Measurement of prostaglandin metabolites is useful in diagnosis of small bowel ulcerations
2019-05-08YuichiMatsunoJunjiUmenoMotohiroEsakiYoichiroHirakawaYutaFuyunoYasuharuOkamoto
Yuichi Matsuno, Junji Umeno, Motohiro Esaki, Yoichiro Hirakawa, Yuta Fuyuno, Yasuharu Okamoto,
Atsushi Hirano, Shigeyoshi Yasukawa, Fumihito Hirai, Toshiyuki Matsui, Shuhei Hosomi, Kenji Watanabe,
Naoki Hosoe, Haruhiko Ogata, Tadakazu Hisamatsu, Shunichi Yanai, Shuji Kochi, Koichi Kurahara,
Tsuneyoshi Yao, Takehiro Torisu, Takanari Kitazono, Takayuki Matsumoto
Abstrac t BACKGROUND We recently reported on a hereditary enteropathy associated with a gene encoding a prostaglandin transporter and referred to as chronic enteropathy associated with SLCO2A1 gene (CEAS). Crohn's disease (CD) is a major differential diagnosis of CEAS, because these diseases share some clinical features. Therefore, there is a need to develop a convenient screening test to distinguish CEAS from CD.AIM To examine whether prostaglandin E major urinary metabolites (PGE-MUM) can serve as a biomarker to distinguish CEAS from CD.METHODS This was a transactional study of 20 patients with CEAS and 98 patients with CD.CEAS was diagnosed by the confirmation of homozygous or compound heterozygous mutation of SLCO2A1. We measured the concentration of PGEMUM in spot urine by radioimmunoassay, and the concentration was compared between the two groups of patients. We also determined the optimal cut-off value of PGE-MUM to distinguish CEAS from CD by receiver operating characteristic(ROC) curve analysis.RESULTS Twenty Japanese patients with CEAS and 98 patients with CD were enrolled.PGE-MUM concentration in patients with CEAS was significantly higher than that in patients with CD (median 102.7 vs 27.9 μg/g × Cre, P < 0.0001). One log unit increase in PGE-MUM contributed to 7.3 increase in the likelihood for the diagnosis of CEAS [95% confidence interval (CI) 3.2-16.7]. A logistic regression analysis revealed that the association was significant even after adjusting confounding factors (adjusted odds ratio 29.6, 95%CI 4.7-185.7). ROC curve analysis revealed the optimal PGE-MUM cut-off value for the distinction of CEAS from CD to be 48.9 μg/g × Cre with 95.0% sensitivity and 79.6% specificity.CONCLUSION PGE-MUM measurement is a convenient, non-invasive and useful test for the distinction of CEAS from CD.
Key words: Chronic enteropathy associated with SLCO2A1 gene; Prostaglandin E major urinary metabolites; Chronic nonspecific multiple ulcers of the small intestine; Crohn's disease; Small intestine
INTRODUCTION
The use of capsule endoscopy and balloon-assisted endoscopy has enabled the precise observation of small bowel mucosal lesions in gastrointestinal disease pathology, such as Crohn's disease (CD), intestinal tuberculosis, and nonsteroidal anti-inflammatory drug (NSAID)-ind uced enteropathy[1-5]. While characteristic end oscopic find ings of small bow el inflammation have been rep orted, it is sometimes d ifficult to correctly diagnose patients by endoscopic findings alone in cases lacking characteristic features of the disease.
We previously reported an independent disease entity termed chronic nonspecific multip le ulcers of the small intestine (CNSU) characterized by multip le small intestinal ulcers of nonspecific histology[6-9]. In addition, we recently found that CNSU is caused by recessive inheritance of the SLCO2A1 gene and p rop osed a new nomenclature for this d isease: Chronic enteropathy associated with SLCO2A1 gene(CEAS)[10]. Therefore, it is now possible to diagnose CEAS based on a combination of clinical features and genetic analysis. How ever, it is sometimes d ifficult to differentiate CEAS from CD because these diseases share clinical features such as p ersistent anemia and hyp op roteinemia, suscep tible age in p uberty, and a pred ominance of ileal involvement. The differentiation betw een CEAS and CD is clinically essential to avoid ineffective therapies because patients with CEAS do not respond to treatment with corticosteroids or biologics[7,9]. Moreover, since the patients with CEAS usually show low inflammatory markers in blood test[11], it is a particularly important problem to distinguish CEAS from CD w ith low disease activity. In clinical p ractice, how ever, it is unrealistic to p erform genetic screening for all p atients suspected as CD or CEAS.
The SLCO2A1 gene encodes a prostaglandin transporter that mediates the cellular uptake of prostaglandins. SLCO2A1 is also the causative gene of primary hypertrophic osteoarthropathy (PHO)[12-14]. An increase in urinary levels of prostaglandin E2(PGE2) and prostaglandin E metabolites in SLCO2A1-deficient PHO individuals has been confirmed[14]. PGE2 is known to have the protective effect on the mucosal injury,how ever it is difficult to directly measure PGE2 level in local tissues or in the blood because of its extremely rap id metabolism[15]. Prostagland in E-major urinary metabolite (PGE-MUM) is know n to be stable and w e have also p reviously d emonstrated an increase in the urinary levels of p rostagland in E metabolites in CEAS patients[10]. Therefore, this study investigated whether PGE-MUM are useful for differentiating CEAS from CD.
MATERIALS AND METHODS
Subjects
Among the participants of our previous stud ies[10,16,17]and the patients diagnosed as CEAS or CD at Kyushu University hospital, w e enrolled 20 Japanese patients w ith CEAS and 98 patients with CD for the present investigation. A diagnosis of CEAS was confirmed based on p ublished clinical criteria[18,19]w ith genetic analysis (Sup p lementary Table 1). The diagnosis of CD was based on the Japanese diagnostic criteria for CD[20]. No patients were administrated prostaglandin analogues or NSAIDs at the time of urine sample collection. All urine and blood samples w ere collected after obtaining w ritten informed consent. This stud y w as app roved by the institutional review board at each collecting site in accordance w ith the Declaration of Helsinki Principles.
Clinical characteristics
Clinical information includ ing age, gender, history of surgery, concomitant drugs,serum C reactive protein (CRP) levels, d isease location, and d isease d uration w as collected from each p articip ating institution. Because the PGE2 metabolite concentration w as reported to increase in cases of thiazide-induced hyponatremia[21]and laxative sennosid e administration[22], w e collected information about the use of these med ications. Because the PGE-MUM concentration w as rep orted to be influenced by age, gender, smoking habit, and pulmonary inflammatory cond itions includ ing chronic fibrosing interstitial pneumonia[15,23], w e also collected information about smoking habit and pulmonary disease. Age was determined at the time of urine samp le collection. Smoking habit w as defined as never, current (> 6 mo on a daily basis), and former (cessation of smoking > 6 mo). Pulmonary d iseases includ ed chronic respiratory d isease, d iffuse lung d isease, and any other lung or airw ay disease. History of surgery was defined as a history of intestinal resection at the time of study enrolment. The site of involvement w as determined by radiographic and/orend oscopic findings, and these patients w ere categorized accord ing to the Montreal classification[24]. Disease d uration w as d efined as the p eriod from the onset of symptoms until study enrolment.
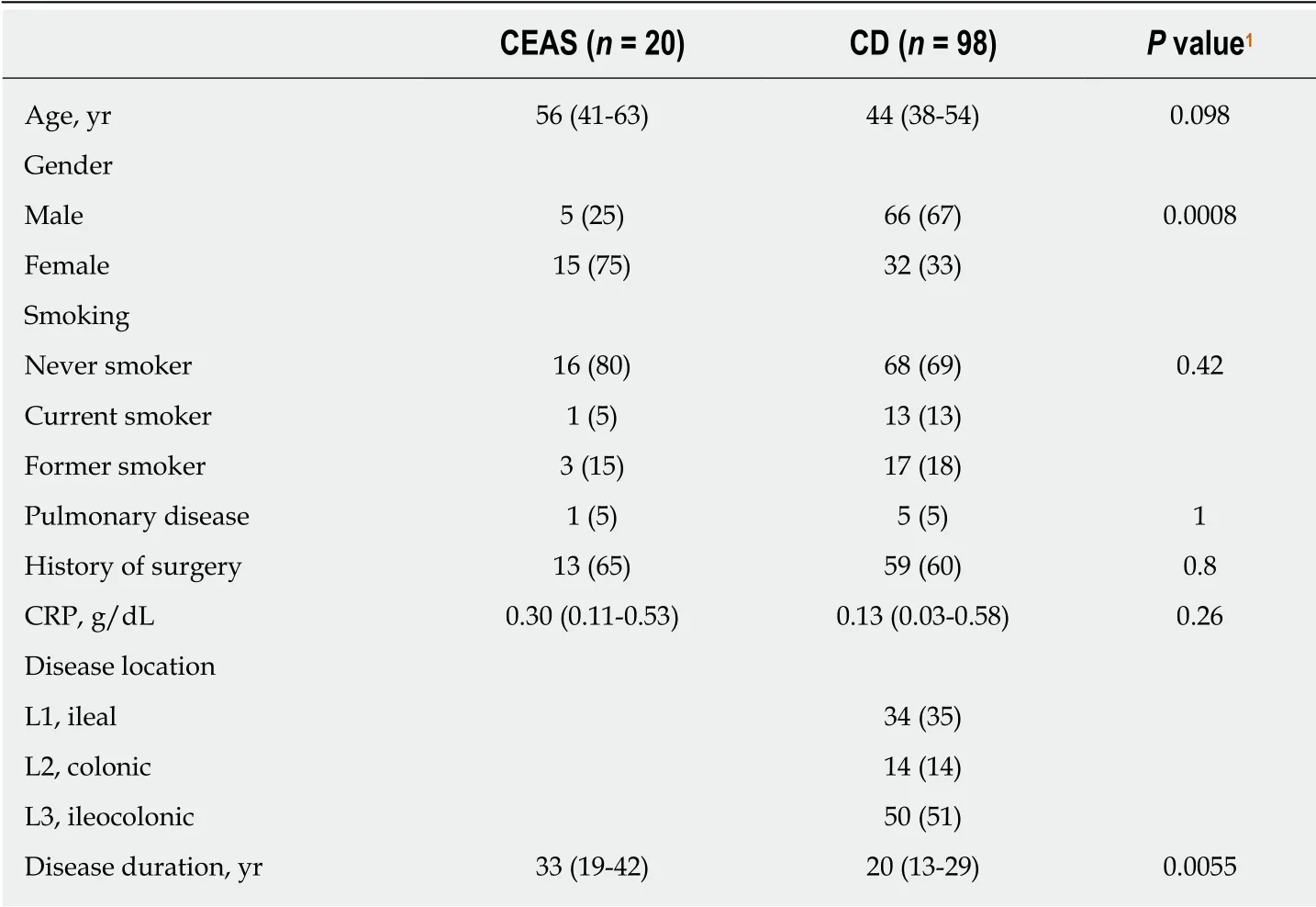
Table 1 Clinical characteristics of the study subjects
Genetic analysis
We used genomic DNA samp les extracted from p erip heral blood in our previous stud ies[10,16,17,25]. In advance, w e checked and confirmed that none of the patients with CD had homozygous or compound heterozygous S LCO2A1 mutations with regards to the major six sites in our p revious rep ort[10], includ ing c.421G>T, c.664G>A,c.940+1G>A, c.1372G>T, c.1461+1G>C, and c.1807C>T. The d iagnosis of CEAS w as confirmed with 90.2% sensitivity by genotyping these 6 mutations on the basis of our previous investigation.
Measurement of prostaglandin E major urinary metabolite concentrations
All PGE-MUM concentrations w ere measured at SRL Inc. (Tokyo, Japan) by using a bicyclic PGE-MUM rad ioimmunoassay kit (Institute of Isotopes Co., Ltd. Budapest,Hungary). PGE-MUM, 7α-hydroxy-5, 11-diketotetranorprosta-1, 16-dioic acid derived from PGE1 and PGE2 can be transformed to bicyclic PGE-MUM by alkali treatment.Each urine samp le w as collected and submitted ind ivid ually accord ing to the manufacturer's protocol described by Fujiwara et al[22]. The urine samples w ere frozen and stored at -20 °C until the assay. PGE-MUM concentrations were corrected by the urinary creatinine concentration.
Statistical analyses
Categorical variables were expressed as the number (%) and continuous variables were expressed as the median (interquartile range). Comparison of the variables between CEAS and CD were performed using the chi-squared test, Fisher's exact probability test, or the Mann-Whitney U-test, where appropriate. The associations betw een clinical factors and the PGE-MUM concentration w ere estimated using univariable linear regression analyses. Because of the skewed distribution of the PGEMUM concentration, values were log-transformed and used for regression analysis.
For the analysis of the clinical significance of PGE-MUM concentration to differentiate CEAS from CD, the logistic regression model was used to estimate the odds ratios (ORs) with 95% confidence intervals (CIs). The diagnostic accuracy of PGE-MUM concentration was evaluated using the area under the curve (AUC) with the receiver operating characteristic (ROC). The optimal cut-off value of PGE-MUM concentration for the diagnosis of CEAS was determined based on the maximum Youden Index (sensitivity + specificity - 1). In addition, sensitivity and specificity were compared using several cut-off values to confirm the diagnostic accuracy of this cut-off value. Statistical analyses w ere p erformed using JMP version 13.0 (SAS Institute, Cary, NC, United States). A P value of 0.05 or smaller w as consid ered statistically significant.
RESULTS
Baseline patient characteristics
The enrolled subjects consisted of 20 p atients w ith CEAS (CEAS group) and 98 patients with CD (CD group). The clinical characteristics of the patients are show n in Table 1. In the CEAS group, female patients were more frequent compared w ith the CD group. The disease duration w as significantly longer in the CEAS group than in the CD group (median 33 vs 20 years, P = 0.0055). There was no significant difference in smoking status or med ical history of pulmonary disease. Because most patients w ith CD were in clinical remission at the time of urine sample collection, the CRP levels remained low in the CD group.
PGE-MUM concentrations
Overall, 118 urinary samples were obtained from CEAS and CD patients. The PGEMUM concentration ranged from 7.7 to 402.0 (median 34.1) μg/g × Cre. Table 2 shows the result of univariable linear regression analysis. A positive history of pulmonary disease and history of surgery were significantly associated with a higher PGE-MUM concentration (P < 0.0001 and P = 0.029, respectively). Serum CRP value w as marginally associated with PGE-MUM concentration (P = 0.078). Factors with a P value < 0.1 were included in the multivariate analysis considering the possibility of confounding factors.
The PGE-MUM concentration was significantly higher in the CEAS group than in the CD group [median interquartile range (IQR): 102.7 (62.0-155.0) μg/g × Cre vs 27.9(19.6-40.0) μg/g × Cre, P < 0.0001, Figure 1]. Crude OR of 1 log unit increase in the PGE-MUM concentration for the distinction between CEAS and CD was 7.3 (95%CI 3.2-16.7, Table 3). This prediction model showed good differentiating ability between CEAS and CD, with an AUC of 0.90 (95%CI 0.82-0.95). This association was significant even after adjusting for possible confounding factors including age, gender, medical history of pulmonary disease, history of surgery, disease duration, and serum CRP value (model 3). The multivariate-adjusted OR was 29.6 (95%CI 4.7-185.7), with an AUC of 0.95 (95%CI 0.86-0.98).
Correlation between PGE-MUM and CD phenotype
The PGE-MUM concentration in the colonic CD group (L2) was significantly lower than in the other groups (median: 16.5 μg/g × Cre vs 29.5 μg/g × Cre, P = 0.036), and the PGE-MUM concentration of the ileal CD group (L1) was significantly higher than in the colonic CD group (median: 32.9 μg/g × Cre vs 16.5 μg/g × Cre, P = 0.017).When we confined the subjects to those with ileal CD, the PGE-MUM concentration in the CEAS group was still significantly higher than that in the ileal CD group (median 102.7 μg/g × Cre vs 32.9 μg/g × Cre, P < 0.0001).
PGE-MUM cut-off level
Figure 2 shows the ROC curve of the PGE-MUM concentration to differentiate CEAS from CD. Based on the ROC analysis, the optimal cut-off value was identified as 48.9 μg/g × Cre, which differentiated CEAS from CD with 95.0% sensitivity and 79.6%specificity (Supplementary Table 2). The AUC was 0.90 (95%CI, 0.82-0.95).
Genetic screening in CD patients with a high PGE-MUM concentration
Among the CD group, six patients had particularly high PGE-MUM concentrations (>100 μg/g × Cre). Although w e confirmed that they d id not harbor the six major mutations in the SLCO2A1 gene, w e screened all 14 coding exons and intron-exon bound aries of the SLCO2A1 gene using Sanger sequencing to exclud e a p ossible misd iagnosis of CEAS. No homozygous or comp ound heterozygous S LCO2A1 mutations were found in the six CD patients.
DISCUSSION
CEAS was initially reported as a novel disease entity referred to as "C" in the Japanese literature in the 1960s[26]. We recently reported it is an autosomal recessive inherited disease[8]caused by loss-of-function mutations in the SLCO2A1 gene[10]. Small bowel lesions in CEAS are characterized by multiple shallow ulcers with a circular oreccentric oblique configuration[18]. In contrast, CD typically d evelops longitudinal ulcers and cobblestoning, and ulcerations are p red ominantly located on the mesenteric side. However, both CEAS and CD involve the ileum w ith occasional ileal stenoses, and the clinical features of CEAS and CD are similar; therefore, it is not easy to differentiate CEAS from CD based on clinical manifestations alone[11]. Although immunohistochemical staining for SLCO2A 1 protein in biopsy specimens from the GI tract was reported to be useful to distinguish CEAS from CD[27,28], a more convenient and non-invasive screening test is required.
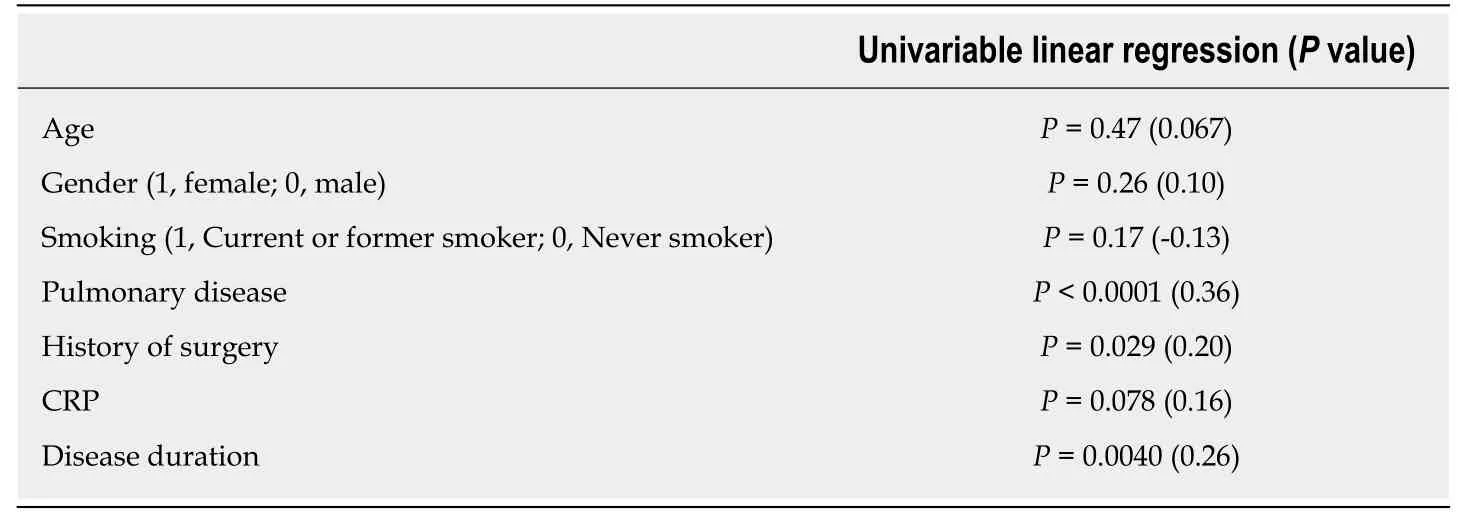
Table 2 Factors associated with prostaglandin E major urinary metabolite concentrations
PGE-MUM, a major urinary metabolite d erived from PGE2, is clinically used to assess the severity of inflammatory conditions such as interstitial pneumonia[23], cystic fibrosis[29], and ulcerative colitis[30,31]. Similarly, urinary PGE2 metabolite concentrations in p atients w ith CD are higher than those in p atients w ith colorectal neoplasms and healthy controls[32]. Indeed, PGE-MUM concentrations in patients with CD (median 27.9, IQR 19.6-40.0 μg/g × Cre) in the present stud y w ere higher than those in Japanese healthy controls (median 13.1, IQR 10.3-17.2 μg/g × Cre) as reported in p revious stud ies[15,23]. Nevertheless, the PGE-MUM concentration, a surrogate marker of physiological PGE2 levels[30], in patients with CEAS was significantly higher than in p atients w ith CD. This d ifference w as still significant even after the adjustment for confounding factors. In addition, we demonstrated that the provisional cut-off value (48.9 μg/g × Cre) of PGE-MUM had a differentiating ability w ith 95.0%sensitivity and 79.6% specificity (AUC 0.90). Therefore, PGE-MUM is useful to distinguish CEAS from CD.
Although prostaglandins are mediators of active inflammation, many experimental stud ies have ind icated that they p lay p ivotal roles in maintaining mucosal homeostasis[33]and mucosal repair[34]of the intestine. PGE2 is mainly d erived from arachidonic acid by cyclooxygenase and PGE synthase and PGE2 acts through four different recep tor subtypes (EP1, EP2, EP3 and EP4), w hich partly determines the various physiological effects of PGE2 includ ing the regulation of acid secretion,bicarbonate secretion, mucus production, and mucosal blood flow[35]. In particular,dimethyl PGE2 (dmPGE2), a stable derivative of PGE2, was reported to exert a potent inhibitory effect against indomethacin-induced intestinal injuries in a rat mod el via activation of EP3 and EP4 receptors[36]. It w as also shown that the deleterious effect of indomethacin on the healing of intestinal ulcers w as induced by an EP4 antagonist and suppressed by the co-administration of PGE2 as w ell as an EP4 agonist[34]. These results indicate that the protective effect of PGE2 on mucosal injury of the intestine is caused by EP3 and EP4 activation.
PHO, which develops skin and bone disorders caused by impaired prostaglandin metabolism, is classified into tw o types: PHOAR2 caused by the SLCO2A1 gene[12],and PHOAR1 caused by the HPGD gene[37]. Prostaglandin-signaling is terminated by the cellular uptake of prostaglandins via the prostaglandin transporter, follow ed by intracellular oxidation by 15-ketoprostaglandin dehyd rogenase (15-PGDH) encod ed by the HPGD gene[38]. Thus, cellular uptake of prostaglandins is the first critical step in inactivating p rostagland ins. The SLCO2A1 gene med iates the d egrad ation of prostaglandins via their intracellular uptake[39,40]. The HPGD gene is also related to the degrad ation of p rostagland ins by encod ing intracellular oxid ation enzymes of 15-PGDH. In both cond itions, significantly elevated p lasma PGE2 levels have been observed[13,37]. Because PGE2 promotes the proliferation of osteoblasts in bone tissue via the exp ression of vascular end othelial grow th factor[41,42], various clinical manifestations of PHO such as digital clubbing, periostosis, acroosteolysis, painful joint enlargement, and thickened skin, are suggested to be the consequence of an overabund ance of PGE2. Although PGE2 is p rotective against gastrointestinal mucosal injuries[35,42], multiple ileal ulcers occur in patients w ith CEAS, in w hom systemic levels of PGE2 are elevated. Consid ering our p revious result that the p rostagland in transporter w as exp ressed on the cellular membrane of vascular end othelial cells[10], it can be p resumed that imp aired p rostagland in use in the intestinal mucosa caused by mutation of the SLCO2A1 gene is a major factor in the pathogenesis of intestinal ulcers in CEAS.
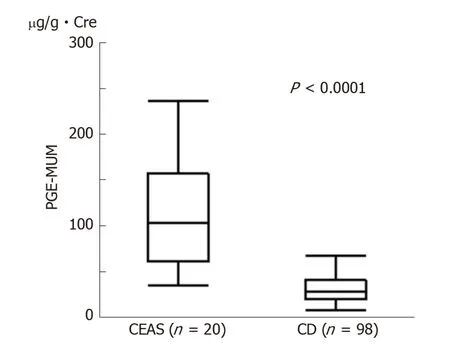
Figure 1 Comparison of Prostaglandin E-major urinary metabolites concentrations between chronic enteropathy associated with SLCO2A1 gene (n = 20) and Crohn's disease (n = 98) groups. Prostaglandin Emajor urinary metabolite concentrations in the chronic enteropathy associated with SLCO2A1 gene group were significantly higher than those in the Crohn's disease group [median (interquartile range): 102.7 (62.0-155.0) µg/g ×Cre vs 27.9 (19.6-40.0) µg/g × Cre, P < 0.0001]. Mann-Whitney U-test. CEAS: Chronic enteropathy associated with SLCO2A1 gene; CD: Crohn's disease; PEG-MUM: Prostaglandin E-major urinary metabolite.
Cryptogenic multifocal ulcerous stenosing enteritis (CMUSE) is another d isease entity that causes gastrointestinal ulcerations resembling CEAS[43,44]. Recently,recessive mutations in the PLA2G4A gene, encoding cytoplasmic phospholipase A 2-α(cPLA2α), which hydrolyses cellular membrane phospholipids into arachidonic acids,have been identified as a cause of CMUSE[44,45]. Because the loss-of-function of cPLA2α decreases systemic levels of PGE2 (approximated by PGE-MUM) and thromboxane A2[45], multiple small bow el ulcers, as w ell as the dysfunction of platelet aggregation are observed in p atients w ith CMUSE[46]. Although p recise analyses of the morphologic features of gastrointestinal lesions in CMUSE have not been reported yet, it seems likely that CEAS and CMUSE share morphologic features regard ing the gastrointestinal lesions. In this sense, the measurement of PGE-MUM concentrations can be assumed to be useful to d ifferentiate CEAS from CMUSE, although genetic testing is mandatory to confirm the diagnosis.
This study had several limitations. First, the sample size of the patients with CEAS was relatively small because CEAS is a rare disease. Therefore, we could not validate the cut-off value for the distinction between the two diseases. Second, because most of our CD patients were in clinical remission, the cut-off value may not be appropriate for the distinction of CEAS from active CD. Indeed, some patients with quiescent CD had markedly high PGE-MUM concentrations. Third, w e could not completely rule out the possibility that the patients w ith CD had recessive mutations in the SLCO2A1 gene. Although w e checked the major six mutations of the SLCO2A1 gene in patients with CD, 9.8% of the mutations might be overlooked based on our previous result[10].
In conclusion, the measurement of PGE-MUM might be a convenient, non-invasive and useful test to differentiate CEAS from CD, although genetic analysis is mandatory for the confirmation of CEAS. Further studies are necessary to clarify the pathogenesis of CEAS to explore therapeutic targets of this disease.
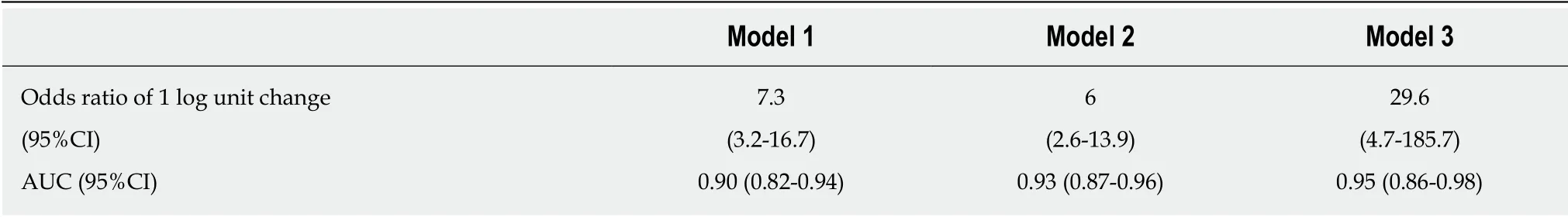
Table 3 Convert odds ratio for prostaglandin E major urinary metabolite
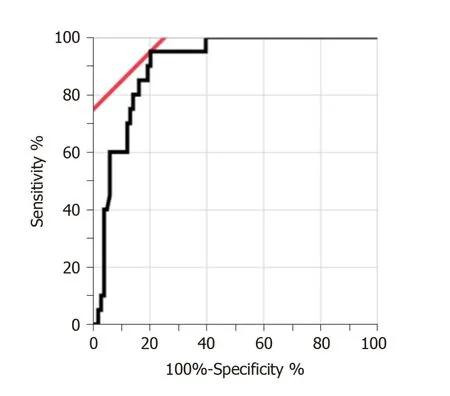
Figure 2 Receiver operating characteristic curve of Prostaglandin E-major urinary metabolites concentrations for differentiation between chronic enteropathy associated with SLCO2A1 gene and Crohn's disease. The area under the receiver operating characteristic curve was 0.90 (95% confidence interval,0.82-0.95). The optimal cut-off value was 48.9 µg/g × Cre.
ARTICLE HIGHLIGHTS
Research background
Chronic enteropathy associated with SLCO2A1 gene (CEAS) is a rare small intestinal disease, but distinct clinical condition from Crohn's disease (CD). To date, we can make a correct diagnosis of CEAS by combination of clinical features and genetic analysis. However, CEAS is sometimes misdiagnosed as CD, because these diseases have some clinical features in common.
Research motivation
A more convenient and non-invasive screening test will help to diagnose CEAS correctly.
Research objectives
To investigate the usefulness of prostaglandin E-major urinary metabolites (PGE-MUM) to distinguish CEAS from CD.
Research methods
Participants were diagnosed CEAS and CD by clinical diagnostic criteria and genetic analysis.All participants' PGE-MUM concentrations were measured by radioimmunoassay. We analyzed differentiating ability of PGE-MUM measurement between CEAS and CD.
Research results
Twenty patients with CEAS and 98 patients with CD were enrolled. It was found that the PGEMUM concentrations of patients with CEAS were significantly higher than those of patients with CD and this correlation was still statistically significant after adjusting the possible confounding factors. Additionally, the present study showed the provisional cut-off value (48.9 μg/g × Cre) of PGE-MUM had a differentiating ability with 95.0% sensitivity and 79.6% specificity (area under the curve 0.90).
Research conclusions
The measurement of PGE-MUM can serve as a useful biomarker to differentiate CEAS from CD,although genetic analysis is mandatory for the diagnosis of CEAS.
Research perspectives
Our study showed the usefulness of PGE-MUM to differentiate CEAS from CD. In the present research, the sample size of the patients with CEAS were relatively small, the cut-off value for the distinction of CEAS from CD should be validated w ith large sample size of patients w ith CEAS in the future research.
ACKNOWLEDGEMENTS
We appreciate the patients for participating in this study. We also appreciate Ms. Risa Tsuneyoshi for technical assistance and Drs. Tomohiko Moriyama, Shin Fujioka,Naoya Kubokura, Yoichiro Nuki, Ema Washio, and Shinichi Kaw ano for their assistance with clinical characterization.
杂志排行
World Journal of Gastroenterology的其它文章
- Precision surgical approach with lymph-node dissection in early gastric cancer
- Update on hepatocellular carcinoma: Pathologists' review
- Application of artificial intelligence in gastroenterology
- Clinical significance of programmed death 1/programmed death ligand 1 pathway in gastric neuroendocrine carcinomas
- Functional role of long non-coding RNA CASC19/miR-140-5p/CEMIP axis in colorectal cancer progression in vitro
- Seven-senescence-associated gene signature predicts overall survival for Asian patients with hepatocellular carcinoma
