med ian serum d rug concentrations w ere 1.8 μg/m L and 9.9 μg/m L, respectively. At baseline 8% of p atients presented antidrug antibod ies to infliximab (ATIs) and 10% to ad alimumab (ATAs);of them 60% and 50% respectively w ere naïve to anti-TNF therapy. In all cases,antibody levels were in the low range (3.9-98 μg/mL). At week 14, ATIs were detected in only one patient (3%) and ATAs in none of them (the test used, ELISA, cannot detect antibod ies in the presence of circulating drug). After one year of treatment,ATIs w ere d etected in 4 p atients (13%) and ATAs in one p atient (4%). All of them were under concomitant immunomodulatory therapy. Median CDEIS at baseline was 6.7 with significant decreases up to 3.2 at w eek 14 (P < 0.001) and up to 3.0 (P < 0.001)after one year of treatment. As illustrated in Figure 2, at w eek 14, 56% of patients achieved end oscopic response and 34% were on MH. At week 46, the corresponding percentages for endoscopic response and MH were 52% and 44%, respectively.
Predictors of endoscopic remission
None of the demographic or baseline d isease characteristics pred icted end oscop ic response or remission at w eek 14. Baseline d isease characteristics w ith p red ictive value of long-term end oscopic response (w k46) in the univariate analysis includ ed disease duration, CDAI and CDEIS. The w eek 14 variables that predicted endoscopic response at week 46 w ere: CRP, a decrease from baseline CDAI of 100 points, CDEIS,the percentage of CDEIS reduction from baseline and the achievement of endoscopic response or MH. Due to an elevated rate of missing values (35% at baseline and 42%at w eek 14), calprotectin w as not included in the logistic regression analysis. In the multivariate analysis, only disease duration and the percentage of CDEIS reduction at week 14, independently predicted MH on the long term.
Pred ictors of MH after 46 w k of treatment w ere identified only at w eek 14 in the univariate analysis, including the CRP and the adalimumab concentration, the CDEIS score, the p ercentage of CDEIS red uction from baseline and the achievement of endoscopic response or MH at week 14. In the multivariate analysis all the endoscopic variables independently predicted MH at w eek 46.
The changes in clinical or biomarker variables from baseline to w eek 14 d id not predict MH on the long term. Ad alimumab or infliximab serum concentrations at week 14 were not associated with clinical or endoscopic outcomes at this time point of evaluation. Serum drug concentration at week 14 did not predict clinical outcomes or endoscopic response at w eek 46. Only adalimumab concentration at week 14 achieved statistical significance to predict MH on the long term (week 46). A concentration of at least 10 μg/m L at w eek (area und er the curve: 0.76) p red icts MH w ith mod erate sensitivity (61%) and good sp ecificity (84%). Drug concentration and anti-d rug antibodies at week 46 were not correlated with clinical or endoscopic outcomes at that time of evaluation.
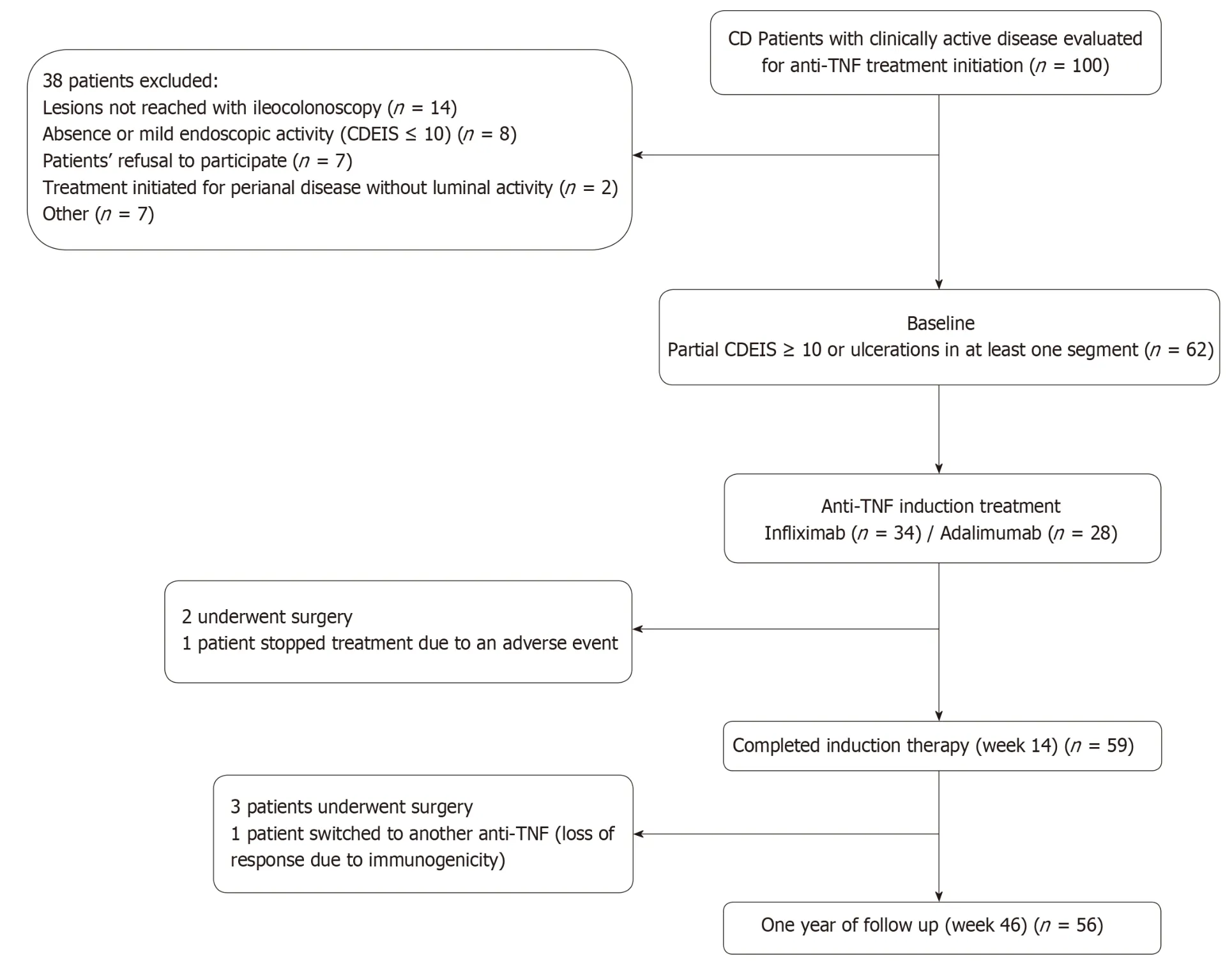
Figure 1 Flow-chart of the study. CD: Crohn's Disease; CDEIS: Crohn's Disease Endoscopic Index of Severity; TNF: Tumor necrosis factor.
In our cohort, only 19% of p atients required treatment intensification before achieving the end p oint of w eek 46. In the logistic regression analysis treatment intensification did not achieve statistical significance as a predictor of MH. To define the best cutoff value for predicting MH after one year of treatment, ROC curves w ere constructed for CDEIS scores at week 14 and for the percentage of CDEIS reduction from baseline to w eek 14. Sensitivity and specificity w ere also calculated for achievement of endoscopic response and MH at week 14 as predictors of long-term MH (Table 3). Relative changes in the endoscopic score from baseline to week 14 had higher accuracy in predicting MH at week 46 than absolute CDEIS values at week 14.Based on the clinical relevance of the evaluated cutoff values, a decrease of at least 80% from baseline CDEIS at week 14 was the best discriminative cutoff value for predicting MH at week 46 with a sensitivity of 59% and 91% specificity (Figure 3). As show n in Table 4, at w eek 14, a red uction of ≥ 80% from baseline CDEIS w as documented in 19 patients (31%) with a positive predictive value (PPV) of 84% and a negative predicted value of 74%. Table 5 shows the comparison of diagnostic accuracy between 50% and 80% CDEIS reduction from baseline to week 14 as a predictor of MH at week 46.
DISCUSSION
In this prospective observational study, we identified predictors of long-term MH in patients w ith CD treated with TNF-α inhibitors. After completing 14 weeks of treatment , w e found that the absolute CDEIS value, the percentage of CDEIS red uction from baseline and the achievement of endoscopic resp onse or MH significantly predict MH after one year of treatment. Of these four predictive factors,achievement of MH after induction treatment w ith an anti-TNF has already been described as a predictor of long-term MH in a series of patients retrospectively evaluated and prospectively validated in a subsequent study[12,13]. In this study, 42 patients with active luminal CD (defined as a SES-CD ≥ 3) treated with an anti-TNF were followed clinically and endoscopically after 3 months and one year of treatment.The authors concluded that MH (defined as a SES-CD ≤ 2) after 3 mo of treatment predicted long-term MH with 88% of sensitivity and 64% of specificity. Our study shows that not only achievement of MH is a predictor of long term MH, but also achievement of endoscopic response after completing induction predicts MH on thelong term, expanding thus the number of patients in w hom a recommend ation of continued anti-TNF therap y is mad e after ind uction w ith high p robability of achieving the therapeutic target of MH at week 46.

Table 1 Patient's demographic characteristics (n = 62) n (%)
The p ercentage of CDEIS reduction as a pred ictor of clinical outcomes has been evaluated in a post-hoc analysis of the SONIC trial[15]. In this stud y, a decrease from the baseline CDEIS of at least 50% at week 26 predicted steroid-free clinical remission at w eek 50 w ith a sensitivity of 73% and a specificity of 46 %. We consid er, though,that objective d emonstration of MH by end oscopy is a more robust end point than steroid-free clinical remission. In addition, a specificity of 46% can lead to a high rate of false positives.
In our cohort, a d ecrease of at least 50% from baseline CDEIS at w eek 14 had a sensitivity of 85% and moderate specificity (66%). In our study, we chose a reduction of 80% from baseline CDEIS as a p red ictor of long term MH since it has better diagnostic accuracy, with mod erate sensitivity (59%) but excellent specificity (91%).Moreover, compared with a 50% reduction, it demonstrated a better PPV and positive likelihood ratio. In cases in which sensitivity is more relevant, a 50% reduction can be chosen as the end point. In the current study, we observed that changes in CDEIS in response to induction therapy are better predictors of long-term efficacy of anti-TNF drugs than absolute values. Absolute values are highly influenced by disease location and extension, w hereas a percentage reduction relative to baseline may be preferable because it is a d ynamic parameter of resp onse and integrates the magnitud e of response to induction therapy.
In this cohort, 84% of p atients that achieved an 80% red uction in CDEIS from baseline to w eek 14 w ere in MH after one year of treatment. In patients w ho d o not achieve this endpoint, potential treatment optimization might be considered by the ad d ition of immunosup p ressive agents in case of anti-TNF monotherap y,intensification of anti-TNF treatment or by consid ering alternative treatment strategies other than TNF-α inhibitors. The effectiveness of these type of interventions remains to be assessed in prospective clinical trials. In our study neither demographic nor disease characteristic predicted achievement of endoscopic response at w eek 14,or MH after ind uction or at w eek 46. This w as also true for clinical disease activity and CRP. Disease d uration has been reported to be a predictor of clinical response[5]and in our study was identified as a predictor of endoscopic response in the long term(the longer the disease duration the less probabilities to achieve endoscopic response).
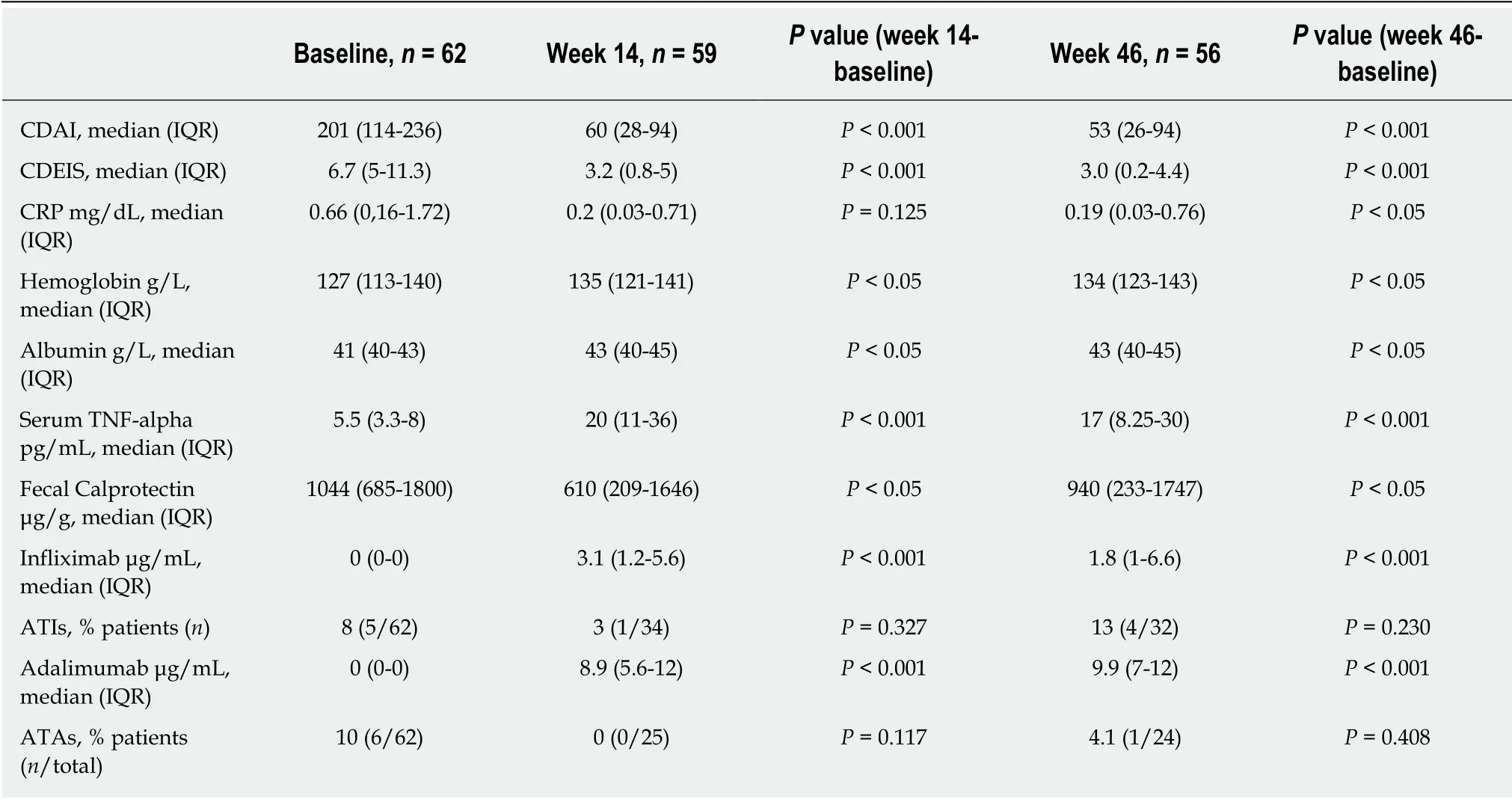
Table 2 Biological, pharmacokinetic, clinical and endoscopic data at baseline and during follow up
In our cohort, adalimumab serum concentration after completing treatment induction has predictive value for the long term MH diagnosis. Previous reports have described an association between adalimumab serum levels and achievement of MH.The cutoff point of this parameter has been situated in at least 4.9 μg/Ml[16]and some retrospective studies or studies with non-systematic measurement of adalimumab serum concentration suggest that the concentration needed to achieve this endoscopic outcome are even higher (> 7.1 μg/m L)[17-19]. In our prospective cohort, an adalimumab concentration of at least 10 μg/mL after induction was best correlated with long term MH with a moderate sensitivity and good specificity, suggesting that patients may need even higher concentrations than previously reported. Measurement of serum drug concentration after induction could be considered as a time point to perform this evaluation for predicting long term endoscopic outcomes. Infliximab serum concentration were not predictive of MH; lack of statistical power, and the fact that a considerable number of patients had low serum infliximab concentrations may have prevented the id entification of the relationship between d rug levels and therapeutic response.
When we analyzed the subgroup of patients that achieved MH at weeks 14 and 46,a recommended concentration of infliximab (equal or higher than 3.0 μg/mL) was observed in a 73% and 29% of patients respectively. The high proportion of patients in MH and low anti-TNF serum concentration during maintenance might indicate that lower drug levels may be sufficient after achieving healing. The current study has several strengths. It has been prosp ectively performed and all p atients had documented endoscopic active disease at baseline. In addition, monitoring of disease activity was prospectively registered using clinical, biological, and endoscopic variables. Our cohort has a lower clinical activity at baseline compared to pivotal registration trials. Indeed, our population probably better reflects clinical practice,where anti-TNF therapies are used not only in patients with severe disease as in pivotal trials, but also in patients with milder forms.
This study has also some limitations that should be acknowledged. It is a single center study with a limited sample size. With regard to biomarkers as predictors of endoscopic response, calprotectin could not be incorporated in the analysis due to a considerable proportion of missing samples, despite proactively reminding the patients to collect them. This is an inherent limitation of this monitoring modality.Escalation of d rug d oses w as triggered by clinical symp toms. This could be considered as a limitation, however, there is no evidence at this moment supporting that treatment intensification based on a target serum drug concentration[20,21]is superior to clinically based dosing. In this scenario, treatment intensification based on clinical decisions represent the current clinical practice in the majority of centers until new evidence is available.
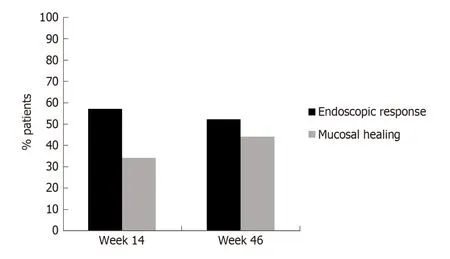
Figure 2 Endoscopic response and mucosal healing during follow-up.
In summary, in patients with CD treated with an anti-TNF, endoscopic evaluation following completion of induction treatment should be considered for determining endoscopic response and predicting long-term outcomes. After 14 weeks of treatment,achievement of a reduction of 80% from baseline CDEIS may be considered a target for optimization of anti-TNF therapy in clinical practice.
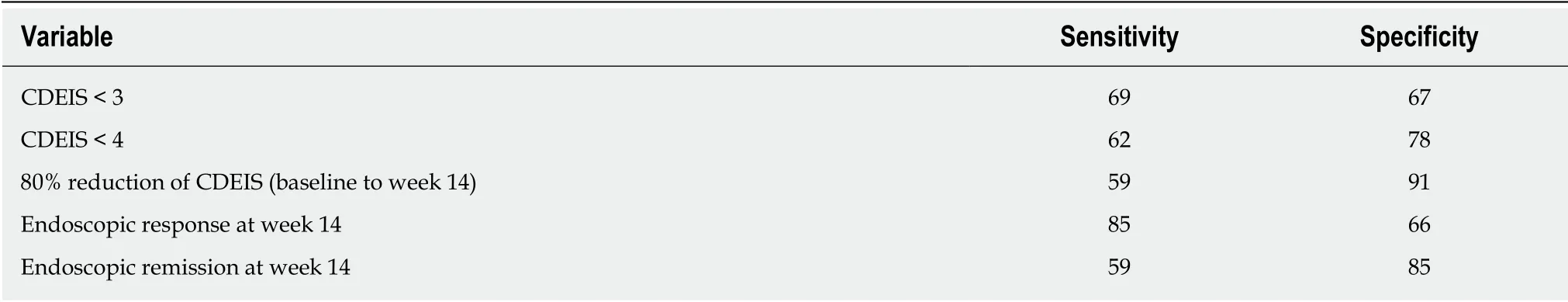
Table 3 Sensitivity and specificity of variables at week 14 as predictors of long-term mucosal healing

Table 4 Diagnostic accuracy of 80% Crohn's Disease Endoscopic lndex of Severity reduction at week 14 for the diagnosis of long-term mucosal healing
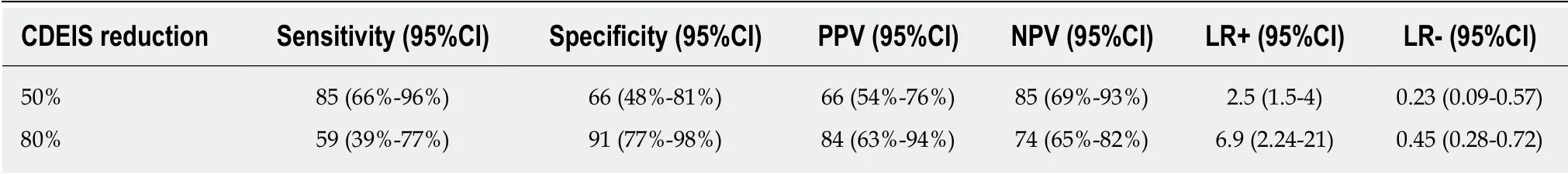
Table 5 Comparison between 50 and 80% Crohn's Disease Endoscopic lndex of Severity reduction from baseline to week 14 as predictors of long-term mucosal healing
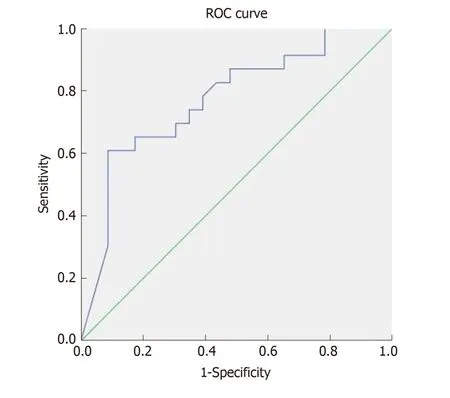
Figure 3 Receiver operating characteristics curves depicting the endoscopic response at week 14 as a predictor of mucosal healing after one year of antitumor necrosis factor treatment. Area under the curve = 0.778, P = 0.001; ROC: Receiver operating characteristics.
ARTICLE HIGHLIGHTS
Research backgroundTumor necrosis factor (TNF) inhibitors are one of the most effective therapies for induction and maintenance of remission in p atients w ith Crohn's d isease (CD), how ever, to d ate, no observational prospective studies have been specifically designed to id entify predictors of mucosal healing (MH), w hich should be the therapeutic target.
Research motivation
Improve know ledge about the predictive factors of response to TNF inhibitors and try to provide more tools to perform a personalized treatment.
Research objectives
The aim of this study was to identify predictors of MH after one year of treatment with TNF-α inhibitors.
Research methods
Prospective observational single center study. Consecutive patients w ith clinically active CD requiring treatment with a TNF-α inhibitor were included. Clinical, biological and endoscopic data were obtained at baseline, weeks 14 and 46.
Research conclusions
Endoscopic response to induction therapy, defined as 80% reduction in global CD Endoscopic Index of Severity (CDEIS), is a robust predictor of long-term MH. End oscopic response is a predictor of MH. Achievement endoscopic response after induction may be consid ered as a therap eutic target for anti-TNF-α therapy. None, it w as a observational stud y to id entify predictors. After induction of remision w ith a TNF inhibitor perform a colonoscopy should be considered to predict long-term outocomes. Endoscopic response predict long-term outcomes.There w ere no hypotheses. After ind uction of remision w ith a TNF inhibitor perform a colonoscopy should be considered to predict long-term outocomes in patients with CD.
Research perspectives
Clinical and biomarker data are not useful predictors of response to TNF-α inhibitors in CD,w hereas endoscopic response to induction therapy, defined as 80% reduction in global CDEIS, is a robust p red ictor of long-term MH. Achievement of this end oscop ic end point may be considered as a therapeutic target for anti-TNF-α therapy.
ACKNOWLEDGEMENTS
This work has been founded in part by Helmsley Charitable Trust 2015PG-IBD005.
杂志排行
World Journal of Gastroenterology的其它文章
- Precision surgical approach with lymph-node dissection in early gastric cancer
- Update on hepatocellular carcinoma: Pathologists' review
- Application of artificial intelligence in gastroenterology
- Clinical significance of programmed death 1/programmed death ligand 1 pathway in gastric neuroendocrine carcinomas
- Functional role of long non-coding RNA CASC19/miR-140-5p/CEMIP axis in colorectal cancer progression in vitro
- Seven-senescence-associated gene signature predicts overall survival for Asian patients with hepatocellular carcinoma
