PbF2∶Er 3+,Yb3+荧光粉的制备和多波长响应双向转换发光机理
2019-04-12马小易王一帆严通廷柏朝晖
马小易 李 彪 王一帆 胡 盼 严通廷 柏朝晖
(长春理工大学材料科学与工程学院,长春 130022)
0 Introduction
Over the past few decades,lanthanide-doped luminescence materials exhibited promising prospects for application in upconversion lasers[1-2],display technologies[3-4],medical treatment[5-6],anti-counterfeit technologies[7-8]and solar cells[9-10].In particular,the application of up-and down-conversion phosphors to solar cells has attracted the attention of a large number of scientific researchers[11-12].In past study,ultraviolet(UV)and infrared light conversion to visible light were realized by doping of different lanthanide ions in different materials[13-16].Yu et al.[17]prepared Er3+-Yb3+co-doped TiO2-xFxupconversion luminescence powder via hydrothermal method. They applied the upconversion-TiO2-xFxinto dye sensitized solar cell(DSSC)and obtained an overall energy conversion efficiency of 7.08%.Zhang et al.[18]prepared SrAl2O4∶Eu2+,Dy3+powder via a combustion method,spectral characterization showed that it can convert ultraviolet(200~400 nm)to visible luminescence (520 nm).The solar conversion efficiency for a DSSC with SrAl2O4∶Eu2+,Dy3+doping (weight ratio of phosphor powder to TiO2was 7∶100)reached 7.938%.However,research on the fluorescence properties of bidirectional conversion luminescent materials that simultaneously convert ultraviolet and infrared light into visible light have rarely been reported.
Compared with other matrix materials,such as oxysulfide and NaYF4,lead fluoride (PbF2)have the advantage of excellent fluorescence response under 1 064 and 1 550 nm excitation,and are a fair good host material for bidirectional conversion luminescence[19-20].In this work,we reported a bidirectional conversion PbF2∶Er3+,Yb3+phosphors,which could convert both UV light (378 nm)and infrared light(808,980,1 064 and 1 550 nm)into visible light(blue,green and red).Therefore,the as-prepared phosphors could improve conversion efficiency of solar cells and be widely used in solar cells.The bidirectional conversion PbF2∶Er3+,Yb3+phosphors were synthesized by the high temperature solid-state reaction method.The luminescence properties of the phosphors were analyzed.In addition,the down-conversion (DC)and up-conversion(UC)multi-wavelength sensitive luminescence mechanisms of the samples were discussed in detail.
1 Experimental
1.1 Preparation
PbF2∶Er3+,Yb3+bidirectional conversion phosphors were prepared via the high temperature solid-state reaction method.PbF2(99.99%),Na2SiF6(99.99%)were purchased from Shanghai Sinopharm Chemical Reagent Company.Er2O3(99.99%),YbF3(99.99%)were purchased from Hawei Ruike Chemical Reagent Company,HNO3(65%~68%(w/w)),HF(40%(w/w))and anhydrous ethanol were purchased from Beijing Chemical Company.Firsly,excess diluted HNO3was added into the Er2O3and stirred on a magnetic stirrer until the oxides were completely dissolved.After cooling,poured the mixed solution into a plastic cup and HF was added into the solution to obtain ErF3precipitates,and washed several times.Then,the molar ratio of PbF2∶ErF3∶YbF3was 80∶2∶18 according to the composition of the compound.Reactants were weighed according to a predetermined molar ratio and thoroughly ground in an agate mortar.Finally,the base materials were placed in a crucible covered with NaSiF6,heated to predetermined temperature(650℃)under a fluoride atmosphere provided by the decomposition of NaSiF6and sintered for a certain time(1.5 h)to get the(Pb0.80Er0.02Yb0.18)F2phosphors.
1.2 Measurements
The XRD patterns were performed using a Rigaku D/max IIB diffractometer which recorded diffraction angel(2θ)from 20°to 80°,Cu Kα1(λ=0.154 06 nm)was used as the radiation source with an accelerating voltage of 40 kV and a working current of 20 mA.The structure refinement data was collected through using the general structure analysis system(GSAS)software.The emission spectra and the intensity-power curves were measured with UV-Vis spectrophotometry using RF-5301PCcoupled 808,980,1 064 and 1 550 nm laser.All the measurement data were performed at room temperature.
2 Results and discussion
2.1 Phase identification and crystal structure

Fig.1 (a)XRD pattern of the(Pb0.80Er0.02Yb0.18)F2 sample;(b)Rietveld refinement of the XRD pattern of(Pb0.80Er0.02Yb0.18)F2 sample;(c)Crystal structure representation of PbF2
Fig.1(a)gives XRDpatternof the(Pb0.80Er0.02Yb0.18)F2phosphors.It is seen that all peak positions were consistent with the standard PDF No.06-0251 of cubic PbF2.The XRD results indicated that the synthesized sample was a cubic PbF2structure with the space group of Fm3m(225).The Er3+and Yb3+ions have successfully entered PbF2host lattice by occupying the Pb2+sites;there was no impurity phase,which demonstrated that the synthetic crystal was pure phase.Fig.1(b)displays the refinement pattern of the(Pb0.80Er0.02Yb0.18)F2phosphor.The final refinement converged with indicator of goodness of fitχ2=1.858,with weighted profile factor Rwp=9.63%,and with profile factor Rp=7.76%,Thecomparison between experimental and calculated results illustrated that sample could better crystallize in the cubic.Fig.1(c)gives the crystal structure of PbF2and 8 coordination of Pb2+,which are distributed in face-centered cubic (FCC)crystal structure.
Table 1 presents the refinement result and structure parameter for (Pb0.80Er0.02Yb0.18)F2phosphors.According to the data acquired by refinement,the occupation ratio of the Er3+/Yb3+ions was 14.08%of the Pb2+position,indicating that the doped Er3+/Yb3+ions could effectively enter the PbF2lattice cell.The lattice parameters are a=b=c=0.585 7 nm,α=β=γ=90°.The crystal structure of resultant sample shifted to high angle in comparison with the peak positions of the standard PbF2crystallographic data (PDF No.06-0251,a=b=c=0.594 nm, α=β=γ=90°),which was mainly causeviabigger radius(Pb2+:0.143 nm)replaced by smaller radius(Er3+:0.117 nm,Yb3+:0.113 nm).
The Er3+and Yb3+ions entered PbF2lattice to replace Pb2+ions to form ErPband YbPb·and FF,and the F-ions in ErF3and YbF3entered PbF2lattice to replace F-ions to form FF.The excess F-ions entered[F8]interspace in unit cell,forming an interstitial Fi′.Because the valence of both Er3+and Yb3+ions are higher than that of Pb2+ions,charge imbalance is produced.The imbalance of charge between replacement and substituted ions leads to formation of vacant ions or interstitial ions[21-22].The possible reactions when Er3+and Yb3+ions entered into lattice sites of Pb2+ions were displayed below:

The relative difference between the ionic radius of Er3+and Yb3+ions and the ionic radius of Pb2+ionsis not more than 15%,which improves the tendency of replacement.More importantly,the presence of[F8]interspace in PbF2cell creates favorable conditions for the entry of F-(0.133 nm)into the lattice either in size(the length of[F8]interspace is 0.297 nm)or energy,so that the interstitial ion is in well coordination with interspace position.

Table 1 Refinement results and structure parameters for(Pb0.80Er 0.02Yb0.18)F2
2.2 Luminescent properties under excitation of 378 nm
Fig.2 exhibits the emission and excitation spectrum of the optimal sample.Fig.2a shows the excitation spectrum of the PbF2∶Er3+,Yb3+sample at 550 nm,which could be excited from the UV to the visible region,and highest peak intensity of the excitation spectrum at 378 nm.Therefore,to obtain a higher DC efficiency,the DC luminescence property of PbF2∶Er3+,Yb3+system at 378 nm excitation was studied.Fig.2b presents the DC spectrum of the sample excited at 378 nm.It could be divided into three parts:(1)A blue emission band with an emission peak near 409 nm;(2)Both peaks are located at the green light emission band near 521 and 550 nm;(3)A red-light emission center around 650 nm.The emission peaks at~409,~521,~550 and ~650 nm correspond to the2H9/2→4I15/2,2H11/2→4I15/2,4S3/2→4I15/2and4F9/2→4I15/2transitions,respectively.
Fig.3 describes the Yb3+-Er3+energy-level diagram of the sample excited at 378 nm.First,the electrons could be pumped from4I15/2to4G11/2by absorbing the energy of near-ultraviolet light at 378 nm,and Er3+ions transfered part of the energy to Yb3+ions,producing Yb3+∶2F5/2→2F7/2transition.Then,because of the narrow energy gap between the4G11/2and2H9/2,the4G11/2energy level in Er3+could easily relax to the2H9/2by non-radiation relaxation.Subsequently,particles in2H9/2returned to4I15/2generating blue luminescence around 409 nm.At this time,Er3+ions at the2H9/2level might relax to the2H11/2,4S3/2,4F9/2levels by the fast multiphoton process[23-24].In the end,Er3+ions decayed from2H11/2,4S3/2and4F9//2to the ground state4I15/2and emitted 521,550 and 650 nm red-green light emission,respectively.

Fig.2 Emission and excitation spectra of(Pb0.80Er0.02Yb0.18)F2 sample

Fig.3 Er3+-Yb3+energy level diagram excited at 378 nm
The cross relaxation (CR1)process results in particles significant reduction at the Er3+ion4S3/2level.Consequently,the emission intensity at 550 nm reduced.The CR2processleadstoasignificant increase in the population of the4F9/2level of Er3+ions.As a result,the 650 nm luminescence intensity was relatively enhanced.
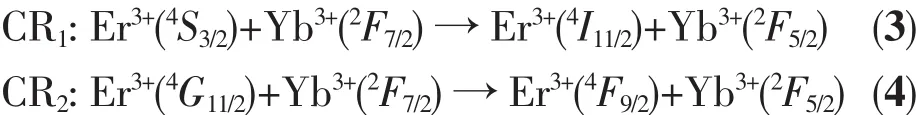
2.3 Upconversion spectral analysis
Fig.4 depicts a series of UC emission spectra of optimal sample excited by different wavelength infrared light.Under excitation of 1 064,980 and 808 nm lasers respectively,the spectra were presented an intense green emission at 540~550 nm as the result of the4S3/2→4I15/2transition of Er3+ions,the weak green emission at 521 nm derived from the2H11/2→4I15/2transition while the weak red emission at 650~665 nm originated from the4F9/2→4I15/2transition.Compared with other luminescence emission spectra,the phosphor presented an intense red emission under 1 550 nm excitation.

Fig.4 Emission spectra of the(Pb0.80Er0.02Yb0.18)F2 excited at different wavelengths
2.3.1 Luminescent mechanism analysis under 1 064 nm excitation
It is known that the emission spectrum of the trivalent rear earth (RE)ions doped UC material varies with excitation power[25].To identify the relative mechanism of the three UC emissions of Er3+ions excited at 1 064 nm at room temperature for the(Pb0.80Er0.02Yb0.18)F2sample.The dependence of emission intensities versus pump power is presented in Fig.5.The result is illustrated in the intensity-power plots,and the values of slopesfor the~521 nm (green),~550 nm(green)and~650 nm(red)were approximately to 2,indicating that the two-photon process was mainly responsible for the observed UC emission under 1 064 nm excitation.

Fig.5 Intensity-power plots of the green and red emissions versus excitation power at 1064 nm
Fig.6 illustrates the simplified Er3+-Yb3+energy level diagram of sample under excitation of 1 064 nm.Er3+ion acted as an activator and was the luminescent center of the sample.It is observed that the2F5/2state of Yb3+ions was very close to the4I11/2state of Er3+ions,therefore,an effective energy transmission process could take place between Er3+and Yb3+ions.Firstly,the2F5/2state of Yb3+ions could be populated through absorbing 1 064 nm infrared light.And the Er3+ion transited from4I15/2to4I11/2level by ground state absorption(GSA)and energy transfer(ET1)from Yb3+ions.Then,the electrons could be pumped from4I11/2state of Er3+ions to4F7/2state through ET2process:

Fig.6 Er3+-Yb3+energy level diagram excited at 1 064 nm

Furthermore,Er3+ions at the4F7/2level might relax to the2H11/2and4S3/2levels by the fast-multiphoton process.Subsequently,Er3+ions at the2H11/2and4S3/2energy level returned to the ground state4I15/2while emitting green light at 521 and 550 nm,respectively.In addition,Er3+ionsat the4I11/2level possibly underwent a non-radiative transition to reach the4I13/2levels,and the Er3+ion transited from the excited state4I13/2to4F9/2level by excited state absorption(ESA)and ET3:

Finally,the electron at the4F9/2level returns to4I15/2ground state and emitted weak red fluorescence at 650 nm.Furthermore,the CR1and CR2processamong Er3+ions enhanced the UC luminescence efficiency of the sample[26]:

2.3.2 Luminescent mechanism analysis under 1 550 nm excitation
Fig.7 depicts the measured power dependence of the three UC emissions of Er3+ions excited by 1 550 nm at room temperature for the(Pb0.80Er0.02Yb0.18)F2sample.The result is illustrated in the intensity-power plots,the green and red emissions exhibited a subcubic(2.675,2.60 and 2.873)power-law behavior for the excitation at 1 550 nm,which indicated that three pumping photons around 1 550 nm participate in the UCexcitation process.
Fig.8 presents the energy-level diagram of the sample excited by 1 550 nm.Yb3+ions could not absorb 1 550 nm infrared light,hence the UC luminescence should depend on the absorption process of Er3+ions and theenergy transfer up-conversion(ETU)process among Er3+ions.When excited by 1 550 nm infrared light,Er3+ions were promoted from the ground state4I15/2to the first excited state4I13/2by GSA.Subsequently,the Er3+ion transited from the excited state4I13/2to4I9/2level by ESA1and ETU1[27-28]:


Fig.7 Intensity-power plots of the green and red emissions versus excitation power at 1 550 nm

Fig.8 Er3+-Yb3+energy level diagram excited at 1 550 nm
Then,the electrons at4I9/2state could transit to2H11/2state through ESA2and transit to4I11/2level by the no-radiation relaxation process,and through the ETU2process transitions to the higher excited state2H11/2:

Finally,the Er3+ions at the2H11/2state relaxed to the4S3/2and4F9/2level by the fast-multiphoton process.The radiation decayed from the2H11/2,4S3/2and4F9/2states to the ground state and generated UC luminescence at 521,540~550 nm and 650~665 nm,respectively.
Compared with other luminescence emission spectra,the red emission of the sample under 1 550 nm excitation was increased.The increase of the red component in the sample was mainly due to the ETU3between the Er3+ion at the4I13/2and4I11/2level[29-30],resulting in an increase of Er3+ions at the4F9/2level.

2.3.3 Luminescent mechanism analysis under 980 nm excitation
The dependence excited by 980 nm is illustrated in the intensity-power plots in Fig.9.The green and red emissions exhibited a sub-square power-law behavior for the excitation at 980 nm,the values of slopes for the ~521 nm green emission,~550 nm green emission and~650 nm red emission are 1.857,1.920 and 1.831,respectively.It is suggested that two pumping photons around 980 nm participate in the UCexcitation process.

Fig.9 Intensity-power plots of the green and red emissions versus excitation power at 980 nm

Fig.10 Er3+-Yb3+energy level diagram excited at 980 nm
Fig.10 describes the simplified Yb3+-Er3+energylevel diagram of the sample under excitation of 980 nm.First,when the sample was irradiated by 980 nm laser,Yb3+ions transited from theground state2F7/2to2F5/2level.Then,the Er3+ion transited from the ground state to4I11/2was through either GSA or ET1process between Yb3+and Er3+ions:

Similarly,the Er3+ion transited from the excited state4I11/2to4F7/2level by ESA1,CR1,ET2:

In addition,Er3+ions at the4F7/2level relaxed to the2H11/2and4S3/2levelsby thefast-multiphoton process.Then,the radiation decayed from the2H11/2and4S3/2levels to the4I15/2ground state and generated green UC emission at 521 and 550 nm,respectively.Red UC emission mainly came from the population of4F9/2level of Er3+ions[31-32].The Er3+ions at4I11/2relaxed to the4I13/2by the fast-multiphoton processand then transit to4F9/2level by ESA2,CR2,CR3and ET3:

Finally,the electronic at4F9/2level decayed to4I15/2ground state and emitted red fluorescence at 650 nm.In summary,the red UC luminescence intensity was not as strong as the green emission,which was due to the large energy gap between the4S3/2and4F9/2energy levels,making it difficult to relax without radiation.
2.3.4 Luminescent mechanism analysis under 808 nm excitation
Fig.11 presents the energy-level diagram of the sampleexcited at808 nm.Firstly,Er3+ionsarepromoted from4I15/2to4I9/2by absorb an 808 nm photon(GSA).Since4I9/2,4I11/2and4I13/2belonged to different spectral branches of the same spectral term,Er3+ions at the4F9/2level relaxed to the4I11/2and4I13/2levels by noradiation transitions process.Subsequently,the Er3+ions at4I13/2could absorb an 808 nm photon to transit to4F7/2and4S3/2level(ESA1,ESA2).Then,the radiation decayed from the4S3/2levels to the4I15/2and generated green UC emission 550 nm.In addition,the CR2phenomenon caused energy transfer between ions at the4I11/2level,Er3+ions at the4F7/2level might relax to2H11/2by the fast-multiphoton process,the radiation decayed from the2H11/2levels to the4I15/2generateed weak green UC emission at 521 nm.Finally,another CR1between4I11/2and4I13/2level resulted in a large accumulation of ions at the4F9/2level,the electronic at4F9/2level decayed to4I15/2and emitted red fluorescence at 650 nm[33].


Fig.11 Er3+-Yb3+energy level diagram excited at 808 nm
3 Conclusions
In summary,we report on the bidirectional conversion properties of Er3+-Yb3+co-doped PbF2prepared by high temperature solid-state reaction method,which may be used for enhancement of the conversion efficiency of solar cells. XRD and structure refinement data showed the Er3+/Yb3+occupied solely Pb2+sites in the crystal host.Through analysis the DC and UC multi-wavelength sensitive luminescence mechanisms of the phosphor,it is found that the resultant phosphor could simultaneously convert both UV light(378 nm)and infrared light(808,980,1 064 and 1 550 nm)into visible light(blue,green and red)which could improve conversion efficiency of solar cells.
Acknowledgments:This project is financially supported by the National Natural Science Foundation of China(Grant No.51602027 and 61307118),the Education Project of Jilin Provincial Department,China(Grant No.JJKH20170607KJ).
Compliance with ethical standards:Conflict of interest:The authors declare that they have no conflict of interest.
