含有5-甲基-3-吡唑甲酸的铅ギ配合物:合成、结构和荧光性质
2019-04-12程美令杨冰心唐李志鹏秦梦娜唐晓艳
程美令 杨冰心 唐李志鹏 秦梦娜 刘 琦,2 唐晓艳,3
(1常州大学石油化工学院,江苏省绿色催化材料与技术重点实验室,常州 213164)(2南京大学配位化学国家重点实验室,南京 210093)(3常熟理工学院化学与材料工程学院,常熟 215500)
0 Introduction
In the past decades,the coordination chemistry of divalent lead has attracted much attention,not only for its toxicity and its occurrence in critical life cycles as a result of its widespread use in the industry[1-5],but also due to its diverse coordination geometries[6-10].In fact,the electronic configuration of Pbギallows the Pb2+cation to exhibit variable coordination numbers from 2 to 10,and diverse coordination geometries.Therefore,the Pbギion also has a tendency to form polynuclear complexes and the potential to provide a stableframework structure[11-13].Particularly,theintrinsic features of Pbギ,the presence of a 6s2outer electron configuration,arouse great interest of chemists in coordination chemistry,supramolecular chemistry,photophysics and photochemistry[1-5,14-16].Meanwhile,several studies focused on the design of chelating ligands for the removal of lead from contaminated water and soils according to the coordination properties leading to preferential binding of Pbギover other essential metal ions[17-21].
Now much effort has been devoted to complexes constructed from heterocyclic carboxylate ligands,such as pyridine carboxylate,pyrazole carboxylate,pyrazine carboxyate and imidazole carboxylate ligands[22-30],due to their strong coordination ability and multicoordination modes by the N and Odonor atoms on the heterocyclic rings and the carboxyl groups.They coordinated to metal ions not only in diverse bridging modes but also in different chelating fashions.For example, 5-methyl-1H-pyrazole-3-carboxylic acid(H2MPCA)has been used to react with many transition metal ions(Cuギ,Coギ,Niギ,Cdギ and Mnギ),generating a lot of complexes,ranging from mononuclear to dinuclear,to trinuclear and then to 1D coordination polymers[30-35].But,the Pbギcomplex containing H2MPCA has been less explored[36].Considering outstanding characters of Pbギ ion and our group′s interest in constructing new complexes based on H2MPCA,we carried out reactions of H2MPCA with Pbギ salts,in the presence of different N-donor ancillary ligands of pyrazine and 1,10-phenanthroline(phen),two Pbギcomplexes,namely,[Pb(HMPCA)2(H2O)2]·H2O(1)and[Pb(HMPCA)2(phen)]·H2O(2),were isolated therefrom.Herein,the syntheses,crystal structures and photoluminescent properties of the complexes 1 and 2 were described.
1 Experimental
1.1 Materials and methods
The ligand H2MPCA was prepared according to the literatures[36-37].All other chemicals were of reagentgrade quality,obtained from commercial sources,and were used as received without further purification.The elemental analysis(C,H and N)was performed on a Perkin-Elmer 2400 SeriesⅡelement analyzer.The infrared spectra were recorded on a Nicolet 460 spectrometer by using KBr pellets.Powder X-ray diffraction (PXRD)determinations were performed on an X-ray diffractometer (D/max 2500 PC,Rigaku)with Cu Kα radiation(λ=0.154 06 nm).The operating voltage and current were 60 kV and 300 mA,respectively.The PXRD measurements were carried out over a 2θrange of 3°~80°in continuous scanning mode.Single-crystal X-ray diffraction measurements of 1 and 2 were carried out with a Bruker Smart ApexⅡCCD diffractometer at 293(2)K.Thermogravimetric analysis (TGA)experiments were carried out on a DuPont thermal analyzer from room temperature to 800℃under N2atmosphere at a heating rate of 10℃·min-1.The luminescent spectra of the solid samples were recorded with a Cary Eclipse spectrometer.
1.2 Preparation of[Pb(HMPCA)2(H 2O)2]·H 2O(1)
A mixture of H2MPCA (0.025 2 g,0.2 mmol),Pb(OAc)2·3H2O(0.076 0 g,0.2 mmol),KOH(0.022 4 g,0.4 mmol),pyrazine(0.016 0 g,0.2 mmol)and H2O(10 mL)was sealed in a 25 mL Teflon-lined autoclave and heated at 180℃for one day,and then cooled to room temperature at a rate of 5℃·h-1.After filtration,the product was washed with distilled water and then dried in vacuum,and colorless crystals of 1 suitable for X-ray diffraction analysis were obtained.Yield:58%(0.029 7 g,based on H2MPCA).Anal.Calcd.for C10H16N4O7Pb(%):C,23.48;H,3.15;N,10.95.Found(%):C,23.51,H,3.28,N,10.89.IR spectrum(KBr pellet,cm-1):3 593(s),3 328(s),3 188(s),3 093(s),2 957(m),2 838(m),1 579(vs),1 480(s),1 411(s),1 368(s),1 331(s),1 281(s),1 180(m),1 118(w),1 000(s),826(m),789(s),725(m),675(s),638(m),538(m),439(s).
1.3 Preparation of[Pb(HMPCA)2(phen)]·H 2O(2)
To the solution of H2MPCA (0.012 6 g,0.1 mmol)in EtOH(4 mL),a solution of Pb(NO3)2(0.033 1 g,0.1 mmol)in deionized water(3 mL)was added.After stirring for 30 min,phen (0.059 5 g,0.3 mmol)was added to the colorless solution,and stirred for further 2 h.The resulting solution was evaporated at room temperature slowly.Colorless block crystals of 2 were obtained after one week.After filtration,the product was washed with deionized water and then dried in vacuum.Yield:70% (0.022 9 g,based on H2MPCA).Anal.Calcd.for C22H20N6O5Pb(%):C,40.30;H,3.07;N,12.82.Found(%):C,40.62 H,2.98,N,12.66.IR spectrum(KBr pellet,cm-1):3 387(m),3 181(m),3 131(s),2 920(m),1 661(w),1 573(s),1 510(m),1 418(s),1 336(s),1 281(m),1 162(w),1 093(w),1 000(m),844(s),807(s),725(m),632(w),538(w),421(w).
1.4 Crystal structure determination
Single-crystal X-ray diffraction measurements of 1 and 2 were carried out with a Bruker Smart Apex CCDdiffractometer at 293(2)K.Intensities of reflections were measured using graphite-monochro-matized Mo Kα radiation(λ=0.071 073 nm)with the φ-ω scans modein arangeof 2.614°< θ< 24.998°(1)or 2.390°<θ< 24.999°(2).The structure was solved by direct methods using the SHELXSof the SHELXTL package and refined with SHELXL[38].H atoms attached to C were placed geometrically and allowed to ride during subsequent refinement with an isotropic displacement parameter fixed at 1.2 times Ueqof the parent atoms.All other hydrogen atoms bonded to O or N atoms were located from difference maps and refined with isotropic thermal parameters 1.5 times of their carrier atoms.The crystallographic data parameters for 1 and 2 are listed in Table 1.
CCDC:1854209,1;1854210,2.
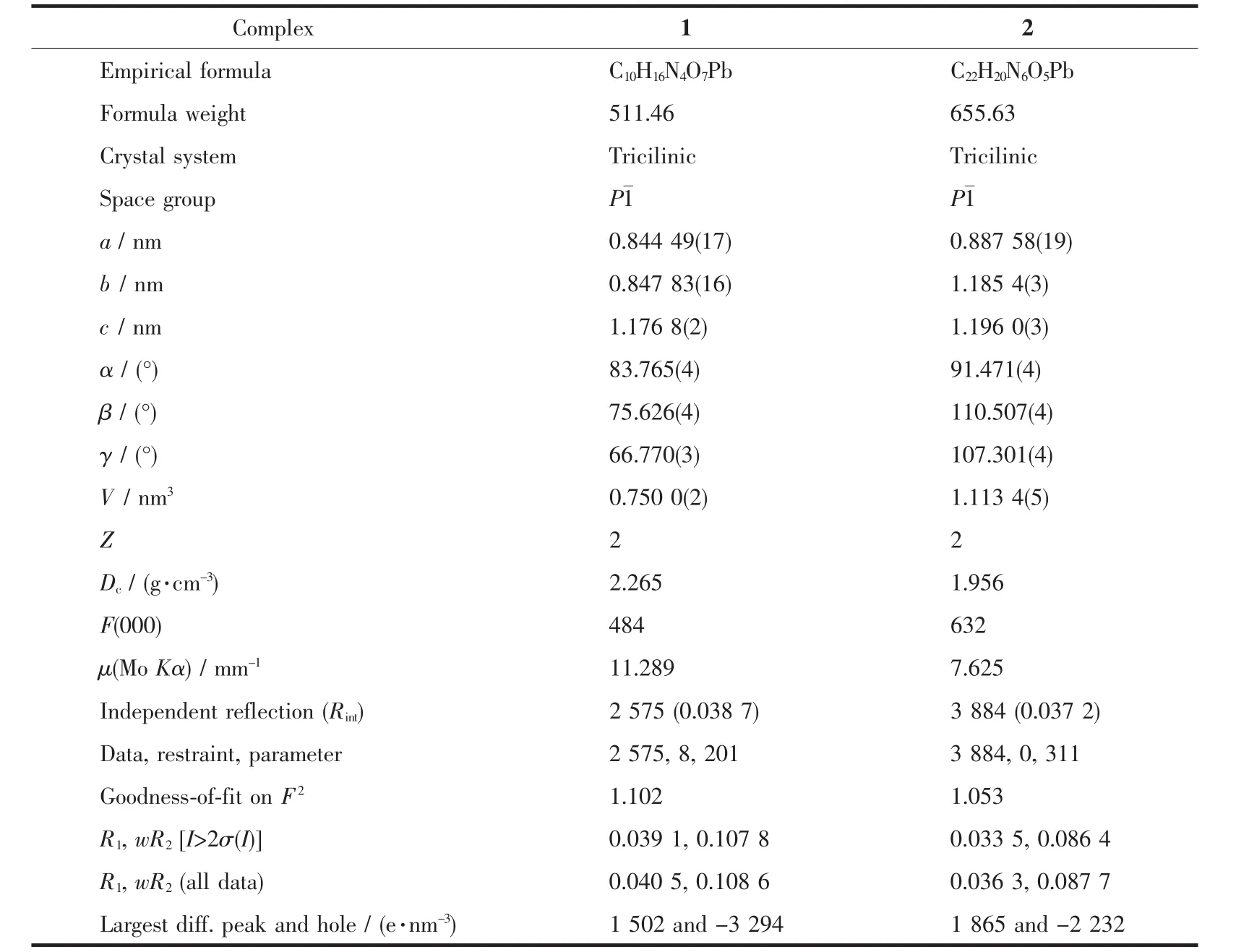
Table 1 Crystal data and structure refinement for 1 and 2
2 Results and discussion
2.1 Synthesis and IR spectra characterization
The dinuclear complex[Pb(HMPCA)2(H2O)2]·H2O(1)was prepared by the hydrothermal reaction of Pbギsalt with H2MPCA,pyrazine and KOH in a molar ratio of 1∶1∶1∶2 at 180 ℃,meanwhile only a powder of unknown composition was obtained under keeping other reaction conditions unchanged and in the absence of pyrazine.In this reaction,pyrazine may play a role in regulating pH value of the reaction system along with KOH.In 1,the H2MPCA ligand partly deprotonated and adopted two coordination modes:N,O-chelating (Scheme 1a)and μ2-κN,O∶κO bridging mode (Scheme 1b).When ancillary ligand phen was introduced to the reaction system,mononuclear complex 2,[Pb(HMPCA)2(phen)]·H2O,was generated by routine solution reaction.In 2,phen acted as chelating ligand,and the HMPCA-ligand also adopted two coordination modes:a μ2-κN,O ∶κO bridging mode(Scheme 1b)and O,O′-chelating mode(Scheme 1c).From the above discussion,we can see that the N ancillary ligands and reaction temperature play an important role in leading to various complexes from dinuclear(1)to mononuclear(2),which may be due to that the phen ligand chelated to Pbギion and increased the steric hindrance around the metal ion.Both 1 and 2 are stable in air and insoluble in water and common organic solvents.In the IR spectra of 1 and 2,the strong and broad absorption bands around 3 200~3 600 cm-1region are assigned as characteristic peaks of OH vibration,indicating that water molecules exist in them (Supporting Information,Fig.S1).In 1 and 2,the sharp bands at 3 140 cm-1are assigned to N-H vibration.The absorption peak between 1 690 and 1 730 cm-1wasnot observed,showingthat all carboxylic groups deprotonated in 1 and 2.The intense bands at 1 330~1 360 cm-1are ascribed to the conjugated C=N stretching vibration.The bands of 1 579 and 1 411 cm-1(1),and 1 573 and 1 418 cm-1(2)indicate the asymmetric and symmetric vibrations of COO-groups.Elemental analyses of 1 and 2 were consistent with the formulas.The identities of 1 and 2 were finally confirmed by X-ray crystallography.
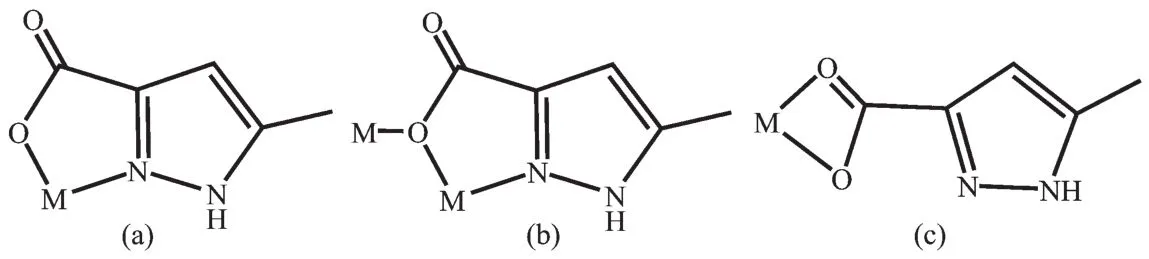
Scheme 1 Different coordination modes of HMPCA-in complexes 1 and 2
2.2 Description of crystal structure of[Pb(HMPCA)2(H 2O)2]·H 2O(1)
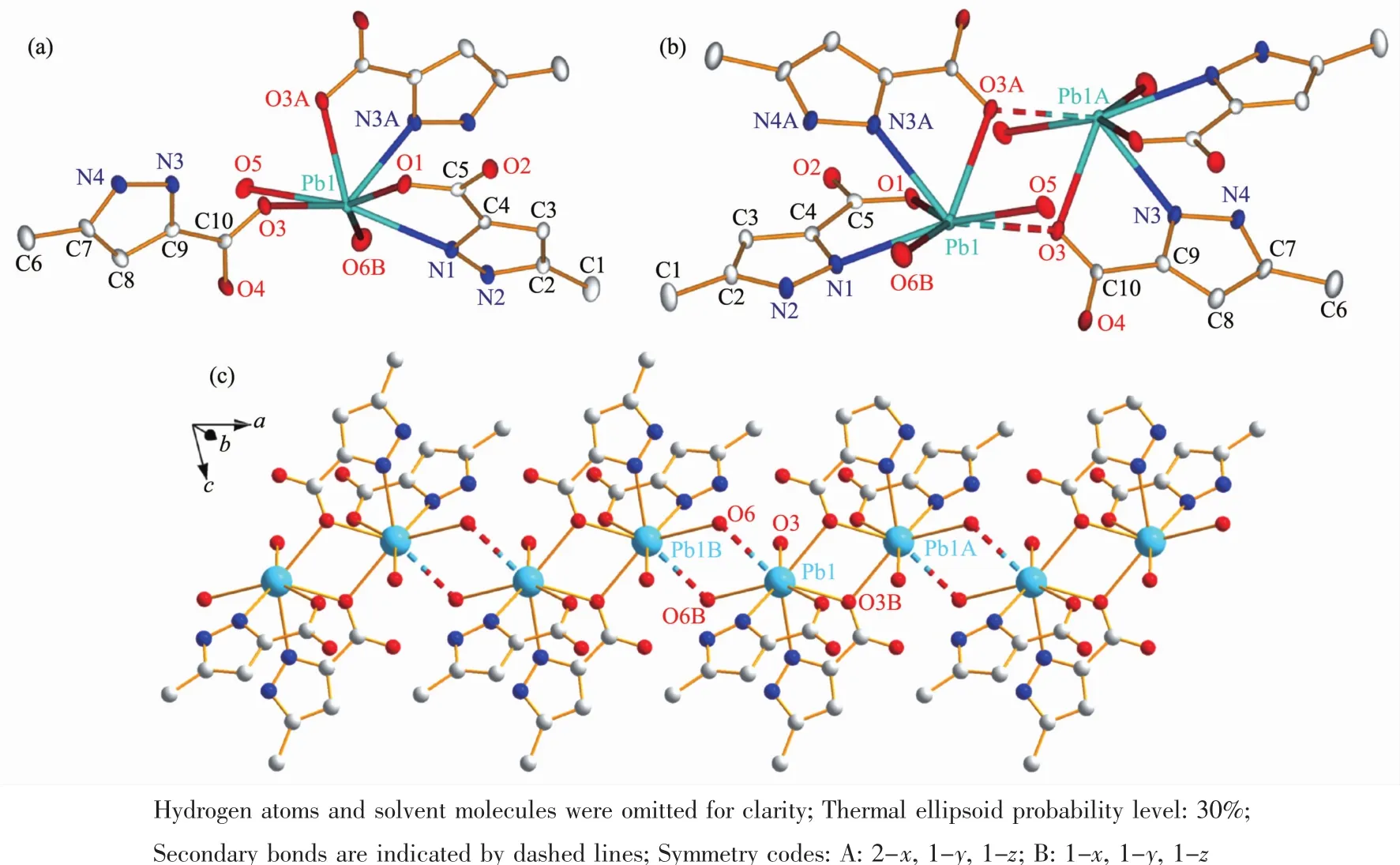
Fig.1 Illustrations of the crystal of 1:(a)coordination environment of Pbギion;(b)dinuclear unit;(c)1D chain constructed by the secondary bond of Pb…O
X-ray crystal structure analysis reveals that 1 crystallizes in the triclinic space group P1.The asymmetric unit consists of one Pbギcation,two HMPCA-anions,two coordinated water molecules and one lattice water molecule.The coordination sphere of Pbギis defined by two nitrogen atoms(N1 and N3A)from two HMPCA-anions,and three oxygen atoms(O1,O3 and O3A)from three HMPCA-anions and two oxygen atoms(O5 and O6B)from coordinated water molecules,leading to a hepta-coordinated hemihedral geometry.It is worth noting that the HMPCA-ligand in 1 adopts two coordination modes.Firstly,the HMPCA-ligand coordinates to the Pbギion in a N,O-chelating fashion(referred to a five-membered chelate ring)through the carboxylate oxygen atom O1 as well as its adjacent nitrogen atom N1 in the pyrazole ring(Scheme 1a and Fig.1a).Secondly,it coordinates to Pbギ ions through a μ2-κN,O∶κO bridging mode(Scheme 1b).As shown in Fig.1b,the bridging HMPCA-anion coordinates to the Pb1 ion through N3A and O3A atoms in the N,O-bonding moiety,and the carboxylate oxygen O3A simultaneously coordinates to the second Pbギion (Pb1A),resulting in the formation of a centrosymmetrical[Pb(HMPCA)(μ2-HMPCA)(H2O)]2unit with the Pb1…Pb1A distance of 0.429 2(1)nm,whichiscomparabletothatin Pbギ-pyridinecarboxylate complex[Pb(INO)Cl](0.430 7 nm,HINO=isonicotinic acid N-oxide)[5],and a little longer than that in the1D coordination polymer[Pb(HMPCA)2]n(0.421 2(4)nm).The Pb1…Pb1A distance is slightly shorter than the sum of the van der Waals radii (0.46 nm),which indicates that there are weak Pb…Pb contacts within the dinuclear units.The Pb-O bond lengths in the Pb2O2rhomb of 1,are 0.265 4(6)nm(Pb(1)-O(3))and 0.260 2(6)nm(Pb(1)-O(3A)),respectively.The mean Pb-OHMPCAbond length of 0.258 6(6)nm is slightly longer than that in[Pb(HMPCA)2]n(0.249 2(3)nm),but a little longer than that in the similar Pb2O2plane in[Pb(INO)Cl](0.267 03(3)nm)[5].The mean Pb-N bond length of 0.264 7(7)nm is close to those in[Pb(H2tpaa)Cl](0.270 2(6)nm,H3tpaa=α,α′,α″-nitrilotri(6-methyl-2-pyridinecarboxylic acid)[10], and [Pb(HMPCA)2]n(0.269 7(4)nm).The distances of Pb-Oaq(from coordinated water)(0.271 7(6)and 0.279 7(1)nm)are somewhat longer than the Pb-OHMPCAbond length.It is interesting to find that,there is a secondary bond of Pb1…O6(0.292 2(1)nm)between the Pb1 ion and the coordinated water molecule O6 of the adjacent dinuclear unit,leading to a 1D chainlike structure(Fig.1c).The lattice water molecule(O7)is embedded in the 1D coordination chain through intermolecular hydrogen bonding interaction N4-H4…O7E(Fig.S2a,Table S2).Then the 1D chains are engaged in hydrogen-bonding interactions between coordination water molecules(O5 and O6)and carboxylate oxygen atoms(O1,O2 and O4),and hydrogen bonds between pyrazole N atom(N2)and carboxylate oxygen atom(O4),forming a 2D supramolecular structure(Fig.S2b).
2.3 Description of crystal structure of[Pb(HMPCA)2(phen)]·H 2O(2)
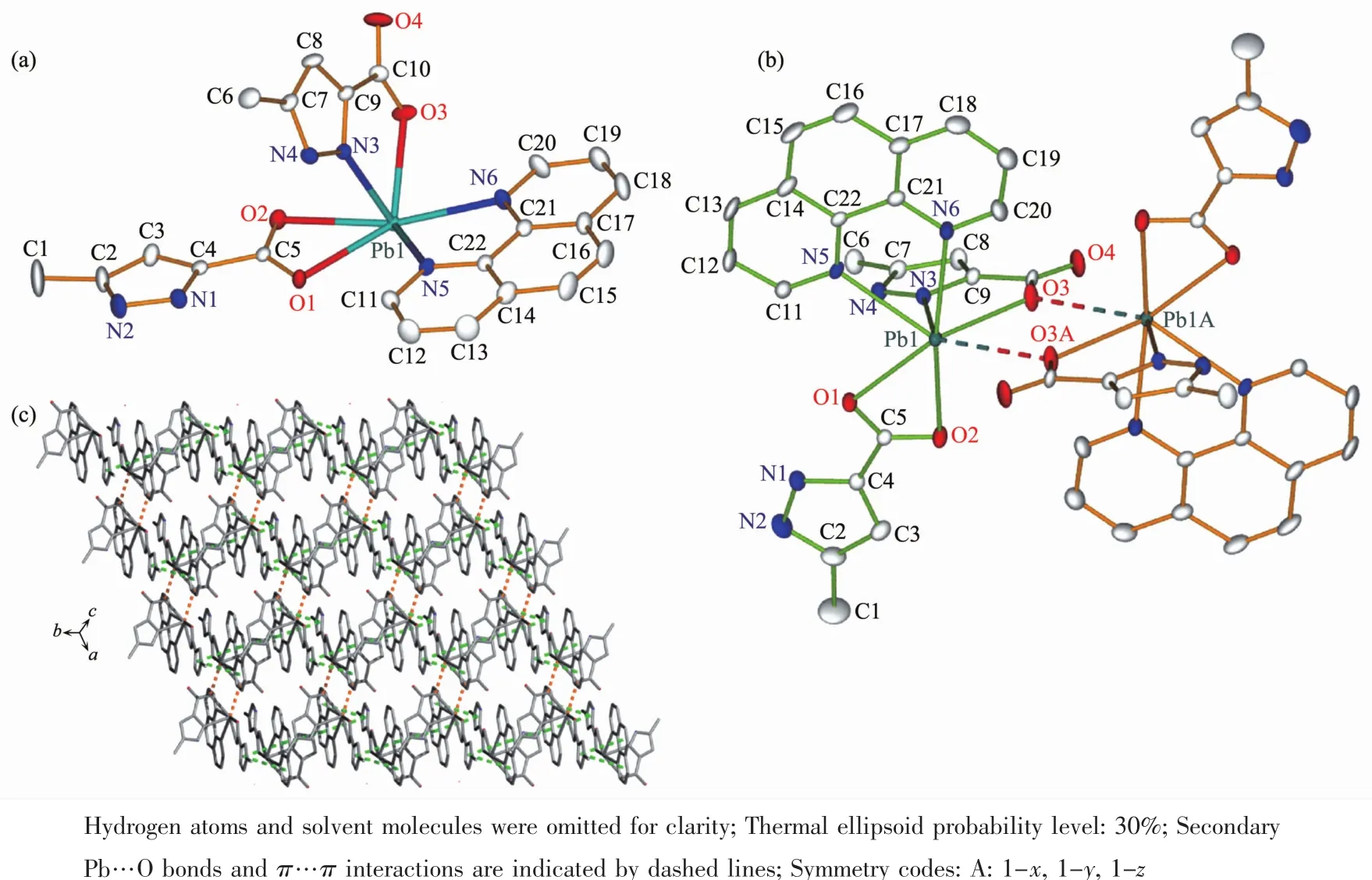
Fig.2 Illustrations of the crystal of 2:(a)coordination environment of Pbギion;(b)dinuclear unit;(c)1D chain constructed byπ…πstacking between phen and pyrazole rings
Complex 2 crystallizes in the triclinic space group P1.The asymmetric unit of 2 consists of one Pbギcation,two HMPCA-anions,one phen ligand and one lattice water molecule.Pbギion is coordinated by two nitrogen atoms(N5 and N6)from the phen ligand,a nitrogen atom (N3)from the HMPCA-anion and three carboxylate oxygen atoms(O1,O2 and O3)from two HMPCA-anions to furnish a hemidirected[PbO3N3]geometry.As shown in Fig.2a,the hexa-coordinated Pb1 ion exhibits a distorted hemidirected geometry.The equatorial positions are occupied by O1,O2,N5 and N6 atoms.The Pb-N(in a range of 0.254 8(5)~0.266 3(4)nm)and Pb-O(in a range of 0.254 8(4)~0.270 9(5)nm)(Table S1)bonds are termed as primary bonds.However,the secondary bond between Pb1 and carboxylate oxygen atom (Pb1…O3A)with a distance of 0.287 1(1)nm(dashed lines in Fig.2b)is longer than the sum of the ionic radii but significantly shorter than the sum of the van der Waals radii(0.354 nm)[39],which can be explained by the presence of an active lone electron pair in the proximity of the O atoms.If the Pb1…O3A bond is taken into account,then the geometry around Pb1 ion can be described as a heptacoordinated [PbO4N3]geometry.In addition,the bond angles around Pb1 are in a range of 48.78(15)°~166.12(17)°,similar to the Pbギ complexes previously reported[40]. The HMPCA-anion in 2 adopts two coordination modes:one is HMPCA-anion coordinates to two Pbギ ions through a μ2-κN,O∶κO bridging mode(Scheme 1b);the other chelates to a Pbギion though two carboxylate oxygen atoms O1 and O2(Scheme 1c),which is slightly different from the N,O-chelating fashion,μ2-κN,O∶κO,O′mode,μ2-κN,O∶κN′mode,μ3-κN,O∶κO,O′∶κO′modeand μ2-κN,O∶κOmode,presented in the reported metal complexes[30-36]and those in 1.By the function of theμ2-HMPCA-anion,two crystallographically equivalent[Pb(HMPCA)2(phen)]units are linked by a pair of Pb…O bonds to generate a centrosymmetrical Pb2O2rhomb with the Pb1…Pb1A distance of 0.468 4(1)nm,which is a little longer than the sum of the van der Waals radii(0.46 nm).This indicates that there is no Pb…Pb contacts within the dinuclear unit,which is different from that in 1.This may be due to that the phen ligands coordinate to the Pbギions and increase the steric hindrance around the metal ions.
By the function of four intermolecularπ…π interactions(Cg1…Cg4B 0.373 6(5)nm,Cg4…Cg1B 0.373 6(5)nm,Cg1…Cg5B 0.368 8(4)nm,Cg5…Cg1B 0.368 8(4)nm,Cg5…Cg5B 0.355 2(4)nm;Cg refers to the ring centroid of pyrazole of HMPCA-ligands,the arene ring and pyridyl of phen ligands,Symmetry codes:B:-x,1-y,1-z),the adjacent dinuclear units are linked to form a 2D layer(Fig.2c).Finally,these 2D layers further packed into a 3D supramolecular framework via three kinds of intermolecular hydrogen bonds:(i)N-H…O and O-H…N hydrogen-bonding interactions between uncoordinated pyrazole N atoms (N1,N2 and N4)and carboxylate oxygen atom(O4)and solvent water molecule(O5);(ii)O-H…O hydrogen-bonding interaction between water molecule(O5)and carboxylate oxygen atom(O4);(iii)C-H…O hydrogen-bonding interactions between C atomsfromphen ligands(C11 and C20)and carboxylate oxygen atoms(O1 and O2)(Table S2,Fig.S3).
2.4 PXRD and thermal analysis
In order to check the phase purity of 1 and 2,the powder X-ray diffraction (PXRD)patterns were recorded at room temperature.As shown in Fig.S4,the experimental PXRD pattern for each complex correlates well with its simulated one generated from single-crystal X-ray diffraction data,confirming the phase purity of the bulk materials.
In order to examine the thermal stability of 1 and 2,thermal gravimetric (TG)analyses were carried out from room temperature to 800℃under nitrogen(Fig.S5).In the TG curve of 1,the first weight loss of 9.50%in the 64~112 ℃ region corresponds to the loss of three water molecules(Calcd.10.56%).Above 301℃,the remaining substance is decomposed gradually,but this degradation does not end upon 800℃.In the TG curve of 2,the first weight loss of 2.46%in the 180~233 ℃ region corresponds to the loss of one water molecule (Calcd.2.75%).Then,the remaining substance is destroyed gradually,but this degradation does not end upon 800℃.
2.5 Luminescent properties
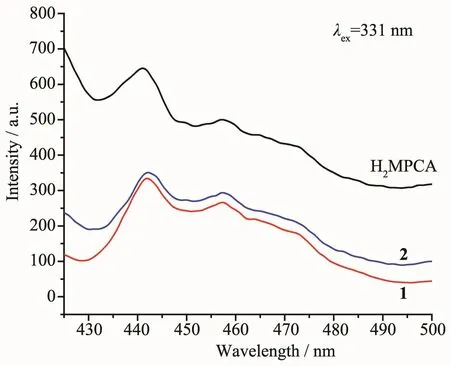
Fig.3 Solid-state emission spectra for complexes 1,2 and ligand H2MPCA at room temperature
The luminescent behaviors of ligand H2MPCA,complexes 1 and 2 were investigated in the solid state at room temperature owing to excellent luminescent properties of Pbギcomplexes[41-42](Fig.3).Upon excitation at 331 nm,the strongest emission peak for the ligand appeared at 441 nm,corresponding to theππ*transition.Both complexes 1 and 2 exhibited a peak centered at 442 nm,resembling that of the ligand.Therefore the origin of the main emissions of complexes 1 and 2 may be attributable to the internal charge transfer of ligand.In addition,the emission intensity of two complexes is obviously weaker than that of the free ligand,which may be due to the fluorescence quenching of no single-electron Pbギion with a certain role[36,43].
3 Conclusions
In summary,two Pbギcomplexes,ranging from dinuclear(1)to mononuclear(2)have been successfully synthesized through the self-assembly of H2MPCA ligand and Pbギsalts in the presence of N ancillary ligands.By the function of Pb…O secondary bond,complexes 1 and 2 are extended to a 1D chain or dinuclear structure,respectively.In 1 and 2,the HMPCA-groups adopt a bridging coordination mode and two chelating coordination modes,among which,the O,O′-chelating mode has never been reported in H2MPCA-based complexes.In addition,two complexes display blue fluorescence in the solid state at room temperature.
Supporting information isavailable at http://www.wjhxxb.cn
