基于Ni12P5纳米粒子的电化学传感器用于灵敏测定葡萄糖
2019-04-12徐金明陶菲菲
徐 雯 周 讯 徐金明 徐 涵 陶菲菲
(1黄山学院化学化工学院,无机功能材料重点实验室,黄山 245041)(2黄山学院分析测试中心,黄山 245041)(3绍兴文理学院化学化工学院,绍兴 312000)
Diabetes is one of the top ten diseases that would cause disability and even death in the world.Glucose concentration in human blood is one of the most key markers for the diagnoses and management of diabetes mellitus.Therefore,it is of paramount importance for developing a simple,sensitive and selective method to assay for glucose.Currently,a myriad of techniques have been utilized to determine glucose,including high performance liquid chromatography (HPLC)[1],chemiluminescence[2], fluorescence[3]and electrochemistry[4-7].Among these methods,the approach of electrochemical-based detection can allow a highsensitive in situ detection with short response time,wide linear range and low cost.Currently,various glucose electrochemical biosensors have been developed and they are typically classified into enzyme-based and non-enzyme-based sensors.In 1962,Clark and Lyons reported the first enzymatic electrochemical biosensor for the determination of glucose[8].Since then,three generations of glucose electrochemical sensors have been developed;they are all fabricated based on the glucose oxidase(GOx)enzymes.The enzyme-based sensor is highly sensitive and selective,but the natural enzymes are usually expensive and difficult to be fixed for the wide applications due to the typical drawbacks including time barrier,hydrophobic energy barrier,size barrier,etc.Especially,the sensor is easily affected by environmental temperature and pH value[9],and the enzyme fixed in the sensor tends to lose its corresponding enzymatic activity in the varied performing systems.Therefore,it is still a challenge to explore effective non-enzyme based glucose electrochemical biosensor to reduce and overcome the intrinsic shortcomings in a facile strategy.
For integrating the non-enzyme based biosensors,electrochemical approach has been certified as an effective way.For examples,noble metals and alloys,transition metal chalcogenides and nanostructured transition metal chalcogenides/carbon composites have been fabricated as electrode materials to detect glucose effectively[10-14].Recently,transition metal phosphides,as a sort of important functional materials,which have attracted wildly great interests in various catalysis reactions for hydro-desulfurization[15],oxygen reduction[16], hydrogen and oxygen evolution via water splitting[17-20],due to their superior and instinct electrical conductivity[21].However,transition metal phosphides have been rarely reported as electrode materials for non-enzyme based glucose detection except for the ones of Ni2P,CoPand NiCoP[22-24].
Herein,it is presented that Ni12P5NPs were synthesized via a modified one-pot hot-solution colloidal preparation method.Then,the obtained Ni12P5NPs which have been constructed to shape a sensitive non-enzyme based glucose sensor exhibited high electrocatalytic activity to glucose oxidation,with the virtues of a quick response time less than 3 s,a broad linear concentration ranging from 0.002 to 4.2 mmol·L-1,a high sensitivity up to 1 572 mA·L·mol-1·cm-2,and a detection limit as low as 0.8 μmol·L-1.The performances were favorably comparable to the most nickel-based catalysts,as seen the literature listed in below discussion.The results demonstrate that the electrode based on Ni12P5NPs can be applied as a glucose sensor with the advantages of high sensitivity,short response time and reproducibility.Additionally,it can be used to detect the glucose in the human blood serum with satisfactory result.
1 Experimental
1.1 Chemical and reagents
Nickelギ acetylacetonatehydrate(Ni(acac)2·x H2O,95%)was bought from Tokyo Chemical Industry(TCI).Oleylamine (OAm,80%~90%),tri-n-octylphosphine(TOP,90%)and 1-octadecene (ODE,90%)were provided by Alfa Aesar.D-(+)-Glucose,dopamine(DA),ascorbic acid(AA),uric acid(UA)and Nafion solution(5%(w/w))were provided by Sigma-Aldrich.Lactose(Lac),fructose(Fru),sodium hydroxide,ethanol and toluene were supplied by Sinopharm Chemical Reagent Ltd.All reagents were used directly as purchased.
1.2 Synthesis of Ni12P5 NPs
The synthesis of the Ni12P5NPs was adopted from Wang′s work with some modifications[25].Briefly,0.128 g Ni(acac)2,1 mL TOP,3 mL OAm and 2 mL ODE were introduced into a 100 mL three-necked flask and continuously magnetically stirred at room temperature under argon flow.Next,the mixed solution was heated to 140℃and held for 30 min to remove impurities such as dissolved oxygen and low boiling point solvent in the solution,and then the device was heated to 270℃ at a rate of 10℃·min-1and held for one hour.Finally,the apparatus was naturally cooled to normal temperature,then the black product was centrifuged five times with a mixed solution of toluene and ethanol and dried at 50℃under vacuum for further use.
1.3 Preparation of the working electrode
The bare glassy carbon electrode (GCE)was polished to the mirror with an Al2O3emulsion having a particle size of 0.3 and 0.05μm,and the electrode was ultrasonically cleaned with water,dilute nitric acid(VH2O∶VHNO3=1∶1,65%(w/w)HNO3)and ethanol each for 1 min.4.0 mg of the prepared Ni12P5powder and 30μL Nafion solution were added to 1.0 mL ethanolwater solution (VH2O∶VEtOH=1∶1), and ultrasonically dispersed for one hour to form a well homogeneous suspension.Then 5.0 μL of the suspension was dropped onto the surface of GCE and dried in air overnight.
1.4 Characterization methods
The Philips Xpert PRO X-ray diffractometer(Cu Kα,λ=0.154 178 nm,40 kV,50 mA,2θ=20°~80°)was used to get the crystalline structure and phase purity of the as-synthesized Ni12P5particles.Transmission electron microscopy (TEM)images reflecting the shape and size were obtained on a Hitachi H-7650 TEM(100 kV).X-ray photoelectron spectroscopy(XPS)were acquired on a Thermo ESCALAB 250 spectrometer(Al Kα,15 kV,10 mA)to determine the chemical compositions and the valence of the Ni12P5NPs.The energy dispersive spectroscopy(EDS)wascharacterized by scanning electron microscope (SEM,Hitachi S3400N,15 kV).
1.5 Electrochemical testing
Cyclic voltammetry(CV)and chronoamperometry(CA)werecarried out on an electrochemical workstation(CHI660E,ChenHua)using a typical three-electrode system,the Ni12P5modified electrode(φ=3 mm)served as the working electrode,a platinum sheet(1 cm2)as an auxiliary electrode and a saturated Ag/AgCl as the reference electrode.Every electrochemical measurement was carried out under normal temperature in 0.1 mol·L-1NaOH solution (pH=13).The water used in experiments was deionized water(18.2 MΩ·cm).
2 Results and discussion
2.1 Characterizations of the Ni12P5 NPs
The crystal structure of the as-prepared Ni12P5NPs was confirmed by XRD,as represented in Fig.1a.The diffraction peaks located at 32.73°,35.81°,38.41°,41.76°,44.42°,46.96°,48.96°,54.04°,56.16°,60.14°,68.59°and 74.85°observed in the pattern could be indexed to(310),(301),(112),(400),(330),(240),(312),(510),(501),(242),(161)and(352)planes of tetragonal Ni12P5(PDF No.74-1381)[26],respectively.There were not any obvious peaks from impurities and deviates,indicating the high purity of the samples.
To reveal the chemical components and electronic state of the Ni12P5NPs,the samples were measured by XPS.As shown in Fig.1b,Ni,P,C and O were present in the product without other impurity.Fig.1c exhibits the XPS spectrum for Ni12P5in the Ni2p.The split peaks at 870 and 873.6 eV accompanied by one satellite peak at 880.4 eV were found in the Ni2p1/2window[27],while the Ni2p3/2region showed three peaks at 852.7,855.7 and 860.9 eV[28].The peaks at 852.7 and 870 eV were attributed to Ni in Ni12P5[29,30],while those at 855.7 and 873.6 eV fitted well with oxidized Ni species[27,30].The peak at 852.7 eV was characteristics of Ni metal(852.5~852.9 eV),indicating that the Ni in Ni12P5has a very small positive charge[24].The P2p XPS spectrum(Fig.1d)exhibited a doublet at 130.35 and 129.55 eV,corresponding to P2p1/2and P2p3/2respectively[30-31]and the peak at 133.2 eV in good agreement with oxidized phosphorus species formed on the surface of Ni12P5because of air contact[32].The binding energy of P2p3/2(129.55 eV)was very close to that of zero-valent P(130.0 eV)[28,33],suggesting that the P in Ni12P5has a very small negative charge.The peaks of O and C could be assigned to the presence of oxygen,water and carbon dioxide molecules absorbed by the surface aswell ashydrocarbonsfromthe XPSmachineitself[34].

Fig.1 (a)XRD patterns of the Ni12P5 NPs;(b)Survey XPSspectrum for Ni12P5 NPs;High-resolution XPSspectra of Ni2p(c)and P2p regions(d)

Fig.2 Typical TEM images of Ni12P5 NPs with low magnification(a)and high magnification(b)
Fig.2a is a representative low-magnified TEM image of the as-synthesized Ni12P5NPs,it is clearly found that the Ni12P5NPs were uniform with monodisperse size.In detail,the size of the monodisperse nanoparticles was determined to be about 9 nm in diameter based on the highmagnification transmission electron microscope image,as seen in Fig.2b. Meanwhile, the chemical composition of the Ni12P5NPs was further measured by energy dispersive X-ray spectroscopy(EDX),as shown in Fig.S1.The result reveals that the nanoparticles were composed of 68.07%(n/n)Ni and 31.93%(n/n)P with an atomic ratio of approximately 2.13∶1 for nickel to phosphorus. This value approaches the stoichiometric ratio of Ni to P in Ni12P5.
2.2 Electrochemical characterization
To evaluate the electrocatalytic activity of the Ni12P5NPs to glucose oxidation,we dropped the Ni12P5NPs on the surface of GCE to form the modified electrode performed in 0.1 mol·L-1NaOH solution.In Fig.3a,there was a pair of redox peaks at 0.606 and 0.429 V observed for the Ni12P5NPs in absence of glucose,which could be connected with the Niバ/Niギ redox couples[22], corfirmed by XPS survey spectrum after CV treatment(Fig.S2~Fig.S4).When adding glucose,the peak potential of glucose oxidation was very close to that of Niギ oxidation to Niバ[13],and the adsorption of glucose and the oxidized intermediates on the active sites of the Ni12P5based electrode,so the anodic peak potential shifted a little in the positive direction[35],but an obvious increase of anodic current density appeared,which shows that there is an electrocatalytic activity of Ni12P5to glucose oxidation.In contrast,in case of GCE without addition of Ni12P5(inset),there was just a featureless voltammetric current within the potential range of interest observed no matter whether glucose was introduced or not.The results confirm that the electrocatalytic activity to glucose oxidation is benefited from the employment of the Ni12P5NPs.Meanwhile,it is noted that the current response of the Ni12P5/GCE electrode was very sensitive when adding glucose in 0.1 mol·L-1NaOH solution,as seen in Fig.3b.It further demonstrates that the Ni12P5/GCE is highly efficient for electrooxidation of glucose.In basic media,anodic scanning can lead to the formation of NiOx/Ni(OH)xon the surface of the Ni12P5NPs[20,30],so it can be concluded the mechanism of the electrocatalysts for Ni12P5as the following equations,equivalent to the one reported in literature[36]:

The effect of scan rate on electrooxidation of glucose was carried by CV with 0.1 mmol·L-1glucose in 0.1 mol·L-1NaOH solution(Fig.4a).When the scan rate increasing from 10 to 150 mV·s-1,the oxidation peak positively shifted,whereas the reduction peak negatively shifted,both the oxidation and reduction peak current density increased continuously,and the current density of oxidation peak showed a good linear relationship with the square root of the scan rate(R2=0.995 8),as shown in Fig.4b.The result clearly indicates that the electrooxidation of glucose on the Ni12P5modified electrode belongs to a diffusioncontrolled process[34].
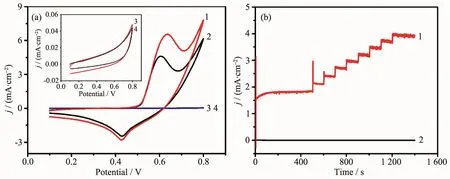
Fig.3 (a)CVs of Ni12P5/GCE(1,2)and bare GCE(3,4)in 0.1 mol·L-1 NaOH with the presence(1,3)and absence(2,4)of 0.1 mmol·L-1 glucose at a scan rate of 50 mV·s-1;(b)Amperometric response of the Ni12P5/GCE(1)and bare GCE(2)at 0.6 V with successive adding glucose of the same concentration in 0.1 mol·L-1 NaOH solution

Fig.4 (a)CV curves of Ni12P5/GCE in 0.1 mol·L-1 NaOH with 0.1 mmol·L-1 glucose at various scan rates;(b)Linear relationship of oxidation current density vs the square root of scan rate
Fig.5a presents the electrochemical response of the electrode in 0.1 mol·L-1NaOH solution at 0.6 V with the continuousinjection of different concentrations of glucose.The modified electrode showed a quick response to the varying concentration of glucose.When adding the glucose,the oxidation current increased immediately and reached the steady-state current in less than 3 s,the current density was linear with the glucose concentration over a wide range of 0.002 to 4.2 mmol·L-1(R2=0.998 9)and the sensitivity was 1 572 mA·L·mol-1·cm-2.The limit of detection was calculated as 0.8 μmol·L-1(signal-to-noise ratio S/N=3),with the results shown in Fig.5b.These values were comparable to most electrochemical glucose sensors constructed on Ni based nanomaterials,as listed in Table 1.
Selectivity is one of the most important parameters for evaluating the performance of sensor.Besides glucose,there are other sugars such as Fru or Lac and active substance such as DA,AA and UA in human blood.The normal value of glucose in the blood(4~7 mmol·L-1)is at least ten times higher than other interferent species (<0.1 mmol·L-1)[44].The experiment was carried out at the Ni12P5modified electrode by adding 1.0 mmol·L-1glucose in 0.1 mol·L-1NaOH with 0.1 mmol·L-1AA,UA,DA,Fru and Lac.As exhibited in Fig.6,the current density increased greatly when adding glucose,whereas the other interferents showed weak current responses.This result suggests that the determination of glucose by our approach is not interfered by the other interferents in the blood,and non-enzymatic glucose biosensor developed on this method exhibits high antiinterference ability.

Fig.5 (a)Amperometric response of the Ni12P5/GCE recorded at 0.60 V in 0.1 mol·L-1 NaOH solution with consecutive addition of various concentrations of glucose;(b)Linear fitting curve between current density and glucose concentrations
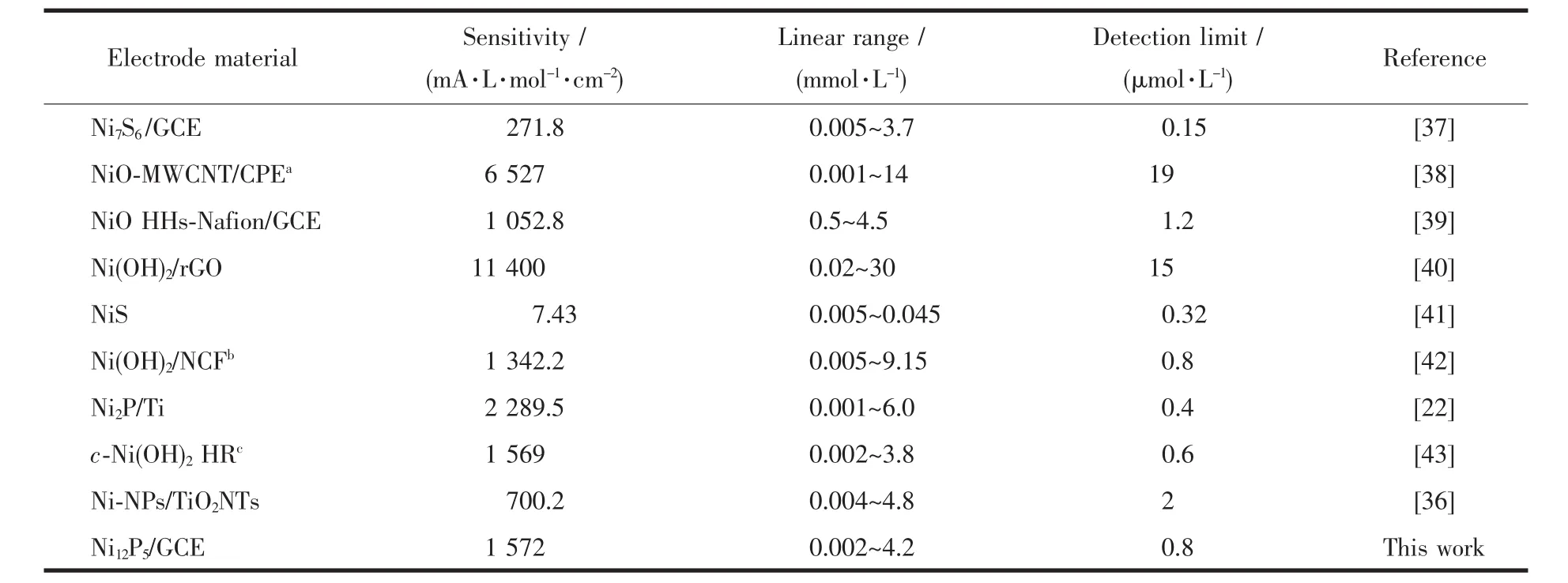
Table 1 Comparison between Ni12P5/GCE and other Ni-based electrochemical sensors for the detection of glucose

Fig.6 (a)Amperometric response of the Ni12P5/GCE with consecutive additions of 1 mmol·L-1 glucose and 0.1 mmol·L-1 DA,UA,AA,Fru in 0.1 mol·L-1 NaOH solution at 0.60 V;(b)Variation in the response current density of Ni12P5/GCE toward 0.1 mmol·L-1 glucose in 0.1 mol·L-1 NaOH for 30 days
To measure the reproducibility,0.1 mmol·L-1glucose was detected under the same conditions using five electrodes fabricated in the same manner,which produced a relative standard deviation (RSD)of 4.97%,showing a high reproducibility of our biosensor.The long-term stability of the sensor based on Ni12P5NPs was determined every five days by CA in 0.1 mol·L-1NaOH solution,the prepared sensor was kept in laboratory atmosphere when not in use.As depicted in Fig.6b,the current density did not show a sharp drop and 91.6%of the initial current response was retained after 30 days.These results demonstrate that the developed sensor is stable with good repeatability and reproducibility.
2.3 Sample determination
To examine the practicality of the biosensor,we applied the Ni12P5/GCE to detect the concentration of glucose in human blood serum obtained from the local hospital.30μL human blood serum was directly diluted with 0.1 mol·L-1NaOH solution,then the amperometric test was performed at an operating potential of 0.6 V and glucose concentration was calculated by linear equation,with results shown in Table 2.The results obtained from our sensor are in accordance with those tested by an automatic biochemical analyzer,and the recovery percentage is close to 100%.It should be confirmed that the developed biosensor can be applied for testing glucose with sufficient accuracy.

Table 2 Test results of glucose in human blood serum(n=3)
3 Conclusions
In summary,a sensitive nonenzymatic electrochemical sensor based on the Ni12P5NPs was designed for the first time to detect glucose.Owing to the high conductivity and excellent catalytic properties,the biosensor based Ni12P5NPs displayed excellent electrocatalytic activity towards oxidation of glucose in alkaline medium,high stability,low detection limit and wide linear range were obtained in the experiment.Especially,the biosensor could greatly eliminate the influence of interfering substances such as AA,DA and UA based on the intensive investigations.Thus,the sensor based on the Ni12P5NPs is promising for the routine detection of glucose.
Supportinginformation isavailableat http://www.wjhxxb.cn
